Meningitis
Authors: Allan R. Tunkel, M.D., Ph.D.
Acute meningitis is a clinical syndrome characterized by the onset of meningeal symptoms over the course of hours up to several days, and is defined by an abnormal number of white blood cells in cerebrospinal fluid (CSF). The acute meningitis syndrome may be caused by a wide variety of infectious agents and may also be a manifestation of noninfectious diseases. Here, I will concentrate on the more common infectious causes of acute meningitis, with emphasis on the bacteria, viruses, and mycobacteria that most typically cause this syndrome.
ETIOLOGY
Bacterial Meningitis
Bacterial meningitis remains a very important disease worldwide (1,2,3,4). The overall annual attack rate for bacterial meningitis, as defined by a surveillance study of 27 states in the United States from 1978 through 1981, was approximately 3.0 cases per 100,000 population, although there was variability based on age, race, and sex (1); the three most common meningeal pathogens (i.e., Haemophilus influenzae, Neisseria meningitides, and Streptococcus pneumoniae) accounted for more than 80% of cases. In a subsequent surveillance study conducted during 1995 in laboratories serving all the acute care hospitals in 22 counties of four states (>10 million population) (3), the incidence of bacterial meningitis decreased dramatically, as a result of a vaccine-related decline in meningitis caused by H. influenzae type b (from 2.9 cases per 100,000 population in 1986 to 0.2 cases per 100,000 population in 1995) such that in the United States, bacterial meningitis is now a disease predominantly of adults rather than infants and children; S. pneumoniae was the most frequent pathogen, accounting for 47% of cases. In a more recent epidemiologic survey, published only in abstract form (4), the incidence of bacterial meningitis declined from 1998 to 2003, with a reduction in pneumococcal meningitis in patients less than 2 years of age.
The isolation frequency of specific pathogens in patients with bacterial meningitis varies depending upon the age of the patient, various predisposing factors, and geographic locale. For example, in neonates, group B streptococcus is the most common etiologic agent. In patients 16 years or older with community-acquired bacterial meningitis (5,6,7), most cases are caused by S. pneumoniae, N. meningitidis, and Listeria monocytogenes. Bacterial meningitis is also a significant problem in hospitalized patients. In one review of 493 episodes of bacterial meningitis in adults 16 years or older at the Massachusetts General Hospital from 1962 through 1988 (5), 40% of episodes were nosocomial in origin, with most cases (38%) caused by gram-negative bacilli. In addition, bacterial meningitis is a major problem in other areas of the world. In the largest review of approximately 4100 cases of bacterial meningitis at Hospital Couta Maia in Salvador, Brazil from 1973 through 1982, the attack rate was 45.8 cases per 100,000 population (8), with H. influenzae, N. meningitidis, and S. pneumoniae accounting for 62% of the cases.
The following sections review the epidemiology and etiology of specific meningeal pathogens.
Streptococcus pneumoniae
S. pneumoniae is now the most frequently observed etiologic agent of community-acquired bacterial meningitis in the United States, accounting for 47% of the total cases and associated with a mortality rate ranging from 19 to 26% (1,2,3). Patients with pneumococcal meningitis often have contiguous or distant foci of infection such as pneumonia, otitis media, mastoiditis, sinusitis, or endocarditis. Serious infection may be observed in patients with various underlying conditions (e.g., splenectomy or asplenic states, multiple myeloma, hypogammaglobulinemia, alcoholism, malnutrition, chronic liver or renal disease, malignancy, and diabetes mellitus) (9,10). The pneumococcus is the most common etiologic agent of meningitis in patients who have suffered basilar skull fracture with CSF leak. In children who develop second episodes of pneumococcal meningitis, screening for congenital immunoglobulin deficiencies should be performed (11).
Neisseria meningitidis
N. meningitidis most commonly causes meningitis in children and young adults and is associated with an overall mortality rate of 3 to 13% (1,2,3). Meningococci of serogroups B, C, and Y account for most of the endemic disease in the United States; during an active and ongoing, laboratory-based, population-based surveillance for meningococcal disease in the United States from 1992-1996, serogroup C caused 35%, serogroup B caused 32%, and serogroup Y caused 26% of cases (12). In contrast, serogroup B accounted for 75% of isolates in Italy in a recent study (13). Disease caused by serogroups A and C may occur in epidemics; group Y strains may be associated with pneumonia. Several outbreaks of invasive meningococcal disease caused by serogroup C have been reported in the United States, Canada and Europe, with most caused by one strain of electrophoretic type 37 (ET-37) termed ET-15. Isolates of the ET-37 complex were also responsible for most cases of sporadic serogroup C meningococcal disease in another study (14). During the outbreak of meningococcal disease coinciding with the Hajj pilgrimage in March 2000, the attack rate of W135 disease was 25 cases per 100,000 pilgrims, with all outbreak-associated isolates of serogroup W135 members of a single clone of the hypervirulent ET-37 complex (15), occurring as a the result of expansion of a clone that had been in circulation since 1970. Respiratory tract infections, with viruses such as influenza virus, may play a role in the pathogenesis of invasive meningococcal disease. Nasopharyngeal carriage of N. meningitidis is an important factor that leads to the development of invasive disease (16). Patients with deficiencies in the terminal complement components (C5, C6, C7, C8, and perhaps C9), the so-called membrane attack complex, have a markedly increased incidence of neisserial infection (17), including that caused by N. meningitidis, although mortality rates in patients with meningococcal disease are lower than those in patients with an intact complement system. Because meningococcal meningitis occurs in ~39% of persons with late complement component deficiencies and 6% of those with properdin deficiencies, it has been suggested that a screening test for complement function (i.e., CH50) should be performed for all patients who have invasive meningococcal infections, with consideration of direct assessment of terminal complement components and properdin proteins (11).
Listeria monocytogenes
L. monocytogenes causes 8% of cases of bacterial meningitis in the United States and carries a mortality rate of 15 to 29% (1,2,3); serotypes 1/2b and 4b have been implicated in up to 80% of meningitis cases caused by this organism. Listerial infection is most common in infants younger than 1 month (up to 10% of cases), adults older than 50 years, alcoholics, cancer patients, those receiving corticosteroid therapy, and immunosuppressed adults (e.g., renal transplant recipients) (18,19). Other predisposing conditions include diabetes mellitus, liver disease, chronic renal disease, collagen-vascular diseases, and conditions associated with iron overload. Although colonization rates are low, pregnant women (who account for 25% of all cases of listeriosis) may harbor the organism asymptomatically in their genital tract and rectum and transmit the infection to their infants. Listeria meningitis is found infrequently in patients with HIV infectiondespite its increased incidence in patients with deficiencies in cell-mediated immunity. Adults less than 50 years of age who present with Listeria meningitis should be screened for HIV infection (11). Meningitis can also occur in previously healthy adults. Outbreaks of Listeria infection have been associated with the consumption of contaminated cole slaw, raw vegetables, milk, and cheese, with sporadic cases traced to contaminated cheese, turkey franks, alfalfa tablets, and processed meats (20), thus pointing to the intestinal tract as the usual portal of entry.
Streptococcus agalactiae
Group B streptococcus is a common cause of meningitis in neonates, with 52% of all cases in the United States reported during the first month of life (3); in the United States, the overall mortality rate ranges from 7 to 27% (1,2,3). Most cases of neonatal meningitis occur after the first week of life. Group B streptococcus has been isolated from the vaginal or rectal cultures of 15 to 35% of asymptomatic pregnant women; the risk of transmission from mother to infant is increased when the inoculum of organisms and the number of sites of maternal colonization are increased and is not related to the route of delivery. Horizontal transmission has also been documented from the hands of nursery personnel to the infant. Risk factors in adults who develop group B streptococcal meningitis include age older than 60 years, diabetes mellitus, pregnancy or the postpartum state, cardiac disease, collagen-vascular diseases, malignancy, alcoholism, hepatic failure, renal failure, previous stroke, neurogenic bladder, decubitus ulcers, and corticosteroid therapy (21,22); in one review of group B streptococcal meningitis in adults, no underlying illnesses were found in 43% of patients (21).
Haemophilus influenzae
H. influenzae was previously isolated in 45 to 48% of all cases of bacterial meningitis in the United States; this organism is now isolated in only 7% of cases (3); the overall mortality rate is 3 to 6% (1,2,3). Most episodes of meningitis previously occurred in infants and children with a peak incidence of 6 to 12 months of age, with 90% of cases caused by capsular type b strains. Isolation of this organism in older children and adults should suggest the presence of certain underlying conditions, including sinusitis, otitis media, epiglottitis, pneumonia, diabetes mellitus, alcoholism, splenectomy or asplenic states, head trauma with CSF leak, and immune deficiency (e.g., hypogammaglobulinemia) (23,24). Recently, a profound reduction in the incidence of invasive infections (including bacterial meningitis) caused by H. influenzae type b in the United States and Western Europe has been seen. This decrease in infection is attributed to the widespread use of conjugate vaccines against H. influenzae type b that have been licensed for routine use in all children beginning at 2 months of age. The benefits of vaccination have also been observed in the developing world. In one trial in The Gambia (25), the annual incidence of H. influenzae type b meningitis before use of the vaccine was 200 cases per 100,000 children less than 1 year of age compared to no cases in 2002.
Aerobic Gram-Negative Bacilli
Aerobic gram-negative bacilli (e.g., Klebsiella spp., Escherichia coli, Serratia marcescens, Pseudomonas aeruginosa, Salmonella spp.) have become increasingly important as etiologic agents in patients with bacterial meningitis (26,27). These agents may be isolated from the CSF of patients after head trauma or neurosurgical procedures and may also be found in neonates, the elderly, immunosuppressed patients, and patients with gram-negative septicemia. Some cases have been associated with disseminated strongyloidiasis in the hyperinfection syndrome, a condition in which meningitis caused by enteric bacteria results from seeding of the meninges during persistent or recurrent bacteremias associated with the migration of infective larvae. Alternatively, the larvae may carry enteric organisms on their surfaces or within their own gastrointestinal tracts as they exit the intestine and subsequently invade the meninges.
Staphylococci
Meningitis caused by Staphylococcus aureus is usually found in early postneurosurgical or post-trauma patients and in those with CSF shunts; other underlying conditions include diabetes mellitus, alcoholism, chronic renal failure requiring hemodialysis, injection drug use, and malignancies (28,29). Thirty-five percent of cases are observed in the setting of head trauma or after neurosurgery, and an additional 20% of patients have underlying infective endocarditis or paraspinal infection. Other sources of community-acquired S. aureus meningitis include patients with sinusitis, osteomyelitis, and pneumonia. Mortality rates have ranged from 14 to 77% in various series. Coagulase-negative staphylococci (e.g., Staphylococcus epidermidis) are the most common causes of meningitis in patients with CSF shunts.
Viral Meningitis
Viruses are the major cause of the acute aseptic meningitis syndrome, a term used to define any meningitis (infectious or noninfectious), particularly one with a lymphocytic pleocytosis, for which a cause is not apparent after initial evaluation and routine stains and cultures of CSF (30). Common viral etiologic agents that cause the acute aseptic meningitis syndrome are discussed in the following sections.
Enteroviruses
Enteroviruses are currently the leading recognizable cause of aseptic meningitis syndrome, accounting for 85 to 95% of all cases in which a pathogen is identified (30). Estimates from the Centers for Disease Control and Prevention (CDC) indicate that 30,000 to 75,000 cases of enteroviral meningitis occur annually in the United States. However, these figures are most likely an underestimation of the true incidence because of underreporting of enteroviral cases from state laboratories to the CDC. Enteroviruses are worldwide in distribution. In temperate climates they appear with a marked summer/fall seasonality, although in tropical and subtropical areas a high year-round incidence is observed. Periods of warm weather and wearing sparse clothing may facilitate the fecal-oral spread of these organisms. In the United States, the 14 most commonly occurring enteroviral serotypes account for more than 80% of isolates (31). In addition, the newly numbered enteroviruses 70 and 71 have been reported to commonly cause central nervous system (CNS) disease (32,33,34). Infants and young children are the primary victims of enteroviral meningitis because they are the most susceptible host population (i.e., absence of previous exposure and immunity) within the community. More than one episode of enteroviral meningitis may develop, although the same enteroviral serotype has not been implicated more than once in any immunocompetent patient. Enteroviruses are also the most common causes of aseptic meningitis in adults (35). Immunodeficiency (specifically congenital or acquired impaired humoral immunity) may predispose to enteroviral meningitis. Cases of enteroviral meningoencephalitis have also been seen in patients treated with the chimeric anti-CD20 monoclonal antibody rituximab (36).
Arboviruses
The most common arthropod-transmitted cause of aseptic meningitis in the United States, until 2002, was St. Louis encephalitis virus, a flavivirus. Aseptic meningitis accounts for about 15% of all symptomatic cases of St. Louis encephalitis and may be as high as 35 to 60% in children, although in patients older than 60 years, encephalitis is the more common finding. These infections are more frequent in warmer months when contact with the insect vector is more likely; vector exposure is more likely to occur indoors than outside because poorly sealed residences appear to be a risk factor. Other arboviruses reported to cause aseptic meningitis include the California encephalitis group of viruses (e.g., La Crosse, Jamestown Canyon, and snowshoe hare viruses, which are bunyaviruses) and the agent of Colorado tick fever, a coltivirus seen in the mountainous and western regions of the United States and Canada. West Nile virus may also cause aseptic meningitis or asymmetric flaccid paralysis, indistinguishable from poliomyelitis, although encephalitis is the most common manifestation (37).
Mumps Virus
In an unimmunized population, mumps is one of the most common causes of aseptic meningitis and encephalitis; symptomatic meningitis is estimated to occur in 10 to 30% of mumps patients overall, and is usually a benign and self-limited process (38). CNS disease caused by mumps virus can occur in patients without evidence of parotitis; 40 to 50% of patients with mumps meningitis have no evidence of salivary gland enlargement. Meningitis is the most common neurologic manifestation of infection with mumps virus. Males are affected two to five times more often than females, and the peak incidence is in children aged 5 to 9 years. Cases of vaccine-associated mumps meningitis have also been reported.
Herpesviruses
Herpesviruses include herpes simplex virus types 1 and 2, varicella-zoster virus, cytomegalovirus, Epstein-Barr virus, and human herpesviruses 6, 7, and 8. Although neurologic complications are known to occur with these viruses, complications associated with herpes simplex viruses are of the most significance. Overall, herpes simplex viruses account for approximately 0.5 to 3% of all cases of aseptic meningitis (39). In patients beyond the neonatal period, it is critical to differentiate between herpes simplex encephalitis, a potentially fatal form of encephalitis, and herpes simplex meningitis, a self-limited syndrome. The syndrome of herpes simplex virus aseptic meningitis is most commonly associated with primary genital infection with herpes simplex virus type 2, developing in 36% of women and 13% of men concomitant with primary infection in one study (40). Meningitis is less likely with recurrences of genital herpes (41). Primary genital infection with herpes simplex virus type 1 and nonprimary genital infection with herpes simplex virus of either type rarely result in meningitis. Acute aseptic meningitis has also been associated with herpes zoster in patients with or without typical skin lesions (42), the latter known as zoster sine herpete. Cases of Mollaret's recurrent meningitis have been associated with herpes simplex virus type 1, herpes simplex virus type 2 (43,44,45) and Epstein-Barr virus (46). Human herpesvirus 6 has also been associated with meningitis in conjunction with roseola infantum; however, this virus can exhibit persistence in the CNS and has been demonstrated in the CSF of asymptomatic persons (47). Cytomegalovirus and Epstein-Barr virus may cause aseptic meningitis in association with a mononucleosis syndrome, particularly in immunocompromised patients.
Human Immunodeficiency Virus
Human immunodeficiency virus (HIV) can cross the meninges early and persist in the CNS after initial infection (48,49). Meningitis associated with HIV may occur as part of the primary infection or occur in an already infected patient; HIV has been isolated from the CSF in some of these cases. However, acute meningitis does not occur in every individual who becomes infected and can be silent. Retrospective studies have noted that an acute meningoencephalitis is observed in 5 to 10% of HIV-infected patients during or after the mononucleosis-like syndrome that heralds the initial infection.
Tuberculous Meningitis
Virtually all tuberculous infections of the CNS are caused by the human tubercle bacillus, Mycobacterium tuberculosis. Much of the data on the incidence of CNS tuberculosis was obtained in the first half of the 20th century, when approximately 5-15% of individuals exposed to tuberculosis developed symptomatic disease; of this number, 5-10% of patients ultimately had CNS involvement (50). Tuberculous meningitis accounts for approximately 15% of extrapulmonary cases or about 0.7% of all clinical tuberculosis in the United States. Factors such as advanced age, immunosuppressive drug therapy, transplantation, lymphoma, gastrectomy, pregnancy, diabetes mellitus, and alcoholism are known to compromise the immune response in patients with smoldering chronic organ tuberculosis, leading to reactivation of latent foci and progression to the clinical syndrome of late generalized tuberculosis (51,52). The advent of HIV infection has also influenced the epidemiology of tuberculosis in the United States (53). Although the majority of tuberculosis cases in HIV-infected patients are pulmonary, extrapulmonary tuberculosis (including CNS disease) occurs in more than 70% of patients with the acquired immunodeficiency syndrome (AIDS) or AIDS discovered soon after the diagnosis of tuberculosis, but in only 24-45% of patients with tuberculosis and less advanced HIV infection (54); this suggests that extrapulmonary tuberculosis appears to be more common in patients with more severe HIV-induced immunosuppression.
CLINICAL MANIFESTATIONS
Bacterial Meningitis
Patients with bacterial meningitis classically present with fever, headache, meningismus, and signs of cerebral dysfunction (i.e., confusion, delirium, or a declining level of consciousness ranging from lethargy to coma) (9,55). In a review of 493 cases of acute bacterial meningitis in adults (5), however, the triad of fever, nuchal rigidity, and change in mental status was found only in two thirds of patients, although all patients had at least one of these findings. The meningismus may be subtle, marked, or acccompanied by Kernig's, Brudzinski's, or both signs (56). However, in a recent prospective study that examined the diagnostic accuracy of meningeal signs in adults with suspected meningitis, the sensitivity of these findings were only 5% for Kernig’s sign, 5% for Brudzinski’s sign, and 30% for nuchal rigidity (57), indicating that they did not accurately distinguish patients with meningitis from those without meningitis, and the absence of these findings did not rule out the diagnosis of bacterial meningitis. Cranial nerve palsies (especially those involving cranial nerves III, IV, VI, and VII) and focal cerebral signs are seen in 10 to 20% of cases. Seizures occur in about 30% of patients. Focal neurologic deficits and seizures arise from cortical and subcortical ischemia, which results from inflammation and thrombosis of blood vessels, often within the subarachnoid space. Papilledema is seen in less than 5% of cases early in infection, and its presence at the time of clinical presentation should suggest an alternative diagnosis. With disease progression, signs of increased intracranial pressure may develop, including coma, hypertension, bradycardia, and palsy of cranial nerve III.
To further characterize the accuracy and precision of the clinical examination in adult patients with acute meningitis, patient data on 845 episodes of acute meningitis (confirmed by lumbar puncture or autopsy) in patients aged 16-95 years were reviewed (58); the majority of patients in this review had acute bacterial meningitis, although 62 had tuberculous or “aseptic” meningitis. The results demonstrated that individual items of the clinical history (i.e., headache, nausea, and vomiting) had a low accuracy for the diagnosis of acute meningitis in adults. However, on review of the accuracy of physical examination findings, the absence of fever, neck stiffness, and altered mental status effectively eliminated the likelihood of acute meningitis; the sensitivity was 99-100% for the presence of one of these findings in the diagnosis of acute meningitis. Despite these findings, physicians should have a low threshold for performance of lumbar puncture in patients at high risk for bacterial meningitis, given the serious nature of this disease.
A specific etiologic diagnosis in patients with bacterial meningitis may be suggested by certain symptoms or signs (55). About 50% of patients with meningococcemia, with or without meningitis, present with a prominent rash located principally on the extremities. Early in the course of illness, the rash is typically erythematous and macular, but it quickly evolves into a petechial phase with further coalescence into a purpuric form. The rash often matures rapidly, with new petechial lesions appearing during the physical examination. In one review of the clinical features of 255 patients with acute meningococcal meningitis (59), a petechial rash was observed in three quarters of the patients, and was more commonly seen in children and adults younger than 30 years (81%) than in patients 30 years of age and older (62%). In patients who have suffered a basilar skull fracture in which a dural fistula is produced between the subarachnoid space and nasal cavity, paranasal sinuses, or middle ear, rhinorrhea or otorrhea may be present secondary to a CSF leak (29); in these patients, meningitis may be recurrent and is most commonly caused by S. pneumoniae. Patients with L. monocytogenes meningitis have an increased tendency to have seizures and focal deficits early in the course of infection, and some patients may present with ataxia, cranial nerve palsies, or nystagmus secondary to rhombencephalitis (18,19). In a large review of 367 episodes of CNS infections caused by L. monocytogenes (19), the most frequent findings were fever (92%) and altered sensorium (65%), with headache reported in only about 50% of patients.
