Fever and Chest Pain
Authors: Jack Perkins, M.D., Stephen Liang, M.D.
INTRODUCTION
Fever and chest pain are two of the most common complaints in the inpatient, outpatient, or emergency department (ED). The differential diagnosis of these 2 entities includes a vast array of possible etiologies as diverse as herpes zoster to pericarditis. The importance of a correct diagnosis can’t be overstated as some patients will require anticoagulation (e.g. pulmonary embolus), while others will benefit from prompt antibiotic therapy (e.g. pneumonia) or even invasive cardiac intervention (acute myocardial infarction). This chapter will outline and briefly discuss the major causes of fever and chest pain while providing a framework with which to pursue a diagnosis. The extensive list of possible etiologies can’t possibly be covered in detail in this chapter, thus it is important to keep in mind other possible etiologies while focusing on the diagnoses that are most likely and have the highest likelihood for significant morbidity and mortality if not properly recognized.
Chest pain has always been one of the most challenging diagnoses in medicine because of the complex innervations in the thorax that rarely allows the nature of the chest pain to lead directly to a diagnosis. Pain perceived in the chest by the patient may originate from the heart, lungs, esophagus, upper GI tract, intercostal muscles, skin, ribs, thoracic vertebrae, or any structures in the mediastinum. Furthermore, various organ systems may share innervations such that a perceived complaint of pain is often not localized to the precise area of pathology. This phenomenon is known as “referred pain” is caused by convergence of thoracic visceral input with somatic afferent fibers which leads the cortex to interpret the pain as coming from the somatic area instead of the visceral location. The result of this ambiguous nature of chest pain is that this complaint does little for the physician except raise their suspicion of a serious underlying pathology and usually leads to an extensive investigation.
Clinical Approach
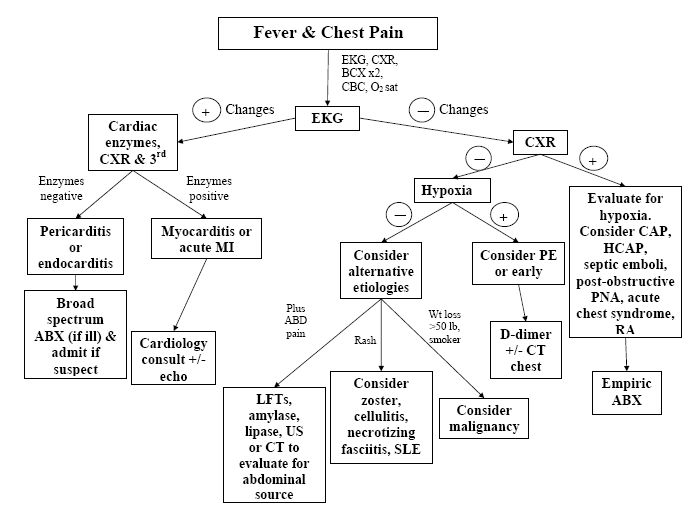
A complete differential diagnosis of patients presenting with fever and chest pain are presented in Tables 1 and 2. All patients who present with these complaints should have an EKG, CXR, blood cultures, CBC and O2 saturation assessment. Depending on the patient age and coronary risk factors, cardiac enzymes may likely be indicated. Based on the EKG results, patients can be divided into normal EKG and abnormal EKG’s. The differential diagnosis and clinical evaluation will vary based on the EKG. A clinical approach based on clinical manifestations and laboratory findings is detailed in algorithm 1 and is a guide on how to approach patients with fever and chest pain.
Hospital Admission
The decision to admit patients presenting with fever and chest pain is generally straightforward. Most patients over the age of 50 will require admission since the list of potential etiologies involves quite a few serious diagnoses that can’t be missed. Firmly establish a diagnosis in the office or emergency department may be challenging. Illnesses such as infective endocarditis, myocarditis, pulmonary embolus and pneumonia may result in hemodynamic and respiratory compromise, necessitating aggressive resuscitation and mechanical ventilation in an intensive care unit. Intravenous antibiotics alone justify admission in most cases when the etiology of the fever and chest pain is felt to be infectious in nature or the patient is ill enough that the consideration of infectious etiologies warrants empiric antibiotic coverage. Conditions such as empyema, infective endocarditis and soft tissue infection may require surgical intervention in an operating suite. Decision-making tools such as the PORT score developed for community-acquired pneumonia may aid with risk stratification and the decision for hospital admission. However, even the pneumonia scoring indices are not sufficiently sensitive to substitute for clinician judgment in determining patients that just are not well enough to risk outpatient therapy. Uncomplicated cases of pericarditis, community-acquired pneumonia, herpes zoster and cellulitis may be treated on an outpatient basis with close primary care follow-up in otherwise healthy adults (e.g. not immunocompromised). However, in the elderly population, clinical presentations are frequently atypical and blunted and fever associated with chest pain in the elderly should almost always warrant admission simply based on the real possibility of a missed diagnosis resulting in significant morbidity and mortality. In summary, a conservative approach to hospital admission for observation is recommended given the high morbidity and mortality of many of the illnesses known to cause fever and chest pain.
CARDIAC ETIOLOGIES
The complaint of fever and chest pain warrants a thorough consideration of cardiac causes. Symptoms such as dyspnea on exertion, orthopnea, and palpitations should be actively identified. Knowledge of a recent viral illness, surgical procedure or intravenous drug use may be helpful in identifying an inciting event. Likewise, risk factors such as a prosthetic valve or previous episodes of endocarditis should be identified. The physical examination may yield a wealth of clues such as a new heart murmur, pericardial rub, petechiae, splenomegaly or peripheral stigmata of embolic disease (e.g. Janeway lesions, Osler nodes, splinter hemorrhages). Electrocardiography should be obtained on all patients with chest pain, regardless of the presence or absence of fever, and may show evidence of myocardial involvement. While most laboratory studies are nonspecific, cardiac biomarkers may reveal active, ongoing myocardial injury. Blood cultures are indispensable to the isolation of causative microorganisms. Echocardiography may be an important diagnostic tool for guiding treatment and the potential need for surgical intervention. Fever and chest pain may occasionally present together in the patient with acute coronary syndrome or aortic dissection. While this would be unusual, the astute physician should always consider these catastrophic cardiac diseases in any patient who presents with chest pain. However, the bulk of the evaluation should focus on the three classic cardiovascular causes of fever and chest pain: pericarditis, myocarditis and infective endocarditis (see Table 2).
Pericarditis
Acute inflammation of the pericardium is known as pericarditis and accounts for approximately 5% of all causes of chest pain presenting to the ED. Acute pericarditis may be complicated by the development of a pericardial effusion that, if significant enough to compress the cardiac chambers, may produce cardiac tamponade. More than 80% of cases of acute pericarditis are idiopathic or presumably viral in origin. Among many others, commonly implicated viruses have included the enteroviruses (Coxsackie A & B), adenovirus, Epstein Barr virus (EBV), cytomegalovirus (CMV), and parvovirus B19. Given the self-limited nature of most cases of acute pericarditis, a definitive etiology is often not pursued. Less common infectious causes of acute pericarditis may include bacterial, tuberculosis and fungal infection. These are more likely to be seen in the immunocompromised patient. Autoimmune diseases such as SLE and rheumatoid arthritis represent some of the most common noninfectious causes of pericarditis. Uremia, trauma and cardiac surgery are other well-known noninfectious causes. While primary tumors of the pericardium are rare, metastatic breast and lung cancer as well as lymphoma may spread to the pericardium, producing large and often hemorrhagic effusions. Pericarditis may occur within days after an acute transmural myocardial infarction as a result of contact with inflamed and healing myocardium. It may also occur weeks to months later as an autoimmune-mediated response known as Dressler’s syndrome.
Acute pericarditis classically presents with sharp, retrosternal chest pain that is sudden in onset. It typically worsens with deep inspiration, with coughing, or when the patient is supine. It may be improved with sitting upright and leaning forward. The pain may radiate to one or both trapezius ridges, as both phrenic nerves traverse the anterior pericardium to innervate these muscle groups. Pain may also be referred to the neck, shoulders, or arms, making it difficult to differentiate from the pain of pulmonary embolism and myocardial ischemia or infarction. Patients frequently present with fever, malaise and myalgias in association with their chest pain, reminiscent of a viral prodrome, though the former may be absent in the elderly. A pericardial friction rub is highly specific for pericarditis and can be heard at one time or another in as many as 85% of cases of acute pericarditis. It is described as a high-pitched raspy sound best auscultated at the left sternal border with the patient leaning forward on end expiration.