Some categories of patients may not manifest many of the classic symptoms and signs of bacterial meningitis (55). For example, neonates with bacterial meningitis usually do not have meningismus (60); in this patient population, clinical clues to the presence of meningitis are temperature instability (hypothermia or hyperthermia), listlessness, high-pitched crying, fretfulness, lethargy, refusal to feed, weak suck, irritability, jaundice, vomiting, diarrhea, or respiratory distress. A change in the child's affect or state of alertness is one of the most important signs of meningitis; seizures are observed in 40% of cases. A bulging fontanelle (seen in one third of cases) usually occurs late during the course of illness. In children 1 to 4 years of age, fever (94%), vomiting (82%), and nuchal rigidity (77%) are the most common initial symptoms (61). Elderly patients, especially those with underlying conditions (e.g., diabetes mellitus or cardiopulmonary disease), may present insidiously with lethargy or obtundation, no fever, and variable signs of meningeal inflammation (62). In one review (63), confusion was very common in elderly patients on initial examination and occurred in 92% and 78% of those withpneumococcal and gram-negative bacillary meningitis, respectively. The diagnosis of bacterial meningitis in neutropenic patients requires a high index of suspicion because symptoms and signs may initially be subtle because of the impaired ability of the patient to mount a subarachnoid space inflammatory response (64). In patients with head trauma, the symptoms and signs of meningitis may be present as a result of the underlying injury and not meningitis. In all of these subgroups of patients, altered or changed mental status should not be ascribed to other causes until bacterial meningitis has been excluded by CSF examination.
Viral Meningitis
Enteroviruses
The clinical manifestations of enteroviral meningitis depend on host age and immune status (31,65). In neonates (2 weeks of age or younger) with proven enteroviral meningitis, fever is a ubiquitous finding and is usually accompanied by any combination of vomiting, anorexia, rash, and upper respiratory symptoms and signs. Neurologic involvement may be associated with nuchal rigidity and a bulging anterior fontanelle, although infants younger than 1 year are less likely to demonstrate meningeal signs. Mental status may be altered, but focal neurologic signs are uncommon. With disease progression, a sepsis-like syndrome characterized by multiorgan involvement, disseminated intravascular coagulation, and cardiovascular collapse may develop. Lack of humoral antibody may contribute to the severity of neonatal infection.
In patients with enteroviral meningitis beyond the neonatal period (older than 2 weeks of age), severe disease and poor outcome are rare. In this patient population the onset of illness is usually sudden, with fever present in 76 to 100% of patients; the fever may be biphasic, initially appearing with nonspecific constitutional symptoms, disappearing, and then reappearing with the onset of meningeal signs; more than half of patients have nuchal rigidity. In adults with enteroviral meningitis, headache (often severe and frontal in location) is nearly always present and photophobia is common. Nonspecific symptoms and signs include vomiting, anorexia, rash, diarrhea, cough, upper respiratory findings (especially pharyngitis), and myalgias. Other clues to the presence of enteroviral disease, in addition to the time of year and known epidemic disease in the community, include the presence of exanthems, myopericarditis, conjunctivitis, and specifically recognizable enteroviral syndromes such as pleurodynia, herpangina, and hand-foot-and-mouth disease (30). Specific clinical stigmata may also be associated with certain enteroviral serotypes (65) – echovirus 9 is associated with scattered maculopapular rashes, herpangina (in particular the finding of painful vesicles on the posterior oropharynx) is associated with coxsackievirus A, and the presence of pericarditis or pleurisy may identify coxsackievirus B.
The duration of illness in enteroviral meningitis is usually less than 1 week, with many patients reporting improvement after lumbar puncture, presumably as a result of a reduction in intracranial pressure. In contrast, during an outbreak of enterovirus 71 infection in Taiwan in patients 3 months to 8.2 years of age, the chief neurologic complaint was rhombencephalitis (seen in 90% of children), which carried a case fatality rate of 14% (33). In another outbreak in young children of enterovirus 71 infection in Perth, Western Australia (34), neurologic syndromes included aseptic meningitis, Guillain-Barre syndrome, acute transverse myelitis, acute cerebellar ataxia, opso-myoclonus syndrome, benign intracranial hypertension, and febrile convulsions.
In persons who are agammaglobulinemic, a chronic enteroviral meningitis or meningoencephalitis may develop and last several years, often with a fatal outcome (31,65). This syndrome has been designated chronic enteroviral meningoencephalitis in agammaglobulinemia (CEMA), a constellation of neurologic symptoms that includes headache, seizures, hearing loss, lethargy/coma, weakness, ataxia, parethesias, and loss of cognitive skills.
Mumps Virus
In patients with mumps, CNS symptoms usually follow the onset of parotitis, when present, by about 5 days; salivary gland enlargement is present in only about 50% of patients. The most frequent clinical manifestation of mumps CNS infection is the triad of fever, vomiting, and headache (38). The fever is usually high and lasts for 72 to 96 hours. Other findings include neck stiffness, lethargy or somnolence, and abdominal pain. Most patients have signs of meningitis, but no evidence of cortical dysfunction. Defervescence is usually accompanied by clinical recovery, with the total duration of illness is usually 7 to 10 days in uncomplicated cases. Mumps may rarely cause encephalitis, seizures, polyradiculitis, polyneuritis, cranial nerve palsies, myelitis, Guillain-Barré syndrome, and fatality.
Herpesviruses
Meningitis associated with herpes simplex virus type 2 is usually characterized by stiff neck, headache, and fever (40). In one review of 27 patients with herpes simplex virus type 2 meningitis 419), neurologic complications were found in 37% of cases and consisted of urinary retention, dysesthesias, paresthesias, neuralgia, motor weakness, paraparesis, concentration difficulties of about 3 months' duration, and impaired hearing. All complications, however, subsided within 6 months in all patients, although recurrent meningitis was documented in five patients. A diffuse vesiculopustular rash may be seen in meningitis caused by varicella-zoster virus. The presence of pharyngitis, lymphadenopathy, and splenomegaly should suggest Epstein-Barr virus infection.
Human Immunodeficiency Virus
HIV-infected patients may present with a typical aseptic meningitis syndrome associated with acute infection (48,49). Other patients may present with an atypical aseptic meningitis that is often chronic, tends to recur, and often includes cranial neuropathies (usually cranial nerves V, VII, and VIII) or long-tract findings. The most common features are headache, fever, and meningeal signs. The illness is self-limited or recurrent rather than progressive.
Tuberculous Meningitis
The clinical picture of tuberculous meningitis is quite variable (50). Children commonly develop nausea, vomiting, and behavioral changes, with headache is seen in fewer than 25% of cases. Seizures are infrequent (seen in 10-20% of children prior to hospitalization), although more than 50% of patients may develop seizures during hospitalization. In adults, the clinical presentation of tuberculous meningitis tends to be more indolent (50,66,67), with an insidious prodrome characterized by malaise, lassitude, low-grade fever, intermittent headache, and changing personality ensues. Within 2-3 weeks, there is development of a meningitic phase manifested as protracted headache, meningismus, vomiting, and confusion. In some adults, the initial prodromal stage may take the form of a slowly progressive dementia over several months or years characterized by personality changes, social withdrawal, and memory deficits. In contrast, patients may also present with a rapidly progressive meningitis syndrome indistinguishable from pyogenic bacterial meningitis (51). A history of prior clinical tuberculosis is infrequent (<20% of cases) in patients with tuberculous meningitis (66,68).
On physical examination, children and adults with tuberculous meningitis present with more uniform findings, although considerable variation does exist (50,51,66,67,68,69,70). Fever is seen in 50-98% of cases, although meningismus and signs of meningeal irritation are absent in 25-80% of children and adults. Focal neurologic signs most frequently consist of unilateral or, less commonly, bilateral cranial nerve palsies, which are seen in up to 30% of patients at presentation; the most frequently affected is cranial nerve VI, followed by cranial nerves III, IV, and VIII. Hemiparesis may result from ischemic infarction in the anterior cerebral circulation, most commonly in the territory of the middle cerebral artery. Funduscopic examination may reveal choroidal tubercles, but these are seen in only about 10% of cases of tuberculous meningitis.
The clinical manifestations of tuberculous meningitis do not appear to be modified in patients with HIV infection (71,72), but HIV co-infection may affect the number and nature of complications including an increased case fatality rate (73). Most HIV-infected patients with tuberculous meningitis present with fever, headache, and altered mentation. Meningeal signs are absent in up to 50% of patients, although in a recent review of patients with tuberculous meningitis admitted to an intensive care unit, 88% had neck stiffness (70).
DIAGNOSIS
Neuroimaging Prior to Lumbar Puncture
The diagnosis of meningitis depends upon examination of cerebrospinal fluid (CSF) obtained after lumbar puncture, although some patients should undergo neuroimaging prior to lumbar puncture because of the possibility of life-threatening brain herniation, which may occur in the patient with elevated intracranial pressure (74,75,76). For example, in patients with intracranial space-occupying lesions, there is a relative pressure gradient with downward displacement of the cerebrum and brainstem that can be increased by lumbar puncture, thereby precipitating brain herniation. The incidence of this complication is unknown, although in one older study that examined the outcome of lumbar puncture in 129 patients with elevated intracranial pressure, 1.2% of patients with papilledema and 12% of patients without papilledema had unfavorable outcomes within 48 hours of the procedure; combining these data with a review of 418 patients with papilledema, the authors concluded that the actual risk of serious complications from lumbar puncture in the presence of papilledema was “much less than 1.2%.” In another study of 302 infants and children with bacterial meningitis found that brain herniation developed in 6% of patients (77), occurring within 8 hours of lumbar puncture in all cases.
In a recent study in 301 adults with bacterial meningitis (78), the following clinical features at baseline that were associated with an abnormal CT scan of the head: age of at least 60 years, history of CNS disease (mass lesion, stroke, and focal infection) immuncompromised state (human immunodeficiency virus infection or acquired immunodeficiency syndrome, immunosuppressive therapy, and transplantation), a history of seizure within one week before presentation, and certain specific neurologic abnormalities (an abnormal level of consciousness, an inability to answer two consecutive questions correctly or to follow two consecutive commands, gaze palsy, abnormal visual fields, facial palsy, arm drift, leg drift, abnormal language). None of these features was present at base line in 96 of the 235 patients who underwent CT scanning. Of these 96 patients, the CT scan was normal in 93 of these patients, yielding a negative predictive value of 97%; of the three remaining patients, only one had mild mass effect on CT and all three underwent lumbar puncture with no evidence of brain herniation. Based on these findings, specific guidelines were recommended for adult patients who should undergo CT scanning prior to lumbar puncture (Table 1) (74). In addition, some authorities would delay lumbar puncture for 30 minutes in patients with short, convulsive seizures, or not perform the lumbar puncture at all in those with prolonged seizure because the seizure may be associated with transient increases in intracranial pressure. This is not the practice in children, however, because seizures occur in up to 30% of children with bacterial meningitis before admission.
Cerebrospinal Fluid Examination
Cerebrospinal fluid (CSF) findings in patients with acute meningitis vary depending upon the etiologic agent (Table 2); these findings are discussed in more detail below.
Bacterial Meningitis
In patients with bacterial meningitis, CSF examination after lumbar puncture is critical to establish the diagnosis (55,74,79). Opening pressure is generally in the range of 200-500 mm H2O, although values may be lower in neonates, infants, and children with acute bacterial meningitis. The CSF appearance may be cloudy, depending upon the presence of significant concentrations of white blood cells, red blood cells, bacteria, and/or protein. The CSF white blood cell concentration is elevated (generally from 1000-5000/mm3), although can range from <100/mm3 to >10,000/mm3. The white blood cell differential is usually that of a neutrophil predominance in CSF, typically between 80% and 95%, although 10% of patients with acute bacterial meningitis present with a lymphocyte predominance (defined as more than 50% lymphocytes or monocytes) in CSF. The CSF glucose concentration is <40 mg/dL in about 50-60% of patients; a CSF:serum glucose ratio of ≤0.4 was 80% sensitive and 98% specific for the diagnosis of bacterial meningitis in children older than 2 months of age. The CSF protein concentration is elevated in virtually all patients with bacterial meningitis.
Viral Meningitis
CSF pleocytosis is almost always present in patients with enteroviral meningitis, although some enteroviruses have been isolated from young infants with clinical evidence of meningitis but no CSF white blood cells (30,65). The cell count is usually 100 to 1000/mm3, although counts in the several thousands have also been reported; higher CSF white blood cell counts have been associated with a greater likelihood of isolating the causative enterovirus. Early in infection, neutrophils may dominate the CSF profile, although this situation quickly gives way to a lymphocytic predominance, usually over the first 6 to 48 hours. However, in a recent retrospective chart review of 158 cases of meningitis (138 aseptic and 20 bacterial), 51% of the 53 patients with aseptic meningitis and duration of symptoms for more than 24 hours had a neutrophil predominance in CSF (80), suggesting that a CSF neutrophil predominance is not useful as a sole criterion in distinguishing between aseptic and bacterial meningitis. Elevated CSF protein and decreased CSF glucose concentrations, if present, are usually mild, although extreme degrees of both have been reported.
Patients with mumps meningitis almost always have CSF pleocytosis that is usually less than 500/mm3, with a differential primarily of mononuclear cells (greater than 80% lymphocytes in 80-90% of patients); the pleocytosis may persist for weeks. CSF protein concentrations are normal in more than half of patients with mumps meningitis in some series (38). The CSF glucose content is normal in most patients, but it may be depressed in up to 25% of cases. Complement fixation and hemagglutination inhibition on serum specimens are the most reliable serologic tests for the diagnosis of mumps; testing of paired acute and convalescent sera should demonstrate a diagnostic fourfold rise in mumps antibody titer. Patients with herpes simplex virus type 2 meningitis also have a lymphocytic meningitis (less than 500/mm3) and a normal glucose content (30). The CSF in HIV-infected patients during the acute retroviral syndrome typically shows a mild lymphocytic pleocytosis (<200/mm3), mildly elevated protein concentrations, and a normal or slightly decreased glucose content (49); these CSF parameters improve and sometimes resolve within 2 weeks.
Tuberculous Meningitis
In patients with tuberculous meningitis, the CSF is typically clear or opalescent (50,51). However, when the CSF is allowed to stand at room temperature or in the refrigerator for a short time, there may form a cobweblike clot that is the classic "pellicle" of tuberculosis, which occurs secondary to the high fibrinogen concentration in the fluid along with the presence of inflammatory cells. A moderate CSF pleocytosis is characteristic of tuberculous meningitis, with 90-100% of patients having >5 white blood cells/mm3. The number of cells seldom exceeds 300/mm3, although exceptions do occur (between 500-1500/mm3 in about 20% of patients) (69). Initially, both lymphocytes and neutrophils predominate, but there is then rapid conversion into a lymphocytic predominance over several weeks. The converse can be seen following the introduction of antituberculous chemotherapy, in which an initial lymphocytic predominance shifts to a neutrophilic predominance on subsequent CSF examinations, the so-called "therapeutic paradox". There is usually a modest depression of CSF glucose, with a median of 40 mg/dL reported in most series; hypoglycorrachia correlates with more advanced stages of clinical disease (50). CSF protein is elevated in the majority of cases, with median values of 150-200 mg/dL; occasionally, CSF protein values in excess of 1-2 g/dL are reported, usually in conjunction with spinal block (68).
Bacterial versus Viral Meningitis
In patients with CSF findings consistent with a diagnosis of bacterial meningitis, but in whom the CSF gram stain and culture are negative, it may be difficult to determine whether the initial CSF studies are consistent with the diagnosis of bacterial meningitis. A combination of test results, however, may permit an accurate prediction of the likelihood of bacterial versus viral meningitis. In one analysis of 422 patients with acute bacterial or viral meningitis, a CSF glucose concentration of <34 mg/dL, a CSF:blood glucose ratio of <0.23, a CSF protein concentration of >220 mg/dL, >2,000 CSF leukocytes/mm3, or >1,180 CSF neutrophils/mm3 were individual predictors of bacterial, rather than viral, meningitis with 99% certainty or better (81). This model was validated in one retrospective review of adult patients with bacterial or viral meningitis (82), although proof of the clinical utility of this model will require a prospective application. This model, however, should not be used to make clinical decisions regarding the initiation of antimicrobial therapy in individual patients with meningitis. Therefore, other diagnostic tests have been examined (see below).
Tests to Distinguish Bacterial from Nonbacterial Meningitis
Lactate
Elevated CSF lactate concentrations may be useful in differentiating bacterial from nonbacterial meningitis in patients who have not received prior antimicrobial therapy. In one study of 78 patients with acute meningitis, CSF lactate concentrations above 4.2 mmol/L were considered as a positive discriminative factor for bacterial meningitis (83), with a the sensitivity of 96%, specificity 100%, positive predictive value of 100% and negative predictive value of 97%. However, despite the high sensitivity and positive predictive value of CSF lactate concentrations in the diagnosis of bacterial meningitis, the results are generally nonspecific and provide little additional diagnostic information. Furthermore, other factors (e.g., cerebral hypoxia/ischemia, anaerobic glycolysis, vascular compromise, metabolism of CSF leukocytes) also may elevate CSF lactate concentrations. Therefore, measurement of CSF lactate concentrations is not recommended for patients with suspected community-acquired bacterial meningitis. However, CSF lactate concentrations were found to be superior to the CSF:blood glucose ratio for suggesting the diagnosis of bacterial meningitis in postoperative neurosurgical patients, in which a CSF lactate concentration of 4.0 mmol/L was used as a cutoff value for the diagnosis (84); the sensitivity was 88%, specificity 98%, positive predictive value 96%, and negative predictive value 94%. CSF lactate concentrations may be valuable in this subgroup of patients, in whom the usual CSF findings of elevated WBC counts (total and differential), gram stain, glucose, and protein are neither sensitive nor specific to reliably distinguish bacterial from a nonbacterial meningeal syndrome.
C-Reactive Protein
C-reactive protein (CRP), made in the liver and secreted within 6 hours of an acute inflammatory reaction, has been measured in patients with meningitis (85). A published meta-analysis has examined the utility of measurement of serum and CSF concentrations of CRP to distinguish bacterial from viral meningitis (86); measurement of serum concentrations of CRP had a sensitivity that ranged from 69% to 99% and a specificity that ranged from 28% to 99%. In another study published after the meta-analysis which included 385 consecutive patients with CSF culture-proven bacterial meningitis and 182 children with proven or presumed bacterial meningitis (87), serum CRP was capable of distinguishing gram stain-negative bacterial meningitis with a sensitivity of 96%, specificity of 93%, and negative predictive value of 99%. CSF concentrations of C-reactive protein have also been evaluated in distinguishing bacterial from viral meningitis (86); sensitivity ranged from 18% to 100% and specificity ranged from 75% to 100%. Measurement of serum CRP may be helpful in patients with CSF findings consistent with meningitis, but in whom the Gram's stain is negative and for whom the physician is considering withholding antimicrobial therapy, based on the data that a normal CRP has a high negative predictive value in the diagnosis of bacterial meningitis.
Procalcitonin
Elevated serum concentrations of procalcitonin, a polypeptide that increases in patients with severe bacterial infection, were shown to be useful in differentiating between bacterial and viral meningitis (85). In one study of 59 consecutive children hospitalized for meningitis (88), the sensitivity of serum procalcitonin (using a cutoff of >5.0 µg/L) was 94% and the specificity was 100% for the diagnosis of bacterial meningitis. In adults, serum concentrations >0.2 ng/mL had a sensitivity and specificity of up to 100% in the diagnosis of bacterial meningitis (89), although false-negative results have been reported by others (sensitivity of 69%) (90). Measurement of serum procalcitonin concentrations is not readily available in clinical laboratories, however, such that recommendations on its use cannot be made at this time.
CSF Tests to Establish an Etiologic Diagnosis of Meningitis
Staining
Gram stain examination of CSF permits a rapid, accurate identification of the causative bacterium in 60-90% of patients with community-acquired bacterial meningitis, and has a specificity of 97% or more (55). The likelihood of visualizing the bacterium on Gram's stain, however, correlates with the CSF concentration of bacteria – concentrations of ≤103 CFU/mL are associated with positive Gram's stain 25% of the time and CSF concentrations >105 CFU/mL leads to positive microscopy in 97% of cases (91); the probability of visualizing bacteria on Gram's stain can be increased up to 100-fold by utilizing cytospin techniques (92). The likelihood of having a positive Gram's stain also depends on the specific bacterial pathogen causing meningitis (76,93). Gram's stains are positive in 90% of cases caused by Streptococcus pneumoniae, 86% of cases caused by Haemophilus influenzae, 75% of cases caused by Neisseria meningitidis, 50% of cases caused by gram-negative bacilli, and in about one-third of patients with meningitis caused by Listeria monocytogenes (19). The yield of CSF Gram’s stain may be about 20% lower in patients who have received prior antimicrobial therapy. Although false-positive CSF Gram's stains may result from observer misinterpretation, reagent contamination, or use of an occluded needle for lumbar puncture (in which an excised skin fragment is contaminated with bacteria), the test is rapid, inexpensive, and highly specific for the etiologic diagnosis of bacterial meningitis (76,94).