A pericardial friction rub may be heard throughout the respiratory cycle and persists even when the patient is asked to hold their breath, distinguishing it from a pleural rub. The commonly held belief that a pericardial rub results from the chafing of the two inflamed pericardial layers against one another is likely inaccurate, as a rub may be heard even in the presence of a large effusion separating the layers. Tachycardia, hypotension, jugular venous distension and pulsus paradoxus (a decrease in systolic blood pressure of more than 10 mmHg with inspiration) are suggestive of cardiac tamponade. While low-grade fevers are common, a temperature above 38º C is concerning for purulent bacterial pericarditis.
Electrocardiography is often diagnostic for acute pericarditis. While the pericardium itself is electrically inert, epicardial inflammation from an overlying pericarditis progresses through four classic stages. Stage 1 is marked by diffuse, upward concave ST-segment elevations with reciprocal ST-segment depressions in aVR and V1 ![]() . PR-segment depression may be seen in most leads with the exception of aVR and V1. These changes are seen within the first hours of initial symptoms and may last up to two weeks before returning to baseline, defined as stage 2. As inflammation and injury progresses into the second and third weeks, stage 3 is characterized by diffuse T-wave inversions. Stage 4 marks the resolution of these T-wave inversions, though some may persist indefinitely. While these four stages are seen less frequently now as a result of early therapy, the presence of diffuse ST-segment elevations seen in stage 1 still remains a cardinal marker of acute pericarditis. Low voltage QRS complexes may hint at the presence of a pericardial effusion.
. PR-segment depression may be seen in most leads with the exception of aVR and V1. These changes are seen within the first hours of initial symptoms and may last up to two weeks before returning to baseline, defined as stage 2. As inflammation and injury progresses into the second and third weeks, stage 3 is characterized by diffuse T-wave inversions. Stage 4 marks the resolution of these T-wave inversions, though some may persist indefinitely. While these four stages are seen less frequently now as a result of early therapy, the presence of diffuse ST-segment elevations seen in stage 1 still remains a cardinal marker of acute pericarditis. Low voltage QRS complexes may hint at the presence of a pericardial effusion.
Laboratory evaluation in the patient with acute pericarditis may reveal an elevated white blood cell count, erythrocyte sedimentation rate and serum C-reactive protein. Renal function should be assessed to exclude uremia as an etiology. In selected cases, tuberculin skin testing, antinuclear antibody and rheumatoid factor may aid diagnosis. Serum biomarkers such as creatine kinase (CK-MB) and serum cardiac troponin I (cTnI) may be elevated in at least a third of the cases of acute pericarditis and likely reflect superficial myocardial inflammation and injury. Significant serum cTnI elevations are only seen in the presence of ST-segment elevation on ECG in pericarditis. Unlike in acute coronary syndrome, elevated serum cTnI is not associated with a poorer prognosis in acute pericarditis. However, persistent serum cTnI elevation for more than two weeks may be suggestive of myocarditis, which does carry a poorer prognosis.
Echocardiography is both appropriate for and frequently obtained in the context of acute pericarditis to evaluate for the presence of a pericardial effusion ![]() . While the discovery of an effusion may help solidify a diagnosis of pericarditis, the absence of one cannot rule it out. In most instances, routine pericardiocentesis has been demonstrated to have very low diagnostic yield. A pericardial effusion with evidence of tamponade however is a clear indication to proceed to pericardiocentesis or surgical drainage. Likewise, an effusion suspected to be secondary to purulent, tuberculosis or neoplastic pericarditis warrants sampling of the pericardial fluid to obtain a definitive diagnosis through bacterial culture, fluid cytology and, if indicated, polymerase chain reaction (PCR) for tubercle bacilli. In cases of ineffective pericardiocentesis, tamponade recurs, or the course of pericarditis is prolonged, pericardial biopsy may be considered.
. While the discovery of an effusion may help solidify a diagnosis of pericarditis, the absence of one cannot rule it out. In most instances, routine pericardiocentesis has been demonstrated to have very low diagnostic yield. A pericardial effusion with evidence of tamponade however is a clear indication to proceed to pericardiocentesis or surgical drainage. Likewise, an effusion suspected to be secondary to purulent, tuberculosis or neoplastic pericarditis warrants sampling of the pericardial fluid to obtain a definitive diagnosis through bacterial culture, fluid cytology and, if indicated, polymerase chain reaction (PCR) for tubercle bacilli. In cases of ineffective pericardiocentesis, tamponade recurs, or the course of pericarditis is prolonged, pericardial biopsy may be considered.
Most cases of acute idiopathic or viral pericarditis respond well to supportive care and symptom relief with non-steroidal anti-inflammatory drugs (NSAIDs) such as aspirin, indomethacin and ibuprophen. Aspirin is preferred in patients with recent myocardial infarction or coronary artery disease, while indomethacin should be avoided because of its adverse impact on coronary blood flow. Colchicine has recently been shown to be a safe and effective adjunct to conventional NSAID therapy in controlling pain and decreasing the risk of recurrent pericarditis. Systemic steroids should be reserved for recurrent pericarditis unresponsive to NSAIDs and colchicine or cases of acute pericarditis linked to an underlying autoimmune or connective tissue disease. Use of steroids in acute pericarditis has been associated with increased likelihood of recurrence.
Myocarditis
Myocarditis can be either an acute or chronic inflammatory process of the myocardium, triggering focal myocyte necrosis and fibrotic deposition. It is a commonly recognized cause of sudden unexplained cardiac death in young adults. Depending on the degree of injury, myocarditis may be complicated by dilated cardiomyopathy and left ventricular dysfunction. In the United States, viral infections remain the most frequently identified cause of acute myocarditis. As in pericarditis, enteroviruses (Coxsackie B), adenovirus, EBV, CMV and parvovirus B19 in addition to other viruses including influenza A, herpes simplex virus 1 (HSV-1), human herpesvirus 6 (HHV-6) and the human immunodeficiency virus (HIV) have been implicated in this disease process. Bacterial and fungal infections make up a small minority of the remaining infectious causes. Worldwide, protozoal infection with Trypanosoma cruzi, better known as Chagas’ disease, remains a predominant cause of acute myocarditis and dilated cardiomyopathy. Noninfectious causes include toxins such as anthracyclines (doxorubicin) and cocaine as well as hypersensitivity reactions to tricyclic antidepressants, antibiotics (penicillins,sulfonamides) and antipsychotics (clozapine). Sarcoidosis, scleroderma and SLE have also been implicated. Recently, an association has been drawn between smallpox vaccination and increased incidence of myocarditis in military personnel after widespread inoculations.
The clinical presentation of myocarditis is highly variable. While some patients may be completely asymptomatic, others present acutely ill with fever, chest pain, myalgias, arthralgias, exertional dypnea, palpitations and syncope. In many cases, these symptoms may be preceded by a nonspecific viral prodrome of respiratory and gastrointestinal complaints as well as fever, malaise and headache. Some may present with symptoms consistent with acutely decompensated heart failure and hemodynamic collapse, suggesting progression to cardiomyopathy. Auscultation of the chest might reveal a third or fourth heart sound, a new heart murmur, or evidence of pulmonary congestion, all suggestive of heart failure. A drug rash might point towards a hypersensitivity reaction as a possible etiology.
Electrocardiographic changes frequently seen in myocarditis are consistent with acute injury or ischemia and may in many instances be mistaken for acute myocardial infarction. ST-segment elevation and depression, T-wave inversions and pathologic Q-waves have all been seen in cases of biopsy-proven myocarditis where myocardial infarction was initially suspected and coronary angiography was subsequently normal. In many of these cases, patients were not only young but had a dearth of coronary risk factors and had yet presented with symptomatology and electrocardiographic changes concerning for myocardial ischemia or infarct. Ventricular arrhythmias and heart block may also manifest with myocarditis and cardiomyopathy.