In contrast to the situation in patients with bacterial meningitis, the identification of tuberculous organisms in CSF by specific stains is difficult because of the small population of organisms. In many series, fewer than 25% of specimens were smear-positive after AFB staining (50,66,68), although one review demonstrated positive smears in 52% of CSF specimens (67). The yield may be increased by staining the pellicle (if present) as well as layering the centrifuged sediment of large CSF volumes onto a single slide with repeated applications until the entire pellet can be stained at once. Obtaining repeated specimens may also increase the yield. In one study, an 86% rate of acid-fast smear positivity was demonstrated when up to four separate specimens were examined for each patient (69), although this result has not been consistently duplicated in the literature, however.
Immunodiagnostic Tests
Several rapid diagnostic tests have been developed to aid in the etiologic diagnosis of bacterial meningitis (93,94). These tests utilize serum containing bacterial antibodies or commercially available antisera directed against the capsular polysaccharides of meningeal pathogens. Available tests include counterimmunoelectrophoresis, coagglutination, and latex agglutination. Depending on the meningeal pathogen, latex agglutination has shown good sensitivity in detecting the antigens of common meningeal pathogens: 78-100% for H. influenzae type b, 67-100% for S. pneumoniae, 69-100% for Streptococcus agalactiae, and 50-93% for N. meningitidis. However, a negative bacterial antigen test does not rule out infection caused by a specific meningeal pathogen. Furthermore, the routine use of latex agglutination for the etiologic diagnosis of bacterial meningitis has recently been questioned (95,96,97), because bacterial antigen testing does not appear to modify the decision to administer antimicrobial therapy and false-positive results have been reported. Some experts, however, would recommend latex agglutination in patients with a negative CSF gram stain this test may also be most useful in the patient pretreated with antimicrobial therapy and whose Gram’s stain and CSF culture are negative.
The diagnosis of enteroviral meningitis by specific immunodiagnostic tests has been studied. However, rapid diagnosis of enterovirus infection by immunoassay techniques has been hampered by the lack of a common antigen among the various serotypes and the low concentrations of virus in body fluids (30,65).
Several newer diagnostic modalities are under development for the diagnosis of tuberculous meningitis (50,98). Some tests utilize biochemical assays to measure some feature of the organism or the host response to it (e.g., bromide partition test, adenosine deaminase assay). Other modalities are immunologic tests that detect mycobacterial antigen or antibody in the CSF [e.g., tuberculostearic acid antigen, enzyme-linked immunosorbent assay (ELISA), latex agglutination]. These immunodiagnostic tests have some promise for rapid and sensitive diagnosis of tuberculous meningitis, although there are problems with the presence of cross-reacting antibodies against nonpathogenic mycobacteria, as well as with the presence of bacterial or fungal antigenic moieties.
Culture
In patients with bacterial meningitis, CSF cultures are positive in 70-85% of patients who have not received prior antimicrobial therapy, but cultures may take up to 48 hours for organism identification so are not helpful in making decisions in the acute management of patients with suspected bacterial meningitis.
A specific virologic diagnosis of enteroviral meningitis depends on isolation of the virus from the CSF in tissue culture (99), although the sensitivity for enteroviral serotypes is only 65 to 75%, largely a result of the inability to grow many coxsackievirus A serotypes (65). The difficulty in isolation of enteroviruses from CSF may also relate to the low enterovirus titers and that no single cell line is optimal for the detection of all members of the genus (31). Furthermore, the time required for identifying an enterovirus from CSF using cell culture is too long to be of clinical utility in establishing the diagnosis because the mean time for enteroviruses to grow is 3.7 to 8.2 days. Although isolation of a nonpolio enterovirus from the throat or rectum of a patient with aseptic meningitis is suggestive of an etiologic diagnosis, a recent study found that non-CSF viral cultures were not helpful in predicting enteroviral CNS infection because enteroviruses were isolated at the same frequency from non-CSF sites in infants in whom enteroviruses were cultured from CSF as in hospitalized infants with an acute illness whose CSF was negative (100). In addition, viral shedding can occur in 7.5% of healthy controls during enterovirus epidemics (30). Mumps virus can be grown from CSF in tissue culture for at least 1 week after the onset of disease, but the sensitivity of this technique is highly variable (30 to 50% if collected from CSF early during the course of mumps CNS infection) (38). Although virus has been cultured from blood and CSF in patients with arboviral meningitis, the diagnosis is usually made by comparison of acute and convalescent sera. HIV has been isolated from the CSF of some patients with neurologic disease (49,101) although it can be isolated from HIV-infected patients without neurologic symptoms or signs (101,102).
In patients with tuberculous meningitis, proof of infection requires isolation of the organism from CSF, although false-negative cultures are common, with mycobacteria isolated from less than 50% of patients with a clinical diagnosis of tuberculous meningitis (50). Higher culture yields may be obtained by processing multiple specimens for each patient; however, even with as many as four CSF specimens, almost 20% of patients with a clinical diagnosis of tuberculous meningitis have negative CSF cultures (69).
Polymerase Chain Reaction
Polymerase chain reaction (PCR) has been utilized to amplify DNA from patients with bacterial meningitis caused by the common meningeal pathogens (N. meningitidis, S. pneumoniae, H. influenzae type b, S. agalactiae, and L. monocytogenes) (55,93). In one study of CSF samples from 54 patients with meningococcal disease or from patients who underwent CSF analysis and did not have meningococcal meningitis (103), the sensitivity and specificity of PCR were both 91%. In another study using a seminested PCR strategy for simultaneous detection of N. meningitidis, H. influenzae, and streptococci in 304 clinical CSF samples (including 125 samples from patients with bacterial meningitis), the diagnostic sensitivity was 94% and specificity 96% (104), although some false-positive results were obtained. The clinical utility of PCR for the diagnosis of bacterial meningitis was also assessed with use of a broad range of bacterial primers, yielding a sensitivity of 100%, specificity of 98.2%, positive predictive value of 98.2%, and negative predictive value of 100% (105). Therefore, broad-based PCR may be useful for excluding the diagnosis of bacterial meningitis, with the potential for influencing decisions to initiate or discontinue antimicrobial therapy. In another study of a multiplex PCR assay for detection of N. meningitidis, S. pneumoniae, and H. influenzae type b DNA (106), the assay had a positive predictive value of 100% and a negative predictive value of 99.1-99.5%. Further refinements of the available techniques may lead to their use in patients with bacterial meningitis in whom the CSF Gram's stain is negative.
PCR is the most promising alternative to viral culture for the diagnosis of enteroviral meningitis. Primers are directed at highly conserved regions of the 5' noncoding region of the viral genome and designed for reverse transcription combined with PCR. Enteroviral reverse transcription–PCR (RT-PCR) has been tested in clinical settings and found to have a sensitivity ranging from 86-100% and specificity from 92 to 100% for the diagnosis of enteroviral meningitis (31,107,108). In addition, the time to identification of the enterovirus using RT-PCR is significantly reduced (hours to a day) compared with cell culture, which may lead to shortened patient hospitalization, less use of antimicrobial agents for treatment of presumptive bacterial meningitis, and reduction of the need for ancillary diagnostic tests (109).
PCR appears promising for the diagnosis of CNS infections caused by herpes simplex virus. With PCR, herpes simplex virus type 2 has been strongly associated with typical cases of Mollaret's meningitis in patients without symptoms or signs of genital infection (44). PCR has also been used to confirm the presence of varicella-zoster viral DNA in the CSF of patients with herpes zoster meningitis (110,111). HIV RNA has been detected in the CSF of patients with meningitic disease (112,113).
The technique of PCR for detecting fragments of mycobacterial DNA in CSF specimens appears to be an equally promising tool (50,73,98). A recent review and meta-analysis determined that the sensitivity and specificity of commercial nucleic acid amplification assays for the diagnosis of tuberculous meningitis was 56% and 98%, respectively (114); these data were also confirmed in another study published after the meta-analysis (115). Before these tests can be considered useful in the diagnosis of tuberculous meningitis, however, large-scale confirmatory studies must first be performed, as the diagnosis of tuberculous meningitis cannot be excluded by a negative result (73).
Neuroimaging
Bacterial Meningitis
Cranial CT or magnetic resonance (MR) imaging does not aid in the diagnosis of acute bacterial meningitis, although one of these modalities should be considered during the course of illness in patients who have persistent or prolonged fever, clinical evidence of increased intracranial pressure, focal neurologic findings or seizures, enlarging head circumference (in neonates), persistent neurologic dysfunction, or persistently abnormal CSF parameters or cultures (55,94). In one study of 352 episodes of community-acquired pneumococcal meningitis in adults (10), cranial CT demonstrated meningitis-associated complications in 39% of episodes, most commonly brain infarction, cerebral edema, and hydrocephalus. However, cranial CT may underestimate the possibility of increased intracranial pressure in patients with pneumococcal meningitis (116), such that ICP monitoring should be considered in patients with prolonged coma. Cranial CT or MR imaging has been recommended at the end of antimicrobial therapy in newborn infants to be certain that no intracranial complications have occurred. In one review of 107 children with bacterial meningitis who underwent CT scanning (117), one or more abnormalities were found in 52% of cases, although most findings did not require specific intervention.
Radiographic studies may be useful in the subset of patients with bacterial meningitis that has occurred as a consequence of a basilar skull fracture with CSF leak (29). CT scanning may detect air-fluid levels, opacification of the paranasal sinuses, or intracranial air; CT scanning with sagittal reconstruction can also be used to document or localize fracture sites. High-resolution CT scanning with water-soluble contrast enhancement of the CSF (metrizamide cisternography) is the best test for defining the site of CSF leakage.
Tuberculous Meningitis
There are no radiologic changes that are pathognomonic for tuberculous infection of the CNS (50,73). On CT scanning, hydrocephalus is frequently present at diagnosis or develops during the course of infection; following the administration of intravenous contrast material, enhancement of the basal cisterns results, with widening and blurring of the basilar arterial structures may be seen. Periventricular lucencies may also be evident, reflecting the presence of periventricular tuberculous exudate and tubercle formation adjacent to the ependyma and choroid. MR imaging with gadolium enhancement has been shown to be more sensitive than CT in detecting the anatomic abnormalities of tuberculous meningitis. MR angiography has also been utilized to detect the characteristic vascular narrowing and the rare complication of aneurysm formation in patients with tuberculous meningitis (118).
INITIAL MANAGEMENT APPROACH FOR BACTERIAL MENINGITIS
The initial management approach to the patient with suspected acute bacterial meningitis depends upon early recognition of the meningitis syndrome, rapid diagnostic evaluation, and emergent antimicrobial and adjunctive therapy. A management algorithm for infants and children is shown in Figure 1, and for adults in Figure 2 (74). Once there is suspicion for acute bacterial meningitis, blood cultures must be obtained and a lumbar puncture performed immediately to determine whether the CSF findings are consistent with the clinical diagnosis. However, there may be circumstances in which the clinician cannot emergently perform the diagnostic lumbar puncture (e.g., secondary to the inability to obtain CSF), or is concerned that the clinical presentation is consistent with a central nervous system (CNS) mass lesion or another cause of increased intracranial pressure and wants to obtain a CT scan of the head prior to lumbar puncture. In those patients, blood cultures must be obtained and appropriate antimicrobial and adjunctive therapy given prior to lumbar puncture, or before the patient is sent to the CT scanner, because a delay in the initiation of therapy introduces the potential for increased morbidity and mortality, if the patient does indeed have acute bacterial meningitis. The choice of empiric antimicrobial therapy in this setting is governed by the patient's age and various conditions that may have predisposed the patient to meningitis (Table 3). Although the yield of CSF cultures and CSF gram stain may be diminished by antimicrobial therapy given prior to lumbar puncture, pretreatment blood cultures and CSF findings (i.e., elevated white blood cell, diminished glucose concentration, and elevated protein concentration) will likely provide evidence for or against the diagnosis of bacterial meningitis. Once CSF analysis is performed, targeted antimicrobial therapy, in adult patients with a positive CSF Gram's stain, can be initiated (Table 4). In children greater than 1 month of age with bacterial meningitis, however, empiric antimicrobial therapy with vancomycin combined with either cefotaxime or ceftriaxone is recommended pending culture results, based on the concern that interpretation of the CSF Gram's stain depends on the expertise of the person reading the slide; some experts would also utilize this strategy in adults with bacterial meningitis. However, a positive CSF Gram’s stain may modify this approach by the addition of another agent (e.g., ampicillin for the presence of gram-positive bacilli) to these two standard drugs. If the Gram’s stain is negative, empiric antimicrobial therapy is given, with choices of agents based on patient age and certain predisposing conditions (Table 3). The choice of specific antimicrobial agents for targeted or empiric therapy is based on the current knowledge of antimicrobial susceptibility patterns of these pathogens. For initial therapy, the assumption should be that antimicrobial resistance is likely. Evidence-based recommendations for specific agents and dosages are reviewed in Tables 5 and 6, respectively.
Timing of Antimicrobial Administration
There are no prospective clinical data on the relationship of the timing of antimicrobial administration of antimicrobial agents to clinical outcome in patients with bacterial meningitis (55,74,119). All existing studies examined only the duration of symptoms, not the duration of meningitis, prior to antimicrobial administration. However, most physicians would intuitively agree that the longer the duration of symptoms in bacterial meningitis, the more likely is the possibility of experiencing an adverse outcome. This concept is supported by results of studies that poor outcome is associated with greater amounts of antigen or a larger number of microorganisms in CSF obtained before initiation of antimicrobial therapy, and that delayed CSF sterilization after 24 hours of antimicrobial therapy is a risk for subsequent neurologic sequelae. The assumption that any delay in administration of antimicrobial therapy might be associated with an adverse clinical outcome has been the basis for malpractice claims against physicians who have been accused of failure to promptly diagnose and treat bacterial meningitis.
Ethical considerations clearly preclude the design of human studies to assess the outcome of patients in whom antimicrobial therapy is deliberated delayed. To address the question of whether delay in diagnosis and treatment affects outcome in bacterial meningitis, several large reviews examined the available published literature. In one review of 4,707 patients in 22 studies, the duration of symptoms before initiation of antimicrobial therapy was compared to subsequent sequelae (120). The studies were heterogeneous with regard to patient demographics, study numbers, causative microorganisms, and length of follow-up. Furthermore, there was often incomplete reporting of relevant data and not all studies contained basic study design components. The author of this review suggested that if the clinical presentation was that of a nonspecific illness (i.e., general non-focal symptoms), a short delay (<3-5 days) did not appear to alter the risk of sequelae or death. However, in the case of fulminant meningitis, a delay in the initiation of antimicrobial therapy seemed unconnected to outcome; and for patients with a history of clinically overt meningitis, an inappropriate delay incrementally increased the risk of permanent injury. In a subsequent literature review of 27 studies (including many of the studies in the previous review) analyzing a total of 5,585 patients up to August 1995, only 20% of all studies specifically defined any "symptoms" in their analysis, and could not identify whether specific "symptoms" denoted a "premeningitis" phase or heralded the onset of bacterial seeding of the CNS (121). The author suggested that because there are no pathognomonic clinical features of bacterial meningitis, opinions based on reviews of an individual patient’s clinical course and symptomatic progression were interpretive at best and could not dictate with certainty when seeding of the CNS occurred.
These issues have also been examined in several retrospective studies. In one retrospective review of 305 patients hospitalized in the United Kingdom with a diagnosis of bacterial meningitis (122), 53 (17.4%) received an antimicrobial agent prior to admission; there was only one death (1.9%) in the 53 patients who received an antimicrobial versus 30 deaths (12%) in the 252 who had not. Based on these results and other studies, the British Infection Society Working Party recommended parenteral administration of appropriate antimicrobial therapy without delay to all adult patients in whom the diagnosis of bacterial meningitis was suspected while arranging urgent transfer to the hospital (123). In another recent retrospective cohort study of 269 adult patients with community-acquired bacterial meningitis in the United States (124), three baseline clinical features were associated with adverse outcome: hypotension, altered mental status, and seizures. These three factors were used to create a prognostic model that predicted clinical outcome, in which patients were stratified into three prognostic stages of low, intermediate, or high risk for adverse outcome based on these clinical features. The results demonstrated that a delay in initiation of antimicrobial therapy after patient arrival in the emergency room was associated with adverse clinical outcome when the patient's condition advanced from a low- or intermediate-risk stage to a high-risk stage of prognostic severity. These data support the assumption that treatment of bacterial meningitis before it advances to a high level of clinical severity improves outcome.
Based on these data, the key factor is to administer antimicrobial therapy before the patient's clinical condition advances to a high level of clinical severity, at which point the patient is less likely to have a full recovery after treatment with appropriate antimicrobial therapy; however, it is not possible to ascertain when the high level of clinical severity is reached. The logical and intuitive approach is to administer antimicrobial therapy as soon as possible after the diagnosis of bacterial meningitis is suspected or proven. This may include administration prior to hospital admission if the patient initially presents outside the hospital. This concept has been supported by three recent retrospective studies which demonstrated a reduction in mortality with early administration of antimicrobial therapy (125), a benefit in terms of neurologic outcome and survival in patients who received antimicrobial therapy before the patient's level of consciousness deteriorated to a Glasgow coma scale lower than 10 (126), and an independent incremental association between delay in administration of antimicrobial therapy and mortality in acute bacterial meningitis (127).
Adjunctive Dexamethasone Therapy
Adjunctive dexamethasone therapy should be administered to certain patients with suspected or proven bacterial meningitis (55,60,74). This is based on experimental animal model studies of meningitis which demonstrated that treatment with bacteriolytic antibiotics may contribute to the inflammatory response in the subarachnoid space, and subsequent cerebral edema and increased intracranial pressure. On the basis of these experimental observations, numerous clinical trials were undertaken to assess the efficacy of adjunctive dexamethasone in patients with bacterial meningitis. However, it must be noted that not all studies were placebo-controlled, various antimicrobial agents were utilized (some of which may not have been adequate for the treatment of bacterial meningitis), dexamethasone was administered at different times in relation to the first antimicrobial dose, and patients had varying levels of illness severity. In making specific recommendations, it is prudent to analyze the data according to patient age group.
Neonates
There is only one published trial that has evaluated the efficacy of adjunctive dexamethasone in neonates with bacterial meningitis (128). In this randomized, but not placebo-controlled trial in 52 full-term neonates, patients were given dexamethasone 10-15 minutes prior to the first antimicrobial dose. The results demonstrated that mortality was 22% in the treated group versus 28% in the control group (P = 0.87). At follow-up examination up until the age of 2 years, 30% of the dexamethasone-treated patients and 39% of the control group had neurologic sequelae. Because the study size was small and under-powered, there are insufficient data to make a recommendation on use of adjunctive dexamethasone in neonates with bacterial meningitis.
Infants and Children
There have been 15 published trials on use of adjunctive dexamethasone in infants and children with bacterial meningitis. Three of the trials were retrospective, but the remainder were prospective, all were randomized, and all but one was placebo-controlled. In a meta-analysis of clinical studies published from 1988 to 1996 (129), adjunctive dexamethasone (0.15 mg/kg every 6 hours for 2-4 days) had confirmed benefit for H. influenzae type b meningitis and, if commenced with or before antimicrobial therapy, suggested benefit for pneumococcal meningitis in children; evidence of clinical benefit was greatest for hearing outcomes. Since publication of the meta-analysis, two additional studies of adjunctive dexamethasone have been published. The first was a retrospective study in children with pneumococcal meningitis and showed that in the dexamethasone group, there was a higher incidence of moderate or severe hearing loss (46% versus 23%; P = 0.016) or any neurologic deficits (55% versus 33%; P = 0.02) (130). However, children in the dexamethasone group more frequently required intubation and mechanical ventilation and had a lower initial CSF glucose concentration, there were no data on use of specific antimicrobial agents in each group, and the dexamethasone was given later than in other studies (i.e., within 60 minutes of the first antimicrobial dose). In a second study which was a randomized, placebo-controlled, double blind trial of adjunctive dexamethasone in children in Malawi (131), the overall number of deaths (31% versus 31%; P = 0.93) and presence of sequelae at final outcome (28% versus 28%; P = 0.97) were not significantly different in the children who received adjunctive dexamethasone. However, the Malawian children enrolled in this trial had severe disease associated with malnutrition and HIV infection, and presented after a delay, which resulted in very high case-fatality rates and significant long-term morbidity, and over one-third of children received antimicrobial therapy before admission and more than 30% were placed on second-line antimicrobial therapy because of inadequate clinical or microbiologic response (133). Adjunctive dexamethasone does not reverse the CNS damage that develops as a result of existent cerebral edema, increased intracranial pressure, or neuronal injury that is present at diagnosis. In another trial performed in Australia in patients 0-14 years of age with had good access to healthcare (133), treatment with adjunctive dexamethasone significantly improved outcome (defined as protection against death or severe morbidity).
Despite some variability in results of published trials, the available evidence supports the use of adjunctive dexamethasone in infants and children with H. influenzae type b meningitis, with therapy initiated 10-20 minutes prior to, or at least concomitant with, the first antimicrobial dose, at 0.15 mg/kg every 6 hours for 2 to 4 days. Adjunctive dexamethasone should not be given to infants and children who have already received antimicrobial therapy, because administration of dexamethasone in this circumstance is unlikely to improve patient outcome. In infants and children with pneumococcal meningitis, there is controversy concerning the use of adjunctive dexamethasone therapy, and the 2003 Report of the Committee on Infectious Diseases of the American Academy of Pediatrics statement for pneumococcal meningitis states that adjunctive therapy with dexamethasone may be considered after weighing the potential benefits and possible risks and that experts vary in recommending the use of corticosteroids in pneumococcal meningitis in children.
Adults
There have been five published trials of adjunctive dexamethasone in adults with bacterial meningitis (74); three were randomized and placebo-controlled, one was randomized but not placebo-controlled, and one was a systemic sampling open cohort study. In four of the five studies, results were inconclusive, but a recently published prospective, randomized, placebo-controlled, double blind multicenter trial did provide important data on use of adjunctive dexamethasone in adults with bacterial meningitis (134). A total of 301 adults (age ≥17 years) were randomized to receive dexamethasone (10 mg every 6 hours for 4 days) or placebo, the first dose administered 15-20 minutes prior to the first antimicrobial dose. At 8 weeks after enrollment, the percentage of patients with an unfavorable outcome (15% versus 25%; P = 0.03) and death (7% versus 15%; P = 0.04) was significantly less in the dexamethasone group. Among the subgroup of patients with pneumococcal meningitis, benefit was evident in those who received adjunctive dexamethasone, with a lower percentage of unfavorable outcome (26% versus 52%; P = 0.006) and death (14% versus 34%; P = 0.02). In all groups, dexamethasone appeared to be the most beneficial in patients with moderate to severe disease on the Glasgow Coma Scale. Based on the available evidence, adjunctive dexamethasone (0.15 mg/kg every 6 hours for 2-4 days with the first dose administered 10-20 minutes before, or at least concomitant with, the first dose of antimicrobial therapy) should be utilized in adults with suspected or proven pneumococcal meningitis (74). Adjunctive dexamethasone should not be given to adult patients who have already received antimicrobial therapy, because administration of dexamethasone in this circumstance is unlikely to improve patient outcome. The data are inadequate to recommend adjunctive dexamethasone to adults with meningitis caused by other bacterial pathogens (135), although some authorities would initiate dexamethasone in all adults since the etiology of meningitis is not always ascertained at initial evaluation (136).
Pneumococcal Meningitis
Despite the clinical trials that have demonstrated the benefits of adjunctive dexamethasone in infants, children, and adults with bacterial meningitis (see above), concerns have been raised about whether use of adjunctive dexamethasone may be harmful in patients with pneumococcal meningitis caused by highly penicillin- or cephalosporin-resistant strains (55,74); these patients may require antimicrobial therapy with vancomycin and the diminished inflammatory response induced by dexamethasone might reduce CSF vancomycin penetration and delay CSF sterilization. The published trials have not examined outcome in patients with these resistant isolates who have received adjunctive dexamethasone. For example, in the recent study in adults cited above (134), only 72% of 108 CSF pneumococcal isolates were submitted for in vitro susceptibility testing and all were susceptible to penicillin, an unusual finding in the United States and many areas of the world. Although it would be optimal to evaluate the efficacy of adjunctive dexamethasone in patients with meningitis caused by highly resistant pneumococci, it is unlikely that this question will be definitively answered in the near future, given the difficulty in enrolling adequate numbers of patients with these resistant strains into a clinical trial (135). Therefore, it is reasonable to administer adjunctive dexamethasone to all adult patients with pneumococcal meningitis, even if the isolate is subsequently found to be highly resistant to penicillin and cephalosporins; careful observation and follow-up are critical to determine whether dexamethasone is associated with adverse clinical outcome.
SPECIFIC ANTIMICROBIAL THERAPY BASED ON ISOLATED PATHOGEN
Bacterial Meningitis
Once a bacterial pathogen is isolated and in vitro susceptibility testing performed, antimicrobial treatment should be modified for optimal therapy. Our recommendations, with alternative suggestions, based on isolated microorganism are listed in Table 5. Recommended dosages of antimicrobial agents in neonates, children, and adults are shown in Table 6. In depth discussion of recommended therapy based on isolated pathogen are in the sections below.
Streptococcus pneumoniae
Therapy for meningitis caused by the pneumococcus has recently been modified according to current pneumococcal susceptibility patterns (55,74,137,138). In the past, pneumococci were uniformly susceptible to penicillin, with MICs of 0.06 µg/mL or less. Numerous reports from throughout the world have now documented strains of pneumococci that are of intermediate susceptibility to penicillin (MIC range of 0.1 to 1.0 µg/mL), as well as strains that are highly resistant to penicillin (MIC of 2.0 µg/mL or higher). Results of recent surveillance studies in the United States show that the prevalence of penicillin-nonsusceptible S. pneumoniae ranges from 25% to more than 50% (139); rates are as high as 60% in some parts of Latin America and as high as 80% in some countries in Asia. Factors reported to predispose to resistance include the patient's age (younger than 10 or older than 50 years), immunosuppression, prolonged hospital stay, children in daycare settings, infection by serotypes 14 and 23, and frequent, prolonged, or prophylactic use of antimicrobial therapy.
Several alternative agents have been examined for the treatment of meningitis caused by penicillin-resistant pneumococci. Chloramphenicol is one agent that has been studied for the treatment of pneumococcal meningitis, although clinical failures with chloramphenicol have been reported in patients with penicillin-resistant isolates, probably because of the poor bactericidal activity of chloramphenicol against these strains; 20 of 25 children had an unsatisfactory outcome (i.e., death, serious neurologic deficit, poor clinical response) in one study (140). Despite susceptibility on disk testing, chloramphenicol minimal bactericidal concentrations of the penicillin-resistant pneumococcal isolates were significantly higher than those for the penicillin-sensitive isolates, with subsequent subtherapeutic bactericidal activity and treatment failure.
Third-generation cephalosporins (i.e., cefotaxime and ceftriaxone) have been considered the treatment of choice in patients with pneumococcal meningitis caused by strains that are of intermediate susceptibility to penicillin. However, some reports of meningitis treatment failure with the third-generation cephalosporins have appeared, and pneumococcal strains have emerged that are resistant to these agents (MIC ≥ 2 µg/mL). When the MIC to the third-generation cephalosporin is 1 µg/mL or less, some patients have been treated successfully with either high-dose cefotaxime or ceftriaxone alone, although one study found that high-dose cefotaxime did not have reliably sufficient CSF bactericidal activity against cephalosporin-resistant pneumococci.
Vancomycin has been evaluated in 11 adult patients with meningitis caused by pneumococcal strains with intermediate susceptibility to penicillin (141). This therapy was associated with clinical failure in 4 patients; no failures occurred in 14 subsequent patients treated with ceftriaxone. In two of the failures, CSF vancomycin concentrations were undetectable at 48 hours, and in a third patient, symptoms recurred on the eighth day of antimicrobial therapy. However, the dosage of vancomycin used in this study (15 mg/kg daily) was below standard recommendations. The concomitant administration of dexamethasone and the subsequent decreased inflammation and poor entry of vancomycin into CSF may have contributed to this negative outcome; this explanation has been supported in an experimental rabbit model of pneumococcal meningitis. These data support the concept that vancomycin should not be used alone for the treatment of pneumococcal meningitis. When used for the treatment of bacterial meningitis, vancomycin should be administered to maintain serum vancomycin trough concentrations of approximately 15-20 µg/mL (74). Of additional concern is the description of S. pneumoniae strains that are tolerant to vancomycin (142,143). A vancomycin- and cephalosporin-tolerant strain of S. pneumoniae was isolated from the CSF of a patient with meningitis who then developed recrudescence of meningitis despite therapy with vancomycin plus a third-generation cephalosporin (144). Because tolerance is a precursor phenotype to the development of resistance, these data have important implications in the use of vancomycin for pneumococcal meningitis.
In view of the data presented above, penicillin can never be recommended as empirical therapy in patients with suspected pneumococcal meningitis. As an empirical regimen, the combination of vancomycin plus a third-generation cephalosporin (either cefotaxime or ceftriaxone) is recommended (74). This combination was synergistic in a rabbit model of penicillin-resistant pneumococcal meningitis and was synergistic, or at least additive, in the CSF of children with meningitis. The addition of rifampin to vancomycin with or without a third-generation cephalosporin has been recommended by some authorities, although clinical data are lacking; rifampin should be added only if the organism is demonstrated to be susceptible, the expected clinical or bacteriologic response is delayed, or the pneumococcal isolate has a cefotaxime/ceftriaxone MIC >2.0 µg/mL. In patients not responding, intrathecal or intraventricular vancomycin also remains a reasonable option (145). Once susceptibility studies of the isolated pneumococcus are performed, antimicrobial therapy can be modified for optimal treatment (see Table 5).
With continued emergence of penicillin- and cephalosporin-resistant strains of S. pneumoniae, other antimicrobial agents have been evaluated for their efficacy in pneumococcal meningitis. Meropenem, a carbapenem with a broad spectrum of in vitro activity, including in vitro activity against penicillin-resistant pneumococci, has been approved by the Food and Drug Administration for the treatment of bacterial meningitis in children 3 months and older. Meropenem has been studied for the treatment of meningitis in both adults and children in several clinical trials (146), with microbiologic and clinical outcomes similar to those with either cefotaxime or ceftriaxone. In one prospective study of 258 children with bacterial meningitis, patients were randomly assigned to receive either meropenem or cefotaxime; there were no significant differences in outcome, with clinical cure (with or without sequelae) in 97% and 96% of patients treated with meropenem and cefotaxime, respectively (147). However, in a recent study of 20 cefotaxime-resistant S. pneumoniae isolates (148), 4 were intermediate and 13 were resistant to meropenem, suggesting that meropenem may not be a useful alternative agent for treatment of pneumococcal isolates that are highly resistant to penicillin and cephalosporins. Further studies are needed to determine the efficacy of meropenem in pneumococcal meningitis caused by penicillin- and cephalosporin-resistant strains, although isolated case reports have shown efficacy.
The fluoroquinolones have generally lacked sufficient in vitro activity against S. pneumoniae to warrant their investigation in the treatment of CNS infections. However, newer agents (moxifloxacin, gemifloxacin, garenoxacin) have shown excellent in vitro activity and have been evaluated in experimental animal models of infection (149). Trovafloxacin has been compared with ceftriaxone with or without vancomycin in a multicenter, randomized, comparative trial enrolling 311 children from 11 countries; S. pneumoniae was isolated in 27% of cases (150). The overall efficacy of both treatment groups was comparable in terms of CSF sterilization (94% in the trovafloxacin group and 96% in the comparator) and clinical success at the end of treatment (75% in the trovafloxacin group and 82% for the comparator). Although trovafloxacin is no longer utilized because of concerns of liver toxicity, these data suggest the potential usefulness of the newer fluoroquinolones in the treatment of bacterial meningitis. However, further clinical trials are needed before these agents can be recommended as first-line therapy for bacterial meningitis. Of concern are the reports of decreased pneumococcal susceptibility to the fluoroquinolones (151) and the development of fluoroquinolone-resistant S. pneumoniae associated with levofloxacin therapy (152), and the report of a case of fatal meningitis caused by a levofloxacin-resistant strain of S. pneumoniae (152). Nevertheless, a combination regimen of a third-generation cephalosporin plus a newer-generation fluoroquinolone may emerge as the treatment option of choice for pneumococcal meningitis in the future.
Neisseria meningitidis
Penicillin G and ampicillin are the antimicrobial agents of choice for meningitis caused by N. meningitidis (55,74), although these recommendations may need to be modified in the future because of trends in the antimicrobial susceptibility of meningococci. Meningococcal strains that are relatively resistant to penicillin G and have a minimal inhibitory concentration (MIC) range of 0.1 to 1.0 µg/mL have recently been reported from several areas (particularly Spain). For example, of 3264 strains of N. meningitidis isolated from blood and CSF in Spain during 1978 to 1985 (154), only one resistant isolate was observed, whereas 9 of 168 (5%) invasive isolates relatively resistant to penicillin G were found in the first 6 months of 1986; this figure reached 20% in 1989. Decreased meningococcal susceptibility to penicillin has also been reported from Greece, Switzerland, Romania, France, Belgium, the United Kingdom, Malawi, South Africa, Canada, Croatia, and Turkey (155,156,157). High-level penicillin resistance resulting from beta-lactamase production has also been reported, and the MICs for these strains may be as high as 256 µg/mL (158). Furthermore, high-levelchloramphenicol resistance (MIC of ≥64 µg/ml) has been described as resulting from the presence of the catP gene on a truncated transposon that has lost mobility because of internal deletions (159); transmission of genetic material between strains of N. meningitidis probably played an important role in dissemination of the gene.
In the United States, meningococcal strains relatively resistant to penicillin have also been described (160,161,162). In a population-based surveillance study of invasive meningococcal disease in selected areas of the United States, 3 of 100 isolates had penicillin MICs of 0.125 µg/mL (160). In another active, population-based surveillance in seven geographically dispersed areas of the United States during 1997 (163), three of 90 isolates were intermediately resistant to penicillin (MIC 0.12 µg/mL), wherease 49 of the remaining 87 isolates had MICs of 0.06 µg/mL. These data indicate the importance of continued surveillance for these resistant strains.
The clinical significance of these isolates is unclear at present because many patients with meningitis caused by relatively penicillin-resistant meningococci have recovered with standard penicillin therapy. However, isolated reports of treatment failure have been described (164,165). In one study from Spain, reduced susceptibility of N. meningitidis to penicillin was seen in 34% of 213 children with meningococcal meningitis (166), and reduced penicillin susceptibility was more frequent in strains responsible for death or sequelae (60 versus 32%, p = .04). Based on these data, some authorities would treat patients with meningococcal meningitis with a third-generation cephalosporin (either cefotaxime or ceftriaxone); these agents are likely to emerge as first-line treatment in the future. Single-dose ceftriaxone has also been found to be non-inferior compared with chloramphenicol when used against epidemic meningococcal meningitis in one study in Niger (167), suggesting that this agent should be utilized during meningococcal epidemics in the developing world.
Listeria monocytogenes
Despite their broad range of in vitro activity, third-generation cephalosporins are inactive in meningitis caused by L. monocytogenes, such that therapy should consist of ampicillin or penicillin G (18,19); the addition of an aminoglycoside should be considered in proven infection because of in vitro synergy and enhanced killing in vivo, as documented in a variety of animal models of Listeria infection. An alternative agent in a penicillin-allergic patient is trimethoprim-sulfamethoxazole, which is bactericidal against Listeria in vitro. Other agents (e.g., chloramphenicol and vancomycin) have varying activity against Listeria in vitro, and their use has been associated with an unacceptably high failure rates in patient with Listeria meningitis. However, intraventricular administration of vancomycin was successful in one case of recurrent L. monocytogenes meningitis. Meropenem is active in vitro and in experimental animal models of L. monocytogenes meningitis and may be a useful alternative if found to be clinically efficacious.
Streptococcus agalactiae
Standard therapy for neonatal meningitis caused by the group B streptococcus is the combination of ampicillin plus an aminoglycoside (60), which is also recommended for adult patients with meningitis caused by this organism (21); this combination is recommended because of documented in vitro synergy and recent reports detailing the presence of penicillin-tolerant strains. Alternative agents are the third-generation cephalosporins; vancomycin is reserved for patients who are allergic to penicillin.
Haemophilus influenzae
Therapy for meningitis caused by H. influenzae type b has been markedly altered by the emergence of beta-lactamase–producing strains. Resistance of H. influenzae to chloramphenicol has also been described; this is of particular concern in developing countries of the world, where chloramphenicol is used as first-line treatment for suspected bacterial meningitis. Even in patients with chloramphenicol-sensitive isolates, a prospective study found chloramphenicol to be bacteriologically and clinically inferior to ampicillin, ceftriaxone, or cefotaxime in the treatment of childhood bacterial meningitis caused predominantly by H. influenzae type b (168). Several studies have documented the efficacy of third-generation cephalosporins (particularly cefotaxime or ceftriaxone) to be similar to that of the combination of ampicillin plus chloramphenicol for bacterial meningitis. Based on these findings, use of a third-generation cephalosporin is recommended as empirical antimicrobial therapy for children with bacterial meningitis. The second-generation cephalosporins should not be used for therapy of bacterial meningitis because a prospective randomized study comparing ceftriaxone with cefuroxime for the treatment of childhood bacterial meningitis documented more rapid CSF sterilization (2 versus 12% of CSF cultures positive at 18 to 36 hours, p = .11) and a lower incidence of hearing impairment (4 versus 17%, p = .05) in the patients receiving ceftriaxone (169). Cefepime has been studied in the treatment of bacterial meningitis (170); in a prospective randomized comparison of cefepime and cefotaxime for the treatment of bacterial meningitis in infants and children (171), cefepime was found to be safe and therapeutically equivalent to cefotaxime and can be considered a suitable therapeutic alternative for the treatment of patients with this disease.
Aerobic Gram-Negative Bacilli
Treatment of bacterial meningitis caused by enteric gram-negative bacilli has been revolutionized by the availability of third-generation cephalosporins. Cefotaxime is preferred over ceftriaxone as the third-generation cephalosporin for use in neonates because it has been used more extensively and is not excreted in bile, which may have an inhibitory effect on the bacterial flora of the intestinal tract (94); ceftriaxone also has increased protein binding. One particular third-generation cephalosporin, ceftazidime, has enhanced in vitro activity against P. aeruginosa and resulted in the cure of 19 of 24 patients in one study of P. aeruginosa meningitis when administered alone or in combination with an aminoglycoside (172). In another study of 10 pediatric patients with Pseudomonas meningitis, 7 patients were cured clinically and 9 were cured bacteriologically when treated with ceftazidime-containing regimens (173). Concomitant intrathecal or intraventricular aminoglycoside therapy should be considered in patients with gram-negative meningitis who are not responding to conventional parenteral therapy. However, this mode of administration is rarely needed at present and was associated with a higher mortality rate than was systemic therapy alone in infants with gram-negative meningitis and ventriculitis.
Several other antimicrobial agents (e.g., aztreonam, imipenem, meropenem, cefepime) have been successfully used in isolated case reports and in small series of patients with meningitis caused by aerobic gram-negative bacilli (55,74). Intravenous colistin (5 mg/kg/day) was successfully used to treat a patient with meningitis caused by a multidrug resistant Acinetobacter baumannii (174); intrathecal colistin was also efficacious in another case of meningitis caused by this same multidrug-resistant organism (175). Additional reports have been published showing that intrathecal or intraventricular colistin alone, or in combination with systemic administration, was well tolerated and could be effective salvage therapy in patients with meningitis caused by strains of A. baumannii that are resistant to conventional antimicrobial therapy (176). The fluoroquinolones (e.g., ciprofloxacin, pefloxacin) have been used successfully in some patients with gram-negative meningitis (149,177,178). The limited published literature on use of the fluoroquinolones suggests that the primary area of usefulness of these agents is for the treatment of multidrug-resistant gram-negative organisms (e.g., P. aeruginosa) or when the response to conventional beta-lactam therapy is slow (e.g., meningitis caused by Salmonella spp.).
Staphylococci
S. aureus meningitis should be treated with nafcillin or oxacillin (28), with vancomycin reserved for patients allergic to penicillin or when methicillin-resistant organisms are suspected or isolated. However, given the likelihood of methicillin-resistant S. aureus in patients with S. aureus meningitis, vancomycin should be utililzed as empiric therapy pending results of in vitro susceptibility testing (179). The addition of rifampin should be considered in patients not responding to therapy. Meningitis caused by coagulase-negative staphylococci, the most commonly encountered organisms in CSF shunt infections, should be treated with vancomycin; rifampin should be added if the patient fails to improve.
Intrathecal Therapy
In patients with bacterial meningitis as a result of CSF shunt infection, direct instillation of antimicrobial agents into the ventricles through either an external ventriculostomy or shunt reservoir is occasionally necessary in patients with shunt infections that are difficult to eradicate or when the patient cannot undergo the surgical components of therapy. No antimicrobial agent has been approved by the FDA for intraventricular use, and the specific indications are not well-defined. Antimicrobial dosages have been used empirically (Table 7), with dosage adjustments and dosing interval based on the ability of the agent to achieve adequate CSF concentrations (180,181,182). After administration of the first intraventricular dose, additional doses can be determined by calculation of the "inhibitory quotient". Prior to administration of the next intraventricular dose, a sample of CSF is withdrawn to obtain the "trough" CSF concentration. The "inhibitory quotient" is then determined by taking the "trough" CSF concentration divided by the MIC of the agent for the isolated bacterial pathogen, and should exceed 10-20 for consistent CSF sterilization. Although not standardized, this approach is reasonable to ensure that adequate CSF concentrations of specific antimicrobial agents are attained.