The serum cardiac biomarker, cardiac troponin I, is reliably elevated in patients with myocarditis early on (within one month) after initial onset of symptoms and is indicative of acute myocyte necrosis. Considered superior to CK-MB, CTnI may however have returned to normal in patients presenting several months after initial myocardial injury.
Echocardiography may demonstrate either global ventricular dysfunction or regional wall motion abnormalities consistent with a nonspecific cardiomyopathy in patients with signs of heart failure. For patients without these signs, the echocardiogram may be normal. Evolving noninvasive diagnostic strategies such as antimyosin scintigraphy and gadolinium-enhanced cardiac magnetic resonance imaging are increasingly useful tools for differentiating acute myocarditis from myocardial infarction.
Definitive diagnosis of myocarditis rests with endomyocardial biopsy. Previously considered to have low sensitivity as a result of the need for multiple biopsies to obtain a diagnostic result, the yield for biopsy has significantly improved with the advent of PCR for specific viral genomes. Immunohistochemical assays for the anti-heart autoantibodies have also helped identify cases of autoimmune-mediated myocarditis.
The treatment of acute myocarditis remains supportive. Hemodynamic optimization of heart failure is paramount and should proceed with diuretics to lower ventricular filling pressures, angiotensin-converting enzyme inhibitors to reduce vascular resistance, and eventually a beta blocker. In severe cases, intravenous inotropes, implantation of a ventricular assist device or even cardiac transplantation may become necessary. The role of immunosuppressive therapy has not borne out and remains limited to myocarditis clearly attributable to systemic autoimmune disease. Antiviral agents such as interferon beta are under evaluation at this time and have shown initial promise.
Myocardial Infarction
Although some disease processes such as myocarditis and endocarditis may cause fever and subsequent myocardial infarction, the emergence of fever may also follow a primarily cardiac event. A patient who suffers an initial cardiac event from atherosclerotic disease may develop a fever. An ST elevation myocardial infarction (STEMI) is more likely to produce a low-grade temperature than a non-ST elevation mocardial infarction (NSTEMI). This is thought to be due to the development of more extensive necrosis with an ST elevation myocardial infarction in comparison with an non-ST elevation mocardial infarction. Fever does not usually present until 1-2 days after onset of the myocardial infarction and is generally less than 101˚ F. The fever associated with myocardial necrosis typically wanes after 48 hours as remodeling begins. A small observational study (n= 40) from Israel showed a trend towards confirming this theory that fever height is related to the size of infarction as measured by echocardiography and serum cardiac enzyme measurements.
Infective Endocarditis
In the last thirty years, the etiology of infective endocarditis has shifted with the eradication of rheumatic fever in much of the industrialized world. While valvular diseases such as mitral valve prolapse and mitral regurgitation remain key risk factors for infective endocarditis, skin flora, primarily staphylococci, now surpass oral streptococci (viridans group streptococci) as the leading cause of infective endocarditis. In particular, Staphylococcus aureus has emerged as the predominant organism responsible for most new cases of infective endocarditis worldwide, with a significant share attributable to methicillin-resistant S. aureus. Together with Enterococcus spp., staphylococci and streptococci comprise more than 80% of all causes of infective endocarditis. Intravenous drug users may also be at heightened risk for infection with Pseudomonas aeruginosa and fungi. In addition to the typical organisms, elderly persons with degenerative valvular disease may be more prone to infection with Streptococcus bovis, which has been associated with gastrointestinal malignancy. Patients with prosthetic valves may develop infective endocarditis from coagulase-negative staphylococci and gram-negative bacteria of the HACEK group. Patients with nosocomial or healthcare-related infections from invasive surgical procedures, infected hardware and long-term hemodialysis represent a growing population at risk for staphylococcal and enterococcal endocarditis as well.