Indications for Repeat Lumbar Puncture
In patients with bacterial meningitis who have responded appropriately to antimicrobial therapy, repeat CSF analysis to document CSF sterilization and improvement of CSF parameters is not routinely indicated. Repeat CSF analysis should be performed, however, in any patient who has not responded clinically after 48 hours of appropriate antimicrobial therapy. This is especially true in the patient with pneumococcal meningitis caused by penicillin- or cephalosporin-resistant strains especially in those who have also received adjunctive dexamethasone therapy (74). The neonate with gram-negative meningitis should undergo repeat lumbar puncture to document CSF sterilization since the duration of antimicrobial therapy is determined, in part, by the result. In patients with CSF shunt infections, the presence of a drainage catheter after shunt removal allows for monitoring of CSF parameters to ensure that the infection is responding to appropriate antimicrobial therapy and drainage.
Outpatient Antimicrobial Therapy
Outpatient antimicrobial therapy may also be appropriate for certain patients with bacterial meningitis. The following criteria have been suggested to guide outpatient antimicrobial therapy (74,183,184): inpatient therapy for at least 6 days; no fever for at least 24-48 hours before initiation of outpatient therapy; no significant neurologic dysfunction, focal findings, or seizure activity; clinically stable or improving condition; intake of all fluids by mouth; first dose of outpatient antimicrobial therapy given under medical supervision and without reaction; access to home health nursing for antimicrobial administration; reliable intravenous line and infusion device, if needed; daily examination by a physician, and an established plan for physician visits, nurse visits, laboratory monitoring, and emergencies; patient and/or family compliance with the program; and a safe environment with access to a telephone, utilities, food, and a refrigerator. Although concerns have been raised about the potential risk of serious complications in patients with bacterial meningitis, complications (when they occur) usually happen within the first 2-3 days of treatment and are exceedingly rare after 3 or 4 days of appropriate antimicrobial therapy. In addition, completion of antimicrobial therapy in a skilled nursing facility may be appropriate for selected patients who need continued care but do not require acute hospitalization.
Duration of Antimicrobial Therapy
The duration of antimicrobial therapy in the patient with bacterial meningitis has often been based more on tradition than on evidence-based data (74,185,186). Recommendations are shown in Table 8. However, it must be emphasized that these guidelines are not standardized and the duration of therapy may need to be individualized based on the patient’s clinical response. Pending further data, intravenous antimicrobial therapy is recommended for the duration of treatment to ensure that adequate CSF concentrations of specific antimicrobial agents are attained.
Viral Meningitis
Specific antiviral chemotherapy is not currently available for the therapy of enteroviral meningitis; treatment is supportive. No specific antiviral therapy also exists for the arboviruses or mumps virus. Recovery of patients with herpes simplex virus type 2 meningitis is usually complete without neurologic sequelae; it is not clear whether antiviral treatment alters the course of mild meningitis, although treatment with acyclovir is generally indicated for primary genital herpes infection (39). Intravenous acyclovir is used if meningitis is associated with genital herpes simplex infection.
Tuberculous Meningitis
CNS tuberculosis was a uniformly fatal disease until the advent of effective chemotherapy. However, the optimal drug regimen, dose, route of administration, and duration of treatment still remain undefined. The most important principle of therapy is that it should be initiated on the basis of strong clinical suspicion and not delayed until proof of infection has been obtained, since outcome of tuberculous meningitis is determined by the clinical stage at admission and the delay in initiating therapy (70). In general, the principles of treatment of CNS tuberculosis do not differ significantly from those for other forms of tuberculosis (50,51,73,98). For treatment of tuberculous meningitis, the American Thoracic Society, in guidelines co-issued with the Infectious Diseases Society of America and the Centers for Disease Control and Prevention, recommends 2 months of isoniazid, rifampin, pyrazinamide and ethambutol followed by 7-10 months of isoniazid and rifampin for treatment of drug-sensitive tuberculous meningitis (187). However, therapy for tuberculous meningitis may need to be individualized, with longer durations of therapy utilized in patients with a higher severity of illness (188). In addition, HIV-1-infected patients may require longer courses of treatment (189). Clinical disease with resistant organisms occurs with higher frequency among immigrants from countries in Asia, Africa, and the Americas; known contacts of drug-resistant cases; homeless and impoverished individuals; and residents of certain geographic areas in the United States (particularly adjacent to the Mexican border) (50). In patients with multidrug-resistant strains of M. tuberculosis, the optimal therapeutic regimen is unknown (73), but should be guided by susceptibility testing and drug penetration into the CSF. Ethionamide crosses both healthy and inflamed meninges, with peak CSF concentrations comparable to those achieved in serum. The fluoroquinolones also penetrate well into CSF and have good in vitro activity against M. tuberculosis, but their published use in tuberculous meningitis is restricted to case reports.
ADJUNCTIVE THERAPIES
Bacterial Meningitis
Reduction of Increased Intracranial Pressure
Patients with bacterial meningitis who have signs of increased intracranial pressure (e.g., altered level of consciousness; dilated, poorly reactive or nonreactive pupils; ocular movement disorders) and who are stuporous or comatose may benefit from the insertion of an intracranial pressure monitoring device (55,136,190); intracranial pressures exceeding 20 mmHg are abnormal and should be treated, and there is rationale for treating smaller pressure elevations (i.e., above 15 mmHg) to avoid larger elevations, so-called plateau waves, that can lead to cerebral herniation and irreversible brain stem injury. Methods available to reduce intracranial pressure include elevation of the head of the bed to 30 degrees to maximize venous drainage with minimal compromise of cerebral perfusion; hyperventilation to maintain the PaCO2 between 27 and 30 mmHg, which causes cerebral vasoconstriction and a reduction in cerebral blood volume; use of hyperosmolar agents (e.g., mannitol) to make the intravascular space hyperosmolar to the brain and permit movement of water from brain tissue into the intravascular compartment; and corticosteroids.
Glycerol, an osmotic dehydrating agent that can be given orally, has been evaluated in a trial of 122 infants and children with bacterial meningitis (191), in which patients were randomized to receive adjunctive intravenous dexamethasone, oral glycerol, dexamethasone plus glycerol, or neither. Seven percent of the glycerol-treated patients and 19% of those not given glycerol had audiologic or neurologic sequelae (p=.052). Despite these positive results, however, further placebo-controlled, blinded studies are required before glycerol can be routinely recommended in patients with bacterial meningitis.
Patients who continue to have elevated intracranial pressures despite the aforementioned measures may be treated with high-dose barbiturate therapy (55,136,190), which decreases cerebral metabolic demands and cerebral blood flow. Barbiturates can also cause vasoconstriction in normal tissue, thereby shunting blood to ischemic tissue and protecting the brain from ischemic insult. During the administration of pentobarbital, the patient is monitored to measure decreases in intracranial pressure, or the dose can be titrated to the development of a burst suppression pattern on the electroencephalogram. This mode of treatment for meningitis and elevated intracranial pressure is of unproven benefit, however, and must be considered experimental.
Surgery
Surgical intervention may be required in some patients with bacterial meningitis. Patients who have suffered a basilar skull fracture with CSF leak may have persistent dural defects that can lead to recurrent episodes of bacterial meningitis. Many leaks will cease spontaneously, but surgery is indicated for leaks that persist for several weeks or in patients who present with delayed or recurrent infection. Surgery is not indicated in the acute phase (before 7 days) of leakage; no difference in outcome is seen when patients with acutely repaired leaks are compared with those whose leaks stop spontaneously within 7 days. Surgical intervention may also be required in patients in whom recurrent meningitis develops from congenital or acquired cranial defects and dermal sinuses (29).
CSF Shunts
In patients with infected CSF shunts, removal of all components of the infected shunt and some component of external drainage, in combination with appropriate antimicrobial therapy, appears to be the most effective treatment (74,182); the ventriculitis of the shunt infection appears to clear more rapidly with the drainage catheter and the presence of the catheter allows continued treatment of the hydrocephalus until the infection has cleared. Success rates are lower when the shunt is treated in situ because the ability of many of these microorganisms to adhere to prostheses and survive antimicrobial therapy. The timing of shunt reimplantation is dependent upon the isolated microorganism, the extent of infection as defined by cultures obtained after externalization, and (occasionally) on CSF findings. In patients with infections caused by coagulase-negative staphylococci and normal CSF findings, the presence of negative CSF cultures after externalization generally confirms that removal of the hardware led to a cure; these patients can be reshunted on the third day after removal. If CSF abnormalities are present and a coagulase-negative staphylococcus is isolated, 7 days of antimicrobial therapy are recommended prior to reshunting as long as repeat CSF cultures are negative and the ventricular protein concentration is appropriate (<200 mg/dL); if repeat cultures are positive, antimicrobial therapy is continued until CSF cultures remain negative for 10 consecutive days before a new CSF shunt is placed. For shunt infections caused by S. aureus, 10 days of negative cultures are recommended prior to reshunting and for gram-negative bacilli, a 10-14-day course of antimicrobial therapy should be utilized, although longer durations may be needed depending upon the clinical response. Some experts also suggest that consideration be given to a 3-day period off antimicrobial therapy to verify clearing of the infection prior to shunt reimplantation, although it may not be necessary in all patients and should be considered optional.
Viral Meningitis
Adjunctive measures have been used in seriously ill patients with enteroviral meningitis. Because enteroviral clearance from the host is antibody mediated, exogenously administered antibody has been examined (31,65). Administration of immune globulin by multiple routes (including directly into the CNS) has led to stabilization or improvement of agammaglobulinemic patients with chronic enteroviral meningitis or meningoencephalitis, although results have varied. In a single randomized trial of intravenous immune globulin plus standard therapy versus standard therapy alone in neonates suspected of having enteroviral infection during the first 2 weeks of life, intravenous immune globulin failed to reduce viremia or improve outcome, although the study enrolled too few patients for a definitive conclusion (192); in this study, 75% of patients had clinical or laboratory evidence of meningitis. Anecdotal reports have appeared on the use of corticosteroids in patients with mumps encephalitis (38), but no benefits have been documented.
Tuberculous Meningitis
Adjunctive therapies for tuberculous meningitis mainly are immunomodulators and surgery.
Corticosteroids
Despite the availability of effective antituberculous chemotherapy, tuberculous meningitis is associated with persistent morbidity and mortality. The most persistently advocated adjunctive agents for tuberculous meningitis are corticosteroids (50,51,73,98), which have been demonstrated to abrogate the signs and symptoms of disease in which patients frequently defervesce, with clearing of sensorium and improvement in well-being, even after only a few doses. A number of studies have suggested that the primary value of corticosteroids may be in their ability to treat or avert the development of spinal block, possibly by lowering the protein content of CSF; an improvement in overall mortality has been ascribed to their specific impact on this poor prognostic sign. Despite the benefits of corticosteroids in reducing mortality, however, a concomitant, almost compensatory increase in significant neurologic sequelae has been seen in survivors with tuberculous meningitis, although two recent studies did not observe this paradoxical finding (193,194). In a recent randomized, double-blind, placebo-controlled trial for patients over 14 years of age with tuberculous meningitis in Vietnam (195), adjunctive dexamethasone improved survival, although probably did not prevent severe disability.
Surgery
Surgical intervention in patients with tuberculous meningitis is primarily aimed at relief of hydrocephalus, with placement of ventriculoatrial or ventriculoperitoneal shunts (73). However, criteria for selection of patients for shunt surgery for hydocephalus complicating tuberculous meningitis remain unclear. One study demonstrated that neurologic status on admission was the most important factor in determining outcome in patients who had undergone shunt surgery for hydrocephalus (196); patients with normal sensorium with or without neurologic deficits were recommended for early shunt surgery, whereas for those with altered sensorium or who were deeply comatose with or without decerebrate-decorticate posturing, external drainage was recommended. A subsequent study by the same authors recommended that patients with altered sensorium undergo shunt surgery whereas those with severe neurologic compromise (deep coma with or without decerebrate-decorticate posturing) have a trial of external drainage with their response determining the need for shunt surgery (197).
PREVENTION OF BACTERIAL MENINGITIS
Immunoprophylaxis
Haemophilus influenzae
Vaccination to prevent infection with specific meningeal pathogens is a very useful measure for decreasing the incidence of bacterial meningitis. For H. influenzae type b, the availability of conjugate vaccines has decreased the number of cases of H. influenzae type b meningitis more than 90%. Three H. influenzae type b conjugate vaccines are now licensed for infant immunization. The American Academy of Pediatrics has recommended vaccine doses at 2, 4, and 6 months of age (198); if PRP-OMP (PedvaxHIB) is administered at 2 and 4 months, a dose at 6 months is not required. A booster dose of vaccine is also administered at 12-15 months of age no matter which vaccine was administered for the primary series. In addition to preventing invasive disease caused by H. influenzae type b, conjugate vaccines are effective in reducing nasopharyngeal colonization and therefore may confer protection to populations not targeted for immunization through herd immunity; this reduction may open ecological niches for non-type b strains, however. In a surveillance study recently conducted in Brazil before and after the introduction of H. influenzae type b conjugate vaccine immunization, the incidence of H. influenzae type b meningitis decreased by 69%, but the incidence of H. influenzae type a meningitis increased 8-fold (199), highlighting the importance of maintaining surveillance to monitor potential increases in disease due to serotype replacement. Of additional concern is the recent report of cases of invasive H. influenzae type b disease in children previously vaccinated against this disease in Nottingham, UK (200). This may be because the vaccination schedule used in the UK is to vaccinate at 2, 3, and 4 months of age; the absence of a booster dose may have led to this increase in invasive disease (201).
Neisseria meningitidis
The availability of vaccines against N. meningitidis has evolved significantly over recent years (202). Until recently in the United States, vaccination with the quadrivalent meningococcal polysaccharide vaccine (against serogroups A, C, Y, and W135) was recommended for patients in certain high-risk groups, but was not recommended for routine use because of the overall low risk of infection, the inability to protect against serogroup B disease, and the inability to provide lasting immunity to young children. Subsequently, because of an increase in invasive meningococcal disease in adolescents and young adults of high school and college age in the United States, the Advisory Committee on Immunization Practices recommended that college freshman dormitory residents and their parents be provided information about meningococcal infection and the benefits of vaccination, and that other undergraduate students wishing to reduce their risk of meningococcal disease can also choose to be vaccinated (203,204).
Based on the success of conjugate H. influenzae type b conjugate vaccines (see above), conjugate vaccines for use against invasive disease caused by meningococci were developed. The United Kingdom became the world’s first country to implement vaccination with a monovalent serogroup C meningococcal vaccine, in which children are vaccinated at 2, 3 and 4 months of age; the efficacy of the meningococcal serogroup C conjugate vaccine in adolescents and toddlers was evaluated after the first 9 months after vaccine introduction in a catch-up program in which toddlers and adolescents received a single dose of CRM197-meningococcal C vaccine (205). The short-term vaccine efficacy in toddlers and adolescents was 92% and 97%, respectively. In an update of the epidemiologic effect of the first 18 months of the UK meningococcal C conjugate vaccine program, the overall reduction of cases of serogroup C disease from 1998-1999 to 2000-2001 was 81% with some variability based on age group (206). In another case-control study in teenagers in the UK to assess vaccine efficacy, the protective effectiveness of the vaccine was 93% (207). In January 2005, a tetravalent meningococcal polysaccharide protein conjugate vaccine (MCV4) with activity against serogroups A, C, Y, and W135 was licensed for clinical use. The Advisory Committee on Immunization Practices recommended routine immunization with this vaccine among persons aged 11-12 years, of adolescents at high school entry if not previously vaccinated, and of college freshman living in dormitories (208). Vaccination was also recommended for other persons at increased risk – military recruits, travelers to areas where meningococcal disease is hyperendemic or epidemic, microbiologists who are routinely exposed to isolates of N. meningitidis, persons with anatomic or functional asplenia, and persons with terminal complement deficiencies. Based on supply problems post-licensure, however, it was recommended that vaccine administration to persons aged 11-12 years be deferred (209).
Streptococcus pneumoniae
A pneumococcal conjugate vaccine is also available and affords protection against seven pneumococcal serotypes that cause invasive disease. A trial of this heptavalent conjugate pneumococcal vaccine in 37,868 infants and children and demonstrated an efficacy of 97.4% in the prevention of invasive pneumococcal disease in fully vaccinated children (210). Based on this data, the American Academy of Pediatrics Committee on Infectious Diseases recommended vaccination of all infants less that 2 years of age with the currently licensed heptavalent pneumococcal conjugate vaccine (Prevnar) (211); the vaccine is admininstered in four doses at 2, 4, 6, and 12-15 months of age. In a study of population-based data from the Active Bacterial Core Surveillance of the CDC on trends in the rate of invasive pneumococcal disease after licensure of the heptavalent pneumococcal vaccine, there was a decline in the rate of invasive pneumococcal disease, with the largest decline (69%) in children under two years of age (212); disease also fell in adults (32% lower for those 20-39 years of age, 8% lower for those 40-64 years of age, and 18% lower for those 65 years of age and older), and in persons with invasive disease caused by pneumococcal strains that were not susceptible to penicillin (35% reduction). In a follow-up report (213), routine vaccination with Prevnar continued to result in statistically significant declines in the incidence of invasive pneumococcal disease in age groups targeted for vaccination, and among older children and adults. Increases in disease caused by pneumococcal serotypes not included in the vaccine occurred in certain populations, but were small when compared with overall declines in vaccine-serotype disease. These findings have also been confirmed in other studies (214). Continued surveillance of pneumococcal serotypes causing invasive disease is critical and may have implications for the serotype formulation of future conjugate vaccines.
Chemoprophylaxis
Haemophilus influenzae
It has become clear that the spread of several types of bacterial meningitis can be prevented by prophylaxis of contacts of cases with antimicrobial agents (55). Several studies have documented the transmission of H. influenzae type b from patients with meningitis to household contacts, with most secondary cases (75%) occurring within 6 days of onset of the index case, although untreated household contacts remain at increased risk for H. influenzae type b disease for at least 1 month after onset in the index case. Daycare outside the home is considered another risk factor for transmission. The rationale for the use of chemoprophylaxis for prevention of secondary disease is eradication of nasopharyngeal colonization of H. influenzae type b, thereby preventing transmission to young, susceptible contacts and the development of invasive disease in those already colonized. In those who require chemoprophylaxis, the recommended agent of choice is rifampin (20 mg/kg daily for 4 days) for all individuals, including adults, in households with at least one unvaccinated or incompletely vaccinated child younger than 48 months. The index patient may also need to receive rifampin prophylaxis. Ampicillin and chloramphenicol, unlike ceftriaxone and cefotaxime, do not effectively eliminate nasopharyngeal colonization. Rifampin is not recommended for pregnant women who are contacts of infected infants because the risk of rifampin to the fetus has not been established. Chemoprophylaxis is not currently recommended for daycare contacts 2 years or older unless two or more cases occur in the daycare center within a 60-day period. However, given the widespread use of the H. influenzae type b conjugate vaccine, chemoprophylaxis to prevent secondary cases of invasive disease is now rarely needed.
Neisseria meningitidis
Chemoprophylaxis is necessary for close contacts of patients with invasive meningococcal disease (55); up to 10% of meningococcal meningitis cases have had contact with another known case. The estimated prevalence of meningococcal carriage in the United States is 5 to 10% under nonepidemic conditions, whereas in closed populations such as military recruits, carriage rates can reach levels of 40 to 90%. Household contacts exposed to a case of meningococcal disease have a 500- to 800-fold to 3000- to 4000-fold increased risk of invasive disease. Secondary systemic meningococcal disease often develops within 5 days of recognition of the index case, with 70 to 80% of secondary cases occurring within 14 days of the primary case; in one report, 9 of 17 (53%) secondary cases occurred 5 to 39 weeks after the primary case. Chemoprophylaxis is recommended for close contacts of the index case, defined as household contacts, daycare center members, and anyone directly exposed to the patient's oral secretions (e.g., through kissing, mouth-to-mouth resuscitation, endotracheal intubation, or endotracheal tube management) (215). Chemoprophylaxis is not recommended for school, work, or transportation contacts. Chemoprophylaxis may also need to be administered to the index case before hospital discharge because certain antimicrobial agents (e.g., high-dose penicillin or chloramphenicol) do not reliably eradicate meningococci from the nasopharynx of colonized patients. Chemoprophylaxis should be administered as soon as possible (ideally within 24 hours) after the case is identified; administration 14 or more days after the onset of illness in the index patient is probably of limited value.
The optimal chemoprophylactic agent to prevent invasive meningococcal disease is controversial (55). The CDC currently recommends the administration of rifampin at 12-hour intervals for 2 days in the following dosages: adults, 600 mg; children 1 month or older, 10 mg/kg; and infants younger than 1 month, 5 mg/kg (215). However, rifampin has several shortcomings, including nasopharyngeal eradication rates of only about 80%, adverse events, necessity for multiple doses over a 2-day period, and emergence of resistant organisms (up to 10 to 27% of isolates), which may then cause invasive disease. In the search for alternative agents, ceftriaxone (intramuscular administration of 250 mg in adults and 125 mg in children) eliminated the serogroup A carrier state in 97% of patients in one study for up to 2 weeks (216), although parenteral administration is required. Additional studies have demonstrated a single dose of oral ciprofloxacin (500 mg in adults) to be very effective in elimination of the nasopharyngeal carriage of N. meningitidis (55). Ciprofloxacin concentrations in nasal secretions have been shown to exceed the MIC90 for meningococci. Ciprofloxacin is not recommended for use in children because of concern regarding cartilage damage. In pregnant patients, ceftriaxone is probably the safest alternative agent for chemoprophylaxis. Azithromycin (500 mg orally once) was also shown to be as effective as a four-dose regimen of rifampin in the eradication of meningococci from the nasopharynx (217). Widespread chemoprophylaxis to low-risk contacts should be discouraged because of the concern over emergence of resistant organisms and possible future limitations on this approach.
Streptococcus agalactiae
The CDC has established guidelines for the prevention of group B streptococcal infection by chemoprophylaxis (218,219). All pregnant women should be screened at 35 to 37 weeks' gestation for anogenital colonization with group B streptococci. Maternal group B streptococcal carriers, identified either antepartum or intrapartum, should receive chemoprophylaxis if one or more of the following risk factors are present: preterm labor at less than 37 weeks' gestation, fever (temperature of 38°C or higher) during labor, or after membranes have been ruptured 18 or more hours during any gestation. Furthermore, previous delivery of a sibling with invasive group B streptococcal disease warrants intrapartum maternal chemoprophylaxis in each subsequent pregnancy. Chemoprophylaxis should consist of intrapartum intravenous ampicillin (2 g initially and then 1 to 2 g every 4 hours) or penicillin G (5 million units initially and then 2.5 million units every 4 hours) until delivery; intravenous clindamycin or erythromycin should be used in penicillin-allergic patients.
Basilar Skull Fracture
A number of studies have used prophylactic antibiotics in patients with basilar skull fractures and CSF leak on the premise that in patients with a dural defect, the CSF is exposed to pathogenic organisms from the nasopharynx, nasal or mastoid sinuses, or external auditory canal. Interpretation and comparison of the various studies examining this question are confounded by multiple variables, including patient selection, choice of antimicrobial agents, and definition of infection. No prospective controlled trials have examined the efficacy of prophylactic antimicrobial agents in these patients, although a meta-analysis suggested that antibiotic prophylaxis did not prevent meningitis in patients with basilar skull fracture (220). Antibiotic use does not appear to change the incidence of post-traumatic bacterial meningitis and may result in the selection and growth of resistant organisms.
REFERENCES
1. Schlech WF III, Ward JI, Band JD, et al. Bacterial meningitis in the United States, 1978 through 1981. The National Bacterial Meningitis Surveillance Study. JAMA. 1985;253:1749–1754. [PubMed]
2. Wenger JD, Hightower AW, Facklam RR, et al. Bacterial meningitis in the United States, 1986: Report of a multistate surveillance study. J Infect Dis. 1990;162:1316–1323. [PubMed]
3. Schuchat A, Robinson K, Wenger JD, et al. Bacterial meningitis in the United States in 1995. N Engl J Med. 1997;337:970–976. [PubMed]
4. Thigpen MC, Rosenstein NE, Whitney CG, et al. Bacterial meningitis in the United States – 1998-2003. In: Program and Abstracts of the 43rd Annual Meeting of the Infectious Diseases Society of America. San Francisco, CA. 2005:46.
5. Durand ML, Calderwood SB, Weber DJ, et al. Acute bacterial meningitis in adults. A review of 493 episodes. N Engl J Med. 1993;328:21–28. [PubMed]
6. Hussein AS, Shafran SD. Acute bacterial meningitis in adults. A 12-year review. Medicine (Baltimore). 2000;79:360-368. [PubMed]
7. McMillan DA, Lin CY, Aronin SI, Quagliarello VJ. Community-acquired bacterial meningitis in adults: categorization of causes and timing of death. Clin Infect Dis. 2001;33:969-975. [PubMed]
8. Bryan JP, de Silva HR, Tavares A, et al. Etiology and mortality of bacterial meningitis in northeastern Brazil. Rev Infect Dis. 1990;12:128–135. [PubMed]
9. Geiseler PJ, Nelson KE, Levin S, et al. Community-acquired purulent meningitis: a review of 1,316 cases during the antibiotic era, 1954–1976. Rev Infect Dis. 1980;2:725–745. [PubMed]
10. Weisfelt M, van de Beek D, Spanjaard L, et al. Clinical features, complications, and outcome in adults with pneumococcal meningitis: a prospective case series. Lancet Neurol. 2006;5:123-129. [PubMed]
11. Overturf GD. Indications for the immunological evaluation of patients with meningitis. Clin Infect Dis. 2003;36:189-194. [PubMed]
12. Rosenstein NE, Perkins BA, Stephens DS, et al. The changing epidemiology of meningococcal disease in the United States, 1992-1996. J Infect Dis. 1999;180:1894-1901. [PubMed]
13. Mastrantonio P, Stefanelli P, Fazio C, et al. Serotype distribution, antibiotic susceptibility, and genetic relatedness of Neisseria meningitidis strains recently isolated in Italy. Clin Infect Dis. 2003;36:422-428. [PubMed]
14. Raymond NJ, Reeves M, Ajello G, et al. Molecular epidemiology of sporadic (endemic) serogroup C meningococcal disease. J Infect Dis. 1997;176:1277–1284. [PubMed]
15. Wilder-Smith A, Goh KT, Barkham T, Paton NI. Hajj-associated outbreak of Neisseria meningitidis serogroup W135: estimates of the attack rate in a defined population and the risk of invasive disease developing in carriers. Clin Infect Dis. 2003;36:679-683. [PubMed]
16. Stephens DS. Uncloaking the meningococcus: dynamics of carriage and disease. Lancet. 1999;353:941-942. [PubMed]
17. Ross SC, Densen P. Complement deficiency states and infection: Epidemiology, pathogenesis and consequences of neisserial and other infections in an immune deficiency. Medicine (Baltimore). 1984;64:243–273. [PubMed]
18. Lorber B. Listeriosis. Clin Infect Dis. 1997;24:1–11. [PubMed]
19. Mylonakis E, Hohmann EL, Calderwood SB. Central nervous system infection with Listeria monocytogenes. 33 years' experience at a general hospital and review of 776 episodes from the literature. Medicine (Baltimore). 1998;77:313–336.[PubMed]
20. Gottlieb SL, Newbern EC, Griffin PM, et al. Multistate outbreak of listeriosis linked to turkey deli meat and subsequent changes in US regulatory policy. Clin Infect Dis. 2006;42:29-36. [PubMed]
21. Dunne DW, Quagliarello V. Group B streptococcal meningitis in adults. Medicine (Baltimore). 1993;72:1–10. [PubMed]
22. Domingo P, Barquet N, Alvarez M, et al. Group B streptococcal meningitis in adults: report of twelve cases and review. Clin Infect Dis. 1997;25:1180–1187. [PubMed]
23. Spagnuolo PJ, Ellner JJ, Lerner PI, et al. Haemophilus influenzae meningitis: the spectrum of disease in adults. Medicine (Baltimore). 1982;61:74–85. [PubMed]
24. Farley MM, Stephens DS, Brachman PS, et al. Invasive Haemophilus influenzae disease in adults. A prospective, population-based surveillance. Ann Intern Med. 1992;116:806–812. [PubMed]
25. Adegbola RA, Secka O, Lahai G, et al. Elimination of Haemophilus influenzae type b (Hib) disease from The Gambia after the introduction of routine immunization with a Hib conjugate vaccine: a prospective study. Lancet. 2005;366:144-150.[PubMed]
26. Cherubin CE, Marr JS, Sierra MF, et al. Listeria and gram-negative bacillary meningitis in New York City, 1972–1979. Am J Med. 1981;71:199–209. [PubMed]
27. Unhanand M, Mustafa MM, McCracken GH Jr, et al. Gram-negative enteric bacillary meningitis: a twenty-one-year experience. J Pediatr. 1993;122:15–21. [PubMed]
28. Schlesinger LS, Ross SC, Schaberg DR. Staphylococcus aureus meningitis: a broad-based epidemiologic study. Medicine (Baltimore). 1987;66:148–156. [PubMed]
29. Kaufman BA, Tunkel AR, Pryor JC, et al. Meningitis in the neurosurgical patient. Infect Dis Clin North Am. 1990;4:677–701. [PubMed]
30. Connolly KJ, Hammer SM. The acute aseptic meningitis syndrome. Infect Dis Clin North Am. 1990;4:599–622. [PubMed]
31. Romero JR. Diagnosis and management of enteroviral infections of the central nervous system. Curr Infect Dis Rep. 2002;4:309-316. [PubMed]
32. Ho M, Chen ER, Parker RA. An epidemic of enterovirus 71 infection in Taiwan. N Engl J Med. 1999;341:929-935.[PubMed]
33. Huang CC, Liu CC, Chang UC, et al. Neurologic complications in children with enterovirus 71 infection. N Engl J Med. 1999;341:936-942. [PubMed]
34. McMinn P, Stratov I, Nagarajan L, Davis S. Neurological manifestations of enterovirus 71 infection in children during an outbreak of hand, foot, and mouth disease in western Australia. Clin Infect Dis. 2001;32:236-242. [PubMed]
35. Rotbart HA, Brennan PJ, Fife KH, et al. Enterovirus meningitis in adults. Clin Infect Dis. 1998;27:896-898. [PubMed]
36. Quartier P, Tournilhac O, Archimbaud C, et al. Enteroviral meningoencephalitis after anti-CD20 (rituximab) treatment. Clin Infect Dis. 2003;36:e47-49. [PubMed]
37. Nash D, Mostashari F, Fine A, et al. The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med. 2001;344:1807-1814. [PubMed]
38. Gnann JW Jr. Meningitis and encephalitis caused by mumps virus. In: Scheld WM, Whitley RJ, Marra CM, eds. Infections of the Central Nervous System. 3rd ed. Philadelphia:Lippincott Williams & Wilkins; 2004:231-241.
39. Corey L, Spear PG. Infections with herpes simplex viruses (second of two parts). N Engl J Med. 1986;314:749–757. [PubMed]
40. Corey L, Adams HG, Brown ZA, et al. Genital herpes simplex virus infection: Clinical manifestations, course, and complications. Ann Intern Med. 1983;98:958–972. [PubMed]
41. Bergström T, Vahlne A, Alestig K, et al. Primary and recurrent herpes simplex virus type 2–induced meningitis. J Infect Dis. 1990;162:322–330. [PubMed]
42. Echevarria JM, Martinez-Martin P, Tellaz A, et al. Aseptic meningitis due to varicella-zoster virus: serum antibody levels and local synthesis of specific IgG, IgM, and IgA. J Infect Dis. 1987;155:959–967. [PubMed]
43. Yamamoto LJ, Tedder DG, Ashley R, et al. Herpes simplex virus type 1 DNA in cerebrospinal fluid of a patient with Mollaret's meningitis. N Engl J Med. 1991;325:1082–1085. [PubMed]
44. Tedder DG, Ashley R, Tyler KL, Levin MJ. Herpes simplex virus infection as a cause of benign recurrent lymphocytic meningitis. Ann Intern Med. 1994;121:334–338. [PubMed]
45. Picard FJ, Dekaban GA, Silva J, Rice GPA. Mollaret's meningitis associated with herpes simplex type 2 infection. Neurology. 1993;43:1722–1727. [PubMed]
46. Graman PS. Mollaret's meningitis associated with acute Epstein-Barr virus mononucleosis. Arch Neurol. 1987;44:1204–1205. [PubMed]
47. Caserta MT, Hall CB, Schnabel K, et al. Neuroinvasion and persistence of human herpesvirus 6 in children. J Infect Dis. 1994;170:1586–1589. [PubMed]
48. McArthur JC. Neurologic manifestations of AIDS. Medicine (Baltimore). 1987;66:407–437. [PubMed]
49. Hollander H, Stringari S. Human immunodeficiency virus–associated meningitis. Clinical course and correlations. Am J Med. 1987;83:813–816. [PubMed]
50. Zugar A. Tuberculosis of the central nervous system. In Scheld WM, Whitley RJ, Marra CM, eds. Infections of the Central Nervous System, 3rd ed. Philadelphia:Lippincott, Williams & Wilkins; 2004:441-459.
51. Leonard JM, Des Prez RM. Tuberculous meningitis. Infect Dis Clin North Am 1990;4:769-787. [PubMed]
52. Singh N, Paterson DL. Mycobacterium tuberculosis infection in solid-organ transplant recipients: impact and implications for management. Clin Infect Dis. 1998; 27:1266-1277. [PubMed]
53. Markowitz N, Hansen NI, Hopewell PC, et al. Incidence of tuberculosis in the United States among HIV-infected persons. Ann Intern Med. 1997;126:123-132. [PubMed]
54. Barnes PF, Bloch AB, Davidson PT, et al. Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med. 1991;324:1644-1650. [PubMed]
55. Tunkel AR. Bacterial meningitis. Philadelphia:Lippincott Williams & Wilkins, 2001.
56. Verghese A, Gallemore G. Kernig's and Brudzinski's signs revisited. Rev Infect Dis. 1987;9:1187–1192. [PubMed]
57. Thomas KE, Hasbun R, Jekel J, Quagliarello VJ. The diagnostic accuracy of Kernig’s sign, Brudzinski’s sign, and nuchal rigidity in adults with suspected meningitis. Clin Infect Dis. 2002;35:46-52. [PubMed]
58. Attia J, Hatala R, Cook DJ, et al. Does this patient have acute meningitis. JAMA. 1999;282:175-181. [PubMed]
59. Anderson J, Backer V, Voldsgaard P, et al. Acute meningococcal meningitis: analysis of features of the disease according to the age of 255 patients. Copenhagen Meningitis Study Group. J Infect. 1997;34:227–235. [PubMed]
60. Saez-Llorens X, McCracken GH Jr. Bacterial meningitis in children. Lancet. 2003;361:2139-2148. [PubMed]
61. Ashwal S, Perkin RM, Thompson JR, et al. Bacterial meningitis in children: Current concepts of neurologic management. Curr Probl Pediatr. 1994;24:267–284.[PubMed]
62. Choi C. Bacterial meningitis in aging adults. Clin Infect Dis. 2001;33:1380-1385. [PubMed]
63. Gorse GJ, Thrupp LD, Nudleman KL, et al. Bacterial meningitis in the elderly. Arch Intern Med. 1989;149:1603–1606. [PubMed]
64. Tunkel AR, Scheld WM. Central nervous system infection in the compromised host. In: Rubin RH, Young LS, eds. Clinical Approach to Infection in the Compromised Host. 4th ed. New York: Kluwer Academic/Plenum Publishers; 2002:163-214.
65. Sawyer MH, Rotbart HA. Viral meningitis and aseptic meningitis syndrome. In: Scheld WM, Whitley RJ, Marra CM, eds. Infections of the Central Nervous System. 3rd ed. Philadelphia:Lippincott, Williams & Wilkins; 2004:75-93.
66. Ogawa SK, Smith MA, Brennessel DJ, et al. Tuberculous meningitis in an urban medical center. Medicine (Baltimore). 1987;63:317-326. [PubMed]
67. Kent SJ, Crowe SM, Yung A, et al. Tuberculous meningitis: a 30-year review. Clin Infect Dis. 1993;17:987-994.[PubMed]
68. Alvarez S, McCabe WR. Extrapulmonary tuberculosis revisited: a review of experience at Boston City and other hospitals. Medicine (Baltimore). 1984;63:25-54. [PubMed]
69. Kennedy DH, Fallon RJ. Tuberculous meningitis. JAMA. 1979;241:264-268. [PubMed]
70. Verdon R, Chevret S, Laissy JP, Wolff M. Tuberculous meningitis in adults: review of 48 cases. Clin Infect Dis 1996;22:982-988 [PubMed]
71. Berenguer J, Moreno S, Laguna F, et al. Tuberculous meningitis in patients infected with human immunodeficiency virus. N Engl J Med. 1992;326:668-672. [PubMed]
72. Dube MP, Holtom PD, Larsen RA. Tuberculous meningitis in patients with and without human immunodeficiency virus infection. Am J Med. 1992;93:520-524. [PubMed]
73. Thwaites GE, Hien TT. Tuberculous meningitis: many questions, too few answers. Lancet Neurol. 2005;4:160-170. [PubMed]
74. Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;29:1267-1284. [PubMed]
75. Marton KI, Gean AD. The spinal tap: a new look at an old test. Ann Intern Med. 1986; 104:840-848. [PubMed]
76. Greenlee JE, Carroll KC. Cerebrospinal fluid in central nervous system infections. In: Scheld WM, Whitley RJ, Marra CM, eds. Infections of the Central Nervous System, 3rd ed. Philadelphia: Lippincott, Williams & Wilkins; 2004;5-30.
77. Horwitz SJ, Boxerbaum B, O’Bell J. Cerebral herniation in bacterial meningitis in childhood. Ann Neurol. 1980; 7:524-528. [PubMed]
78. Hasbun R, Abrahams J, Jekel J, Quagliarello VJ. Computed tomography of the head before lumbar puncture in adults with suspected meningitis. N Engl J Med. 2001; 345:1727-1733. [PubMed]
79. Bonadio WA. The cerebrospinal fluid: physiologic aspects and alterations associated with bacterial meningitis. Pediatr Infect Dis J. 1992; 11:423-432. [PubMed]
80. Negrini B, Kelleher KJ, Wald ER. Cerebrospinal fluid findings in aseptic versus bacterial meningitis. Pediatrics. 2000;105:316-319. [PubMed]
81. Spanos A, Harrell FE Jr, Durack DT. Differential diagnosis of acute meningitis. An analysis of the predictive value of initial observations. JAMA. 1989; 262:2700-2707. [PubMed]
82. McKinney WP, Heudebert GR, Harper SA, et al. Validation of a clinical prediction rule for the differential diagnosis of acute meningitis. J Gen Intern Med. 1994; 9:8-12. [PubMed]
83. Genton B, Berger JP. Cerebrospinal fluid lactate in 78 cases of adult meningitis. Intensive Care Med. 1990; 16:196-200. [PubMed]
84. Leib SL, Boscacci R, Gratzl O, Zimmerli W. Predictive value of cerebrospinal fluid (CSF) lactate level versus CSF/blood glucose ratio for the diagnosis of bacterial meningitis following neurosurgery. Clin Infect Dis. 1999; 29:69-74. [PubMed]
85. Nathan BR, Scheld WM. The potential roles of C-reactive protein and procalcitonin concentrations in the serum and cerebrospinal fluid in the diagnosis of bacterial meningitis. In: Current Clinical Topics in Infectious Diseases, vol 22. Remington JS, Swartz MN, eds. Oxford: Blackwell Science Ltd. 2002:155-65.
86. Gerdes LU, Jorgensen PE, Nexo E, Wang P. C-reactive protein and bacterial meningitis: a meta-analysis. Scand J Clin Lab Invest. 1998; 58:383-393. [PubMed]
87. Sormunen P, Kallio MJT, Kilpi T, Peltola H. C-reactive protein is useful in distinguishing Gram stain-negative bacterial meningitis from viral meningitis in children. J Pediatr. 1999; 134:725-729. [PubMed]
88. Gendrel D, Raymond J, Assicot M, et al. Measurement of procalcitonin levels in children with bacterial or viral meningitis. Clin Infect Dis. 1997; 24:1240-1242. [PubMed]
89. Viallon A, Zeni F, Lambert C, et al. High sensitivity and specificity of serum procalcitonin levels in adults with bacterial meningitis. Clin Infect Dis. 1999; 28:1313-1316. [PubMed]
90. Schwarz S, Bertram M, Schwab S, et al. Serum procalcitonin levels in bacterial and abacterial meningitis. Crit Care Med. 2000; 28:1828-1832. [PubMed]
91. La Scolea LJ Jr, Dryja D. Quantitation of bacteria in cerebrospinal fluid and blood of children with meningitis and its diagnostic significance. J Clin Microbiol. 1984; 19:187-190. [PubMed]
92. Chapin-Robertson K, Dahlberg SE, Edberg SC. Clinical and laboratory analyses of cytospin-prepared Gram stains for recovery and diagnosis of bacteria from sterile body fluids. J Clin Microbiol. 1992; 30:377-380. [PubMed]
93. Gray LD, Fedorko DP. Laboratory diagnosis of bacterial meningitis. Clin Microbiol Rev. 1992; 5:130-145. [PubMed]
94. Feigin RD, McCracken GH Jr, Klein JO. Diagnosis and management of meningitis. Pediatr Infect Dis J. 1992; 11:785-814. [PubMed]
95. Maxson S, Lewno MJ, Schutze GE. Clinical usefulness of cerebrospinal fluid bacterial antigen studies. J Pediatr. 1994; 125:235-238. [PubMed]
96. Hayden RT, Frenkel LD. More laboratory testing: greater cost but not necessarily better. Pediatr Infect Dis J. 2000; 19:290-292. [PubMed]
97. Tarafdar K, Rao S, Recco RA, Zaman MM. Lack of sensitivity of the latex agglutination test to detect bacterial antigen in the cerebrospinal fluid of patients with culture-negative meningitis. Clin Infect Dis. 2001; 33:406-408. [PubMed]
98. Sinner SW, Tunkel AR. Approach to the diagnosis and management of tuberculous meningitis. Curr Infect Dis Rep. 2002;4:324-331. [PubMed]
99. Chonmaitree T, Baldwin CD, Lucia HL. Role of the virology laboratory in diagnosis and management of patients with central nervous system disease. Clin Microbiol Rev. 1989;2:1–14. [PubMed]
100. Johnson GM, McAbee GA, Seaton ED, et al. Suspect value of non-CSF viral cultures in the diagnosis of enteroviral CNS infections in young infants. Dev Med Child Neurol. 1992;34:876–884. [PubMed]
101. Hollander H, Levy JA. Neurologic abnormalities and recovery of human immunodeficiency virus from cerebrospinal fluid. Ann Intern Med. 1987;106:692–695. [PubMed]
102. Chalmers AC, Aprill BS, Shephard H. Cerebrospinal fluid and human immunodeficiency virus. Findings in healthy, asymptomatic, seropositive men. Arch Intern Med. 1990;150:1538–1540. [PubMed]
103. Ni H, Knight AI, Cartwright K, et al. Polymerase chain reaction for diagnosis of meningococcal meningitis. Lancet. 1992; 340:1432-1434. [PubMed]
104. Radstrom P, Backman A, Qian N, et al. Detection of bacterial DNA in cerebrospinal fluid by an assay for simultaneous detection of Neisseria meningitidis, Haemophilus influenzae, and streptococci using a seminested PCR strategy. J Clin Microbiol. 1994; 32:2738-2744. [PubMed]
105. Saravolatz LD, Manzor O, VanderVelde N, et al. Broad-range bacterial polymerase chain reaction for early detection of bacterial meningitis. Clin Infect Dis. 2003; 36:40-45. [PubMed]
106. Tzanakaki G, Tsopanomichalou M, Kesanopoulos K, et al. Simultaneous single-tube PCR assay for the detection of Neisseria meningitidis, Haemophilus influenzae type b and Streptococcus pneumoniae. Clin Microbiol Infect. 2005;11:386-390.[PubMed]
107. Rotbart HA. Diagnosis of enteroviral meningitis with the polymerase chain reaction. J Pediatr. 1990;117:85–89. [PubMed]
108. Sawyer MH, Holland D, Aintablian N. Diagnosis of enteroviral central nervous system infection by polymerase chain reaction during a large community outbreak. Pediatr Infect Dis J. 1994;13:177–182. [PubMed]
109. Ramers C, Billman G, Hartin M, et al. Impact of a diagnostic cerebrospinal fluid enterovirus polymerase chain reaction test on patient management. JAMA. 2000;283:2680-2685. [PubMed]
110. Echevarria JM, Cases I, Tenoirio A, et al. Detection of varicella-zoster virus–specific DNA sequences in cerebrospinal fluid from patients with acute aseptic meningitis and no cutaneous lesions. J Med Virol. 1994;43:331–335. [PubMed]
111. Shoji H, Honda Y, Murai I, et al. Detection of varicella-zoster virus DNA by polymerase chain reaction in cerebrospinal fluid of patients with herpes zoster meningitis. J Neurol. 1992;239:69–70. [PubMed]
112. Pratt RD, Nichols S, McKinney N, et al. Virologic markers of human immunodeficiency virus type 1 in cerebrospinal fluid of infected children. J Infect Dis. 1996;174:288–293. [PubMed]
113. Conrad AJ, Schmid P, Syndulko K, et al. Quantifying HIV-1 RNA using the polymerase chain reaction on cerebrospinal fluid and serum of seropositive individuals with and without neurologic abnormalities. J Acquir Immune Defic Syndr. 1995;10:425–435. [PubMed]
114. Pai M, Flores LL, Pai N, et al. Diagnostic accuracy of nucleic acid amplification tests for tuberculous meningitis: a systematic review and meta-analysis. Lancet Infect Dis. 2003;3:633-643. [PubMed]
115. Thwaites GE, Caws M, Chau TT, et al. Comparison of conventional bacteriology with nucleic acid amplification (amplified mycobacterium direct test) for diagnosis of tuberculous meningitis before and after inception of antituberculous chemotherapy. J Clin Microbiol. 2004:42:996-1002. [PubMed]
116. Winkler F, Kastenbauer S, Yousry TA, et al. Discrepancies between brain CT imaging and severely raised intracranial pressure proven by ventriculostomy in adults with pneumococcal meningitis. J Neurol. 2002;249:1292-1297. [PubMed]
117. Friedland IR, Paris MM, Rinderknecht S, et al. Cranial computed tomographic scans have little impact on management of bacterial meningitis. Am J Dis Child. 1992;146:1484–1487. [PubMed]
118. Gupta RK, Gupta S, Singh D, et al. MR imaging and angiography in tuberculous meningitis. Neuroradiology. 1994;36:87-92. [PubMed]
119. Short WR, Tunkel AR. Timing of administration of antimicrobial therapy in bacterial meningitis. Curr Infect Dis Rep. 2001;3:360-364. [PubMed]
120. Radetsky M. Duration of symptoms and outcome in bacterial meningitis: an analysis of causation and the implications of a delay in diagnosis. Pediatr Infect Dis J. 1992;11:694-698. [PubMed]
121. Bonadio WA. Medical-legal considerations related to symptom duration and patient outcome after bacterial meningitis. Am J Emerg Med. 1997;15:420-423. [PubMed]
122. The Research Committee of the British Society for the Study of Infection. Bacterial meningitis: causes for concern. J Infect. 1995;30:89-94. [PubMed]
123. Begg N, Cartwright KAV, Cohen J, et al. Consensus statement on diagnosis, investigation, treatment, and prevention of acute bacterial meningitis in immunocompetent adults. J Infect. 1999;39:1-15. [PubMed]
124. Aronin SI, Peduzzi P, Quagliarello VJ. Community-acquired bacterial meningitis: risk stratification for adverse clinical outcome and effect of antibiotic timing. Ann Intern Med. 1998;129:862-869. [PubMed]
125. Miner JR, Heegaard W, Mapes A, Biros M. Presentation, time to antibiotics, and mortality of patients with bacterial meningitis at an urban county medical center. J Emerg Med. 2001;21:387-392. [PubMed]
126. Lu CH, Huang CR, Chang WN, et al. Community-acquired bacterial meningitis in adults: the epidemiology, timing of appropriate antimicrobial therapy, and prognostic factors. Clin Neurol Neurosurg. 2002;104:352-358. [PubMed]
127. Proulx N, Frechette D, Toye B, et al. Delays in the administration of antibiotics are associated with mortality from adult acute bacterial meningitis. Q J Med. 2005;98:291-298. [PubMed]
128. Daoud AS, Baticha A, Al-Sheyyab M, et al. Lack of effectiveness of dexamethasone in neonatal bacterial meningitis. Eur J Pediatr. 1999;158:230-233. [PubMed]
129. McIntyre PB, Berkey CS, King SM, et al. Dexamethasone as adjunctive therapy in bacterial meningitis. A meta-analysis of randomized clinical trials since 1988. JAMA. 1997;278:925-931. [PubMed]
130. Arditi M, Mason EO Jr, Bradley JS, et al. Three-year multicenter surveillance of pneumococcal meningitis in children: clinical characteristics, and outcome related to penicillin susceptibility and dexamethasone use. Pediatrics. 1998;102:1087-1097. [PubMed]
131. Molyneux EM, Walsh AL, Forsyth H, et al. Dexamethasone treatment in childhood bacterial meningitis in Malawi: a randomized controlled trial. Lancet. 2002;360:211-218. [PubMed]
132. McCracken GH Jr. Rich nations, poor nations, and bacterial meningitis. Lancet. 2002;360:183.[PubMed]
133. McIntyre PB, Macintyre CR, Gilmour R, Wang H. A population based study of the impact of corticosteroid therapy and delayed diagnosis on the outcome of childhood pneumococcal meningitis. Arch Dis Child. 2005;90:391-396. [PubMed]
134. de Gans, van de Beek D. Dexamethasone in adults with bacterial meningitis. N Engl J Med. 2002;347:1549-1556. [PubMed]
135. Tunkel AR, Scheld WM. Corticosteroids for everyone with meningitis? N Engl J Med. 2002;347:1613-1615. [PubMed]
136. van de Beek D, de Gans J, Tunkel AR, Wijdicks EFM. Community-acquired bacterial meningitis in adults. N Engl J Med. 2006;354:44-53. [PubMed]
137. Kaplan SL, Mason EO Jr. Management of infections due to antibiotic-resistant Streptococcus pneumoniae. Clin Microbiol Rev. 1998;11:628–644. [PubMed]
138. Kaplan SL. Management of pneumococcal meningitis. Pediatr Infect Dis J. 2002;21:589-591. [PubMed]
139. Appelbaum PC. Resistance among Streptococcus pneumoniae: implications for drug selection. Clin Infect Dis. 2002;34:1613-1620. [PubMed]
140. Friedland IR, Klugman KP. Failure of chloramphenicol therapy in penicillin-resistant pneumococcal meningitis. Lancet. 1992;339:405–408. [PubMed]
141. Viladrich PF, Gudiol F, Linares J, et al. Evaluation of vancomycin for therapy of adult pneumococcal meningitis. Antimicrob Agents Chemother. 1991;35:2467–2472. [PubMed]
142. Novak R, Henriques B, Charpentier E, et al. Emergence of vancomycin tolerance in Streptococcus pneumoniae. Nature. 1999;399:590-593. [PubMed]
143. Gilmore MS, Hoch JA. A vancomycin surprise. Nature. 1999;399:524-527. [PubMed]
144. McCullers JA, English BK, Novak R. Isolation and characterization of vancomycin-tolerant Streptococcus pneumoniae from the cerebrospinal fluid of a patient who developed recrudescent meningitis. J Infect Dis. 2000;181:369-373.[PubMed]
145. Ahmed A. A critical evaluation of vancomycin for treatment of bacterial meningitis. Pediatr Infect Dis J. 1997;16:895–903. [PubMed]
146. Bradley JS, Scheld WM. The challenge of penicillin-resistant Streptococcus pneumoniae meningitis: current antibiotic therapy in the 1990s. Clin Infect Dis. 1997;24(Suppl 2):S213–S221. [PubMed]
147. Odio CM, Puig JR, Feris JM, et al. Prospective, randomized, investigator-blinded study of the efficacy and safety of meropenem vs. cefotaxime therapy in bacterial meningitis in children. Pediatr Infect Dis J. 1999;18:581-590. [PubMed]
148. Buckingham SC, Davis Y, English BK. Pneumococcal susceptibility to meropenem in a mid-South children’s hospital. South Med J. 2002;95:1293-1296. [PubMed]
149. Cottagnoud P, Tauber MG. Fluoroquinolones in the treatment of meningitis. Curr Infect Dis Rep. 2003;5:329-336. [PubMed]
150. Saez-Llorens X, McCoig C, Feris JM, et al. Quinolone treatment for pediatric bacterial meningitis: a comparative study of trovafloxacin and ceftriaxone with or without vancomycin. Pediatr Infect Dis J. 2002;21:14-22. [PubMed]
151. Chen DK, McGeer A, de Azavedo JC, et al. Decreased susceptibility of Streptococcus pneumoniae to fluoroquinolones in Canada. N Engl J Med. 1999;341:233-239. [PubMed]
152. Urban C, Rahman N, Zhao X, et al. Fluoroquinolone-resistant Streptococcus pneumoniae associated with levofloxacin therapy. Clin Infect Dis. 2001;184:794-798. [PubMed]
153. Wortmann GW, Bennett SP. Fatal meningitis due to levofloxacin-resistant Streptococcus pneumoniae. Clin Infect Dis. 1999;29:1599-1600. [PubMed]
154. Saez-Nieto JA, Lujan R, Berron S, et al. Epidemiology and molecular basis of penicillin-resistant Neisseria meningitidis in Spain: a 5-year history (1985–1989). Clin Infect Dis. 1992;14:394–402. [PubMed]
155. van Deuren M, Brandtzaeg P, van der Meer JWM. Update on meningococcal disease with emphasis on pathogenesis and clinical management. Clin Microbiol Rev. 2000;13:144-166. [PubMed]
156. Boras A, Bozinovic D, Tenover FC, Popovic T. First report of Neisseria meningitidis intermediately resistant to penicillin in Croatia. J Clin Microbiol. 2001;39:823. [PubMed]
157. Punar M, Eraksoy H, Cagatay AA, et al. Neisseria meningitidis with decreased susceptibility to penicillin in Istanbul, Turkey. Scand J Infect Dis. 2002;34:11-13. [PubMed]
158. Oppenheim BA. Antibiotic resistance in Neisseria meningitidis. Clin Infect Dis. 1997;24(Suppl 1):S98–S101[PubMed].
159. Galimand M, Gerbaud G, Guibourdenche M, et al. High-level chloramphenicol resistance in Neisseria meningitidis. N Engl J Med. 1998;339:868–874. [PubMed]
160. Jackson LA, Tenover FC, Baker C, et al. Prevalence of Neisseria meningitidis relatively resistant to penicillin in the United States, 1991. J Infect Dis. 1994;169:438–441. [PubMed]
161. Buck GE, Adams M. Meningococcus with reduced susceptibility to penicillin isolated in the United States. Pediatr Infect Dis J. 1994;13:156–157. [PubMed]
162. Woods CR, Smith AL, Wasilauskas BL, et al. Invasive disease caused by Neisseria meningitidis relatively resistant to penicillin in North Carolina. J Infect Dis. 1994;170:453–456. [PubMed]
163. Rosenstein NE, Stocker SA, Popovic T, et al. Antimicrobial resistance of Neisseria meningitidis in the United States, 1997. Clin Infect Dis. 2000;30:212-213. [PubMed]
164. Casado-Flores J, Osona B, Comingo P, Barquet N. Meningococcal meningitis during penicillin therapy for meningococcemia. Clin Infect Dis. 1997;25:1479. [PubMed]
165. Goldani LZ. Inducement of Neisseria meningitidis resistance to ampicillin and penicillin in a patient with meningococcemia treated with high doses of ampicillin. Clin Infect Dis. 1998;26:772. [PubMed]
166. Cubells CL, Garcia JJG, Martinez JR, Otin CL. Clinical data in children with meningococcal meningitis in a Spanish hospital. Acta Paediatr. 1997;86:26–29. [PubMed]
167. Nathan N, Borel T, Djibo A, et al. Ceftriaxone as effective as long-acting chloramphenicol in short-course treatment of meningococcal meningitis during epidemics: a randomized non-inferiority study. Lancet. 2005;366:308-313. [PubMed]
168. Peltola J, Anttila M, Renkonen OV, The Finnish Study Group. Randomised comparison of chloramphenicol, ampicillin, cefotaxime, and ceftriaxone for childhood bacterial meningitis. Lancet. 1989;1:1281–1287.
169. Schaad UB, Suter S, Gianella-Borradori A, et al. A comparison of ceftriaxone and cefuroxime for the treatment of bacterial meningitis in children. N Engl J Med. 1990;322:141–147. [PubMed]
170. Saez-Llorens X, O’Ryan M. Cefepime in the empiric treatment of meningitis in children. Pediatr Infect Dis J. 2001;20:356-361. [PubMed]
171. Saez-Llorens X, Castano E, Garcia R, et al. Prospective randomized comparison of cefepime and cefotaxime for treatment of bacterial meningitis in infants and children. Antimicrob Agents Chemother. 1995;39:937–940. [PubMed]
172. Fong IW, Tomkins KB. Review of Pseudomonas aeruginosa meningitis with special emphasis on treatment with ceftazidime. Rev Infect Dis. 1985;7:604–612. [PubMed]
173. Rodriguez WJ, Khan WN, Cocchetto DM, et al. Treatment of Pseudomonas meningitis with ceftazidime with or without concurrent therapy. Pediatr Infect Dis J. 1990;9:83–87. [PubMed]
174. Jimenez-Mejias ME, Pichardo-Guerrero C, Marquez-Rivas FJ, et al. Cerebrospinal fluid penetration and pharmacokinetic/pharmacodynamic parameters of intravenously administered colistin in a case of multidrug-resistant Acinetobacter baumannii meningitis. Eur J Clin Microbiol Infect Dis. 2002;21:212-214. [PubMed]
175. Vasan W, Desmery P, Ilutovich S, et al. Intrathecal use of colistin. J Clin Microbiol. 2000;38:3523. [PubMed]
176. Katragkou A, Roilides E. Successful treatment of multidrug-resistant Acinetobacter baumannii central nervous system infections with colistin. J Clin Microbiol. 2005;43:4916-4917. [PubMed]
177. Kremery V Jr, Filka J, Uher J, et al. Ciprofloxacin in treatment of nosocomial meningitis in neonates and in infants: report of 12 cases and review. Diagn Microbiol Infect Dis. 1999;35:75-80. [PubMed]
178. Lipman J, Allworth A, Walis SC. Cerebrospinal penetration of high doses of intravenous ciprofloxacin in meningitis. Clin Infect Dis. 2000;31:1131-1133. [PubMed]
179. Chang WN, Lu CH, Wu JJ, et al. Staphylococcus aureus meningitis in adults: a clinical comparison of infections caused by methicillin-resistant and methicillin-sensitive strains. Infection. 2001;29:245-250. [PubMed]
180. Bayston R, Hart CA, Barnicot M. Intraventricular vancomycin in the treatment associated with cerebrospinal fluid shunting and drainage. J Neurol Neurosurg Psych. 1987;50:1419-1423. [PubMed]
181. Wen DY, Bottini AG, Hall WA, Haines SJ. The intraventricular use of antibiotics. Neurosurg Clin North Am. 1992;3:343-354. [PubMed]
182. Tunkel AR, Kaufman BA. Cerebrospinal fluid shunt infections. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases, 6th ed. Philadelphia: Elsevier Science; 2005:1126-1132.
183. Waler JA, Rathore MH. Outpatient management of pediatric bacterial meningitis. Pediatr Infect Dis J. 1995;14:89-92. [PubMed]
184. Tice AD, Strait K, Ramey R, et al. Outpatient parenteral antimicrobial therapy for central nervous system infections. Clin Infect Dis. 1999;29:1394-1399. [PubMed]
185. Radetsky M. Duration of treatment in bacterial meningitis: a historical inquiry. Pediatr Infect Dis J. 1990;2:2-9.[PubMed]
186. O’Neill P. How long to treat bacterial meningitis. Lancet. 1993;341:530. [PubMed]
187. American Thoracic Society joint statement. Treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603-662.
188. Humphries M. The management of tuberculous meningitis. Thorax. 1992;47:577-581. [PubMed]
189. Havlir DV, Barnes PF. Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med. 1999; 340:367-373. [PubMed]
190. Lyons MK, Meyer FB. Cerebrospinal fluid physiology and the management of increased intracranial pressure. Mayo Clin Proc. 1990;65:684–707. [PubMed]
191. Kilpi T, Peltola H, Jauhianinen T, et al. Oral glycerol and intravenous dexamethasone in preventing neurologic and audiologic sequelae of childhood bacterial meningitis. Pediatr Infect Dis J. 1995;14:270-278. [PubMed]
192. Abzug MJ, Keyserling HL, Lee ML, et al. Neonatal enterovirus infection: virology, serology, and effects of intravenous immune globulin. Clin Infect Dis. 1995;20:1201–1206. [PubMed]
193. Girgis NI, Farid Z, Kilpatrick ME, et al. Dexamethasone adjunctive treatment for tuberculous meningitis. Pediatr Infect Dis J. 1991;10:179-183. [PubMed]
194. Schoeman JF, Van Zyl LE, Laubscher JA, Donald PR. Effect of corticosteroids on intracranial pressure, computed tomographic findings, and clinical outcome in young children with tuberculous meningitis. Pediatrics. 1997;99:226-231.[PubMed]
195. Thwaites GE, Bang ND, Dung NH, et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med. 2004;351:1741-1751. [PubMed]
196. Palur R, Rajshekhar V, Chandy MJ, et al. Shunt surgery for hydrocephalus in tuberculous meningitis: A long term follow up study. J Neurosurg. 1991;74:64-69. [PubMed]
197. Mathew JM, Rajshekhar V, Chandy MJ. Shunt surgery in poor grade patients with tuberculous meningitis and hydrocephalus: Effects of response to external ventricular drainage and other variables on long term outcome. J Neurol Neurosurg Psych. 1998;65:115-118. [PubMed]
198. Centers for Disease Control and Prevention. Recommendations for use of Haemophilus influenzae b conjugate vaccines and a combined diphtheria, tetanus, pertussis, and Haemophilus b vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 1993;42:1-15.
199. Ribeiro GS, Reis JN, Cordeiro SM, et al. Prevention of Haemophilus influenzae type b (Hib) meningitis and emergence of serotype replacement with type a strains after introduction of Hib immunization in Brazil. J Infect Dis. 2003;187:109-116. [PubMed]
200. Garner D, Weston V. Effectiveness of vaccination for Haemophilus influenzae type b. Lancet. 2003;361:395-437. [PubMed]
201. Steinhoff M, Goldblatt D. Conjugate Hib vaccines. Lancet. 2003;361:360-361. [PubMed]
202. Wildes SS, Tunkel AR. Meningococcal vaccines. A progress report. Biodrugs. 2002;16:321-329. [PubMed]
203. Harrison LH. Preventing meningococcal infection in college students. Clin Infect Dis. 2000;30:648-651. [PubMed]
204. American Academy of Pediatrics, Committee on Infectious Diseases. Meningococcal disease prevention and control strategies for practice-based physicians (addendum: recommendations for college students). Pediatrics. 2000;106:1500-1504.
205. Ramsay MA, Andrews N, Kaczmarski EB, et al. Efficacy of meningococcal serogroup C conjugate vaccine in teenagers and toddlers in England. Lancet. 2001;357:195-196. [PubMed]
206. Miller E, Salisbury D, Ramsay M. Planning, registration, and implementation of an immunization campaign against meningococcal serogroup C disease in the UK: a success story. Vaccine. 2001;20:S58-67. [PubMed]
207. Bose A, Coen P, Tully J, et al. Effectiveness of meningococcal C conjugate vaccine in teenagers in England. Lancet. 2003;361:675-676. [PubMed]
208. Centers for Disease Control and Prevention. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 2005;54(RR-7):1-21.
209. Centers for Disease Control and Prevention. Limited supply of meningococcal conjugate vaccine, recommendation to defer vaccination of persons aged 11-12 years. MMWR Dispatch. 55/May 19, 2006.
210. Black S, Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr Infect Dis J. 2000;19:187-195. [PubMed]
211. American Academy of Pediatrics, Committee on Infectious Diseases. Policy statement: recommendations for the prevention of pneumococcal infections, including use of pneumococcal conjugate vaccine (Prevnar), pneumococcal polysaccharide vaccine, and antibiotic prophylaxis. Pediatrics. 2000;106:362-366.
212. Whitney CG, Farley MM, Hadler J, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737-1746. [PubMed]
213. Centers for Disease Control and Prevention. Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease – United States, 1998-2003. MMWR. 2005;54:893-7.
214. Haddy RI, Perry K, Chacko CE, et al. Comparison of incidence of invasive Streptococcus pneumoniae disease among children before and after introduction of conjugated pneumococcal vaccine. Pediatr Infect Dis J. 2005;24:320-323. [PubMed]
215. Centers for Disease Control and Prevention. Control and prevention of meningococcal disease and control and prevention of serogroup C meningococcal disease: Evaluation and management of suspected outbreaks. MMWR. 1997;46(RR-5):1–11.
216. Schwartz B, Al-Tobaiqi A, Al-Ruwais A, et al. Comparative efficacy of ceftriaxone and rifampicin in eradicating pharyngeal carriage of group A Neisseria meningitidis. Lancet. 1988;1:1239–1242. [PubMed]
217. Girgis N, Sultan Y, Frenck RW Jr, et al. Azithromycin compared with rifampin for eradication of nasopharyngeal colonization by Neisseria meningitidis. Pediatr Infect Dis J. 1998;17:816-819. [PubMed]
218. Centers for Disease Control and Prevention. Prevention of perinatal group B streptococcal disease: A public health perspective. MMWR. 1996;45(RR-7):1–24. [PubMed]
219. Schuchat A. Group B streptococcus. Lancet. 1999;353:51-56.[PubMed]
220. Villalobos T, Arango C, Kubilis P, Rathore M. Antibiotic prophylaxis after basilar skull fracture. Clin Infect Dis. 1998;27:364–369.[PubMed]
Tables
Figure 1. Management Algorithm for Infants and Children with Suspected Bacterial Meningitis.
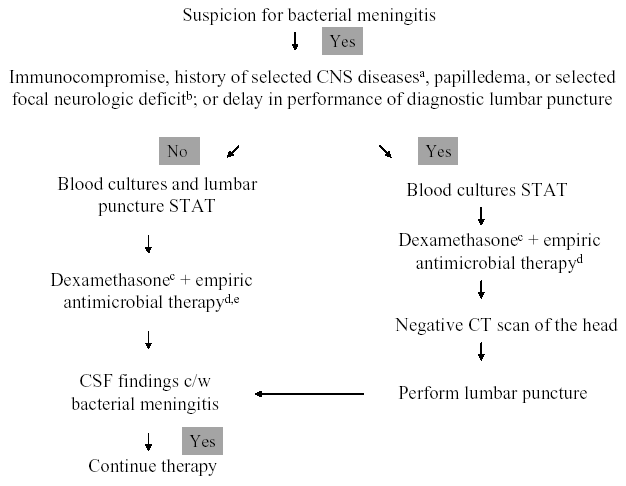
aIncludes those with CSF shunts, hydrocephalus, trauma, after neurosurgery, or various space-occupying lesions
bPalsy of cranial nerve VI or VII is not an indication to delay lumbar puncture.
cSee text for recommendations for use of adjunctive dexamethasone in infants and children with bacterial meningitis.
dSee Table 3.
eDexamethasone and antimicrobial therapy should be administered immediately after CSF is obtained.
Figure 2. Management Algorithm for Adults with Suspected Bacterial Meningitis.
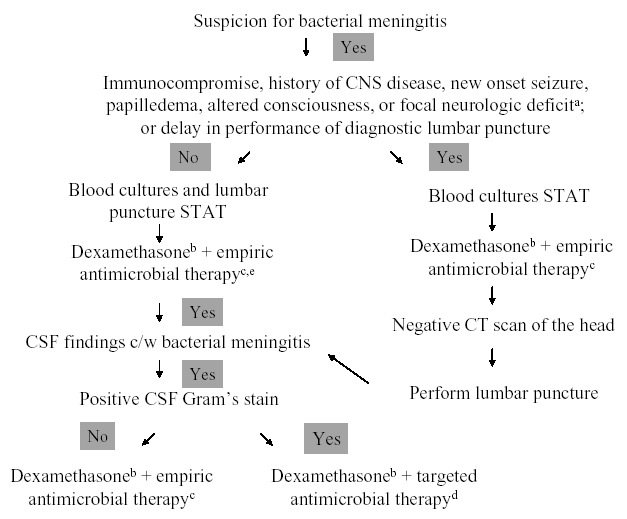
aSee Table 1.
bSee text for specific recommendations for use of adjunctive dexamethasone in adults with bacterial meningitis.
cSee Table 3.
dSee Table 4.
eDexamethasone and antimicrobial therapy should be administered immediately after CSF is obtained.
Table 1. Recommended Criteria for Adult Patients with Suspected Bacterial Meningitis Who Should Undergo CT Scanning prior to Lumbar Puncture.a
|
aIn the absence of all of these criteria, lumbar puncture can be performed without the need for CT scanning.
bHIV infection or AIDS, immunosuppressive therapy, post-transplantation
cMass lesion, stroke, or focal infection
dWithin 1 week of presentation; some authorities would not perform LP in patients with prolonged seizures, or would delay LP for 30 minutes in patients with short, convulsive seizures.
ePresence of venous pulsations suggests absence of increased intracranial pressure.
fIncluding dilated non-reactive pupil, abnormalities of ocular motility, abnormal visual fields, gaze palsy, arm or leg drift.
Table 2. Cerebrospinal Fluid (CSF) Findings in Patients with Meningitis.
| CSF Parameter | Bacterial | Virala | Tuberculous |
|---|---|---|---|
| Opening pressure (mm H2O) | 200-500b | <250 | 180-300 |
| White blood cell count (mm3) | 1000-5000c | 50-1000 | 50-300 |
| White blood cell differential | Neutrophils | Lymphocytesd | Lymphocytese |
| Glucose (mg/dL) | <40f | >45 | <45 |
| Protein (mg/dL) | 100-500 | <200 | 50-300 |
aPrimarily nonpolio enteroviruses (echoviruses and coxsackieviruses).
bValues exceeding 600 suggest the presence of cerebral edema, intracranial suppurative foci, or communicating hydrocephalus.
cRange may be <100 to >10,000
dMay have a neutrophil predominance early in infection, but gives way to a lymphocyte predominance over the first 6-48 hours.
eIn patients receiving antituberculous chemotherapy, an initial lymphocyte predominance may become neutrophilic on subsequent CSF examination.
fThe CSF:serum glucose ration is <0.40 in the majority of patients.
Table 3. Recommendations for Empiric Antimicrobial Therapy of Purulent Meningitis Based on Patient Age and Specific Predisposing Condition.
Predisposing Factor |
Common Bacterial Pathogens |
Antimicrobial Therapy |
|---|---|---|
Age |
||
<1 month |
Streptococcus agalactiae, Escherichia coli,Listeria monocytogenes, Klebsiella spp. |
Ampicillin + cefotaxime; or ampicillin + an aminoglycoside |
1-23 months |
Streptococcus pneumoniae, Neisseria meningitidis, Streptococcus agalactiae,Haemophilus influenzae, Escherichia coli |
Vancomycin + a third-generation cephalosporina,b |
2-50 years |
Neisseria meningitidis, Streptococcus pneumoniae |
Vancomycin + a third-generation cephalosporina,b |
>50 years |
Streptococcus pneumoniae, Neisseria meningitidis, Listeria monocytogenes,aerobic gram-negative bacilli |
Vancomycin + ampicillin + a third-generation cephalosporina,b |
Head trauma |
||
Basilar skull fracture |
Streptococcus pneumoniae, Haemophilus influenzae, group A b–hemolytic streptococci |
Vancomycin + a third-generation cephalosporina |
Penetrating trauma |
Staphylococcus aureus, coagulase-negative staphylococci (especially Staphylococcus epidermidis), aerobic gram-negative bacilli(including Pseudomonas aeruginosa) |
Vancomycin + cefepime; or vancomycin + ceftazidime; or vancomycin + meropenem |
Postneurosurgery |
Aerobic gram-negative bacilli(includingPseudomonas aeruginosa), Staphylococcus aureus, coagulase-negative staphylococci (especially Staphylococcus epidermidis) |
Vancomycin + cefepime; or vancomycin + ceftazidime; or vancomycin + meropenem |
Cerebrospinal fluid shunt |
Coagulase-negative staphylococci (especiallyStaphylococcus epidermidis), Staphylococcus aureus, aerobic gram-negative bacilli(including Pseudomonas aeruginosa), Propionibacterium acnes |
Vancomycin + cefepimec; or vancomycin + ceftazidimec; or vancomycin + meropenemc |
aCeftriaxone or cefotaxime.
bSome experts would add rifampin if dexamethasone is also given.
cIn infants and children, vancomycin alone is reasonable unless Gram’s stain reveals the presence of gram-negative bacilli.
Table 4. Recommendations for Antimicrobial Therapy in Adult Patients with Presumptive Pathogen Identification by Positive Gram’s Stain.a
| Microorganism | Recommended Therapy | Alternate Therapies |
|---|---|---|
| Streptococcus pneumoniae | Vancomycin + a third-generation cephalosporinb,e | Meropenem; fluoroquinolonec |
| Neisseria meningitidis | Third-generation cephalosporinb | Penicillin G; ampicillin;chloramphenicol; fluoroquinolone; aztreonam |
| Listeria monocytogenes | Ampicillind or penicillin Gd | Trimethoprim-sulfamethoxazole; meropenem |
| Streptococcus agalactiae | Ampicillind or penicillin Gd | Third-generation cephalosporinb |
| Haemophilus influenzae | Third-generation cephalosporinb | Chloramphenicol; cefepime; meropenem; fluoroquinolone |
| Escherichia coli | Third-generation cephalosporinb | Cefepime; meropenem; aztreonam; fluoroquinolone; trimethoprim-sulfamethoxazole |
aIn children, ampicillin is added to the standard therapeutic regimen of cefotaxime or ceftriaxone plus vancomycin when L. monocytogenes is considered, and an aminoglycoside if a gram-negative enteric pathogen is of concern.
bCeftriaxone or cefotaxime.
dAddition of an aminoglycoside should be considered.
eSome experts would add rifampin if dexamethasone is also given.
Table 5. Recommendations for Specific Antimicrobial Therapy in Bacterial Meningitis Based on Isolated Pathogen and Susceptibility Testing.
Microorganism |
Standard Therapy |
Alternative Therapies |
|---|---|---|
Penicillin MIC <0.1 mg/mL |
Penicillin G or ampicillin |
Third-generation cephalosporina; chloramphenicol |
Penicillin MIC 0.1-1.0mg/mLb |
Third-generation cephalosporina |
|
Penicillin MIC >2.0 mg/mL |
Vancomycin + a third-generation cephalosporina,c |
Fluoroquinoloned |
|
ceftriaxone MIC >1.0 mg/mL |
Vancomycin + a third-generation cephalosporina,c |
Fluoroquinoloned |
Penicillin MIC <0.1 mg/mL |
Penicillin G or ampicillin |
Third-generation cephalosporina; chloramphenicol |
Penicillin MIC 0.1-1.0 mg/mL |
Third-generation cephalosporina |
Chloramphenicol; fluoroquinolone; meropenem |
Ampicilline or penicillin Ge |
Trimethoprim-sulfamethoxazole; meropenem |
|
Ampicilline or penicillin Ge |
Third-generation cephalosporina |
|
Escherichia coli and other Enterobacteriaceaeg |
Third-generation cephalosporina |
Aztreonam; fluoroquinolone; meropenem; trimethoprim-sulfamethoxazole; ampicillin |
Cefepimee or ceftazidimee |
Aztreoname; ciprofloxacine; meropeneme |
|
b-lactamase-negative |
Ampicillin |
Third-generation cephalosporina; cefepime; chloramphenicol, fluoroquinolone |
b-lactamase-positive |
Third-generation cephalosporine |
Cefepime; chloramphenicol, fluoroquinolone |
Vancomycin; meropenem |
||
Vancomycinf |
Trimethoprim-sulfamethoxazole; linezolid |
|
Vancomycinf |
||
aCeftriaxone or cefotaxime.
bCeftriaxone/cefotaxime susceptible isolates.
cConsider addition of rifampin if ceftriaxone MIC is >2 mg/mL.
dMoxifloxacin.
eAddition of an aminoglycoside should be considered.
fConsider addition of rifampin.
gChoice of a specific antimicrobial agent must be guided by in vitro susceptibility testing.
Table 6. Recommended Dosages of Antimicrobial Therapy in Patients with Bacterial Meningitis.
| Total Daily Dose (dosing interval in hours) | ||||
|---|---|---|---|---|
| Antimicrobial Agent | Neonates (0-7 d)a | Neonates (8-28 d)a | Infants and children | Adults |
| Amikacinb | 15-20 mg/kg (12) | 30 mg/kg (8) | 20-30 mg/kg (8) | 15 mg/kg (8) |
| Ampicillin | 150 mg/kg (8) | 200 mg/kg (6-8) | 300 mg/kg (6) | 12 g (4) |
| Aztreonam | 6-8 g (6-8) | |||
| Cefepime | 150 mg/kg (8) | 6 g (8) | ||
| Cefotaxime | 100-150 mg/kg (8-12) | 150-200 mg/kg (6-8) | 225-300 mg/kg (6-8) | 8-12 g (4-6) |
| Ceftazidime | 100-150 mg/kg (8-12) | 150 mg/kg (8) | 150 mg/kg (8) | 6 g (8) |
| Ceftriaxone | 80-100 mg/kg (12-24) | 4 g (12-24) | ||
| Chloramphenicol | 25 mg/kg (24) | 50 mg/kg (12-24) | 75-100 mg/kg (6) | 4-6 g (6)c |
| Ciprofloxacin | 800-1200 mg (8-12) | |||
| Gentamicinb | 5 mg/kg (12) | 7.5 mg/kg (8) | 7.5 mg/kg (8) | 5 mg/kg (8) |
| Meropenem | 120 mg/kg (8) | 6 g (8) | ||
| Moxifloxacin | 400 mg (24)d | |||
| Nafcillin | 75 mg/kg (8-12) | 100-150 mg/kg (6-8) | 200 mg/kg (6) | 9-12 g (4) |
| Oxacillin | 75 mg/kg (8-12) | 150-200 mg/kg (6-8) | 200 mg/kg (6) | 9-12 g (4) |
| Penicillin G | 0.15 mU/kg (8-12) | 0.2 mU/kg (6-8) | 0.3 mU/kg (4-6) | 24 mU (4) |
| Rifampin | 10-20 mg/kg (12) | 10-20 mg/kg (12-24)e | 600 mg (24) | |
| Tobramycinb | 5 mg/kg (12) | 7.5 mg/kg (8) | 7.5 mg/kg (8) | 5 mg/kg (8) |
| Trimethoprim-sulfamethoxazolef | 10-20 mg/kg (6-12) | 10-20 mg/kg (6-12) | ||
| Vancomycing | 20-30 mg/kg (8-12) | 30-45 mg/kg (6-8) | 60 mg/kg (6) | 30-45 mg/kg (8-12) |
aSmaller doses and longer intervals of administration may be advisable for very low birth weight neonates (<2000 g).
bNeed to monitor peak and trough serum concentrations.
cHigher dose recommended for patients with pneumococcal meningitis.
dNo data on optimal dosage needed in patients with bacterial meningitis.
eMaximum daily dose of 600 mg.
fDosage based on trimethoprim component.
gMaintain serum trough concentrations of 15-20 mg/mL.
Table 7. Recommended Dosages of Antimicrobial Agents Administered by the Intraventricular Route.a
| Antimicrobial Agent | Daily Intraventricular Dose |
|---|---|
| Vancomycin | 5-20 mgb |
| Gentamicin | 1-8 mg |
| Tobramycin | 5-20 mg |
| Amikacin | 5-50 mgc |
| Polymyxin B | 5 mgd |
| Colistin | 10 mg |
| Quinupristin/dalfopristin | 2-5 mg |
aThere are no specific data that define the exact dose of an antimicrobial agent that should be administered by the intraventricular route.
bMost studies have utilized a 10 mg or 20 mg dose.
cThe usual daily intraventricular dose is 30 mg.
dDosage in children is 2 mg daily.
Table 8. Duration of Antimicrobial Therapy in Bacterial Meningitis Based on Isolated Pathogen.
| Microorganism | Duration of Therapy (days) |
|---|---|
| Neisseria meningitidis | 7 |
| Haemophilus influenzae | 7 |
| Streptococcus pneumoniae | 10-14 |
| Streptococcus agalactiae | 14-21 |
| Aerobic gram-negative bacillia | 21 |
| Listeria monocytogenes | >21 |
aDuration in the neonate is 2 weeks beyond the first sterile CSF culture or at least 3 weeks, whichever is longer.
What's New
Tangden T, et al. Neurosurgical Gram-Negative Bacillary Ventriculitis and Meningitis: A Retrospective Study Evaluating the Efficacy of Intraventricular Gentamicin Therapy in 31 Consecutive Cases. Clin Infect Dis 2011;52:1310-1316.
Pelkonen T, Roine I, et al. Risk factors for death and severe neurological sequelae in childhood bacterial meningitis in sub-Saharan Africa. Clin Infect Dis. 2009 Apr 15;48(8):1107-10.
Gruber TJ, et al. Pediatric Neurosurgical Practice Patterns Designed to Prevent Cerebrospinal Fluid Shunt Infection. Pediatr Neurosurg. 2009;45:456-60.
Ramirez-Avila L et al. Eosinophilic Meningitis due to Angiostrongylus and Gnathostoma Species.Clin Infect Dis. 2009; 48:322–327.
Guided Medline Search For:
Reviews
Gilbert DN. Use of Plasma Procalcitonin Levels as an Adjunct to Clinical Microbiology. J Clin Microbiol 2010;48:2325-2329.
Bradley JS. Corticosteroids for bacterial meningitis.
Tunkel AR, et al. The Management of Encephalitis: Clinical Practice Guidelines by the Infectious Disease Society of America. Clin Infect Dis 2008;47:303-327.
Guided Medline Search For Recent Reviews
History
Abi-Fadel F, et al. Mollaret's meningitis: 65 years of history. Intern Emerg Med 2012;7 (Suppl 1):S15-S16.
Ward MA, et al. Josef Brudzinski and Vladimir Mikhailovich Kernig: Signs for Diagnosing Meningitis. Clin Med & Res 2010;8:13-17.
Ruben R. Bacterial meningitic deafness: historical development of epidemiology and cellular pathology. Acta Oto-Laryngologica 2008;128:388-392.
Guided Medline Search For Historical Aspects
Table of Contents
- Etiology
- Clinical Manifestations
- Diagnosis
- Initial Management Approach for Bacterial Meningitis
- Specific Antimicrobial Therapy Based on Isolated Pathogen
- Adjunctive Therapies
- Prevention of Bacterial Meningitis