Fever remains the most common clinical presentation of infective endocarditis. While the fever may be remitting in nature, it may exceed 40º C in cases of acute infective endocarditis and can be accompanied by chills and rigor. It may also be absent in the elderly and in patients with congestive heart failure, chronic renal failure, liver disease or infective endocarditis caused by less virulent organisms. Systemic symptoms such as myalgias, fatigue, malaise, night sweats, anorexia, nausea and vomiting may be seen and often predominate in subacute presentations. Up to 10% of patients with infective endocarditis may report chest pain. Heart murmurs may be heard in as much as 85% of patients. In many instances, these murmurs are preexisting. However, a new, altered or changing murmur has a strong predictive value for infective endocarditis when taken in context with established bacteremia. Peripheral embolic manifestations can be nonspecific for infective endocarditis and are increasingly infrequent due to earlier diagnosis and treatment. Classic Janeway lesions are nontender, erythematous vesicles or pustules seen on the palms and soles while Osler’s nodes are painful, subcutaneous nodules found on the pulp of the digits. Splinter hemorrhages may be seen in the nail beds of the fingers and toes. Petechiae may be seen in the conjuctiva and buccal mucosa. Pale, oval lesions surrounded by hemorrhage, known as Roth’s spots, may be noted on examination of the retina. Splenomegaly may be evident in delayed presentation or late diagnosis of endocarditis.
Blood cultures remain the cornerstone of diagnosis for infective endocarditis. Three or more sets of blood cultures should be drawn at least one hour apart (fresh stick each time preferable) prior to initiation of antibiotics. Cultures may be drawn regardless of whether the patient is febrile. Blood cultures may be negative in about 15% of cases of infective endocarditis. While this may related to prior administration of antibiotics, fastidious organisms such as Coxiella burnetti (Q fever), HACEK organisms, and fungi may require additional serologic or molecular techniques to identify. Laboratory evaluation is otherwise nonspecific. Inflammatory markers such as the erythrocyte sedimentation rate and C-reactive protein may be elevated. Leukocytosis and a normocytic anemia may or may not be present, depending on the severity of the presentation. Urinalysis may reveal proteinuria, hematuria or casts suggestive of an immune complex glomerulonephritis that may be seen with infective endocarditis.
All patients with suspected infected endocarditis should have an electrocardiogram performed. New atrio-ventricular, fascicular or bundle-branch block may signify perivalvular invasion and potential abscess formation. Aortic valve involvement is most common. Echocardiography should be employed to evaluate for intracardiac masses or vegetations ![]() , valve competence and myocardial abscess. It is reasonable to perform a transthoracic echocardiogram (TTE) first if the patient is clinically stable or if there is low clinical suspicion for infective endocarditis. Transesophageal echocardiography (TEE) should be considered if the patient is a difficult imaging candidate for TTE or there is moderate to high clinic suspicion for infective endocarditis. A negative TTE in the setting of deteriorating clinical course should prompt TEE in light of its higher sensitivity for identifying vegetations and abscesses. False-negative results in both TTE and TEE may occur if vegetations are small or have embolized. Large, mobile vegetations (> 10 mm), particularly on the anterior mitral leaflet, have the greatest potential to embolize and have been linked with increased mortality.
, valve competence and myocardial abscess. It is reasonable to perform a transthoracic echocardiogram (TTE) first if the patient is clinically stable or if there is low clinical suspicion for infective endocarditis. Transesophageal echocardiography (TEE) should be considered if the patient is a difficult imaging candidate for TTE or there is moderate to high clinic suspicion for infective endocarditis. A negative TTE in the setting of deteriorating clinical course should prompt TEE in light of its higher sensitivity for identifying vegetations and abscesses. False-negative results in both TTE and TEE may occur if vegetations are small or have embolized. Large, mobile vegetations (> 10 mm), particularly on the anterior mitral leaflet, have the greatest potential to embolize and have been linked with increased mortality.
Remarkably, the four elements characterizing infective endocarditis first described by Sir William Osler in 1885 remain relatively unchanged: persistent bacteremia with an appropriate infectious microorganism, predisposing factors, active endomyocardial involvement and vascular phenomena. Given the protean nature of its presentation, multiple criteria have evolved throughout the years to aid with accurate diagnosis. The widely accepted Duke criteria provide a framework for diagnosis of infective endocarditis that has a sensitivity of roughly 80% (see Table 3).
In 2005, the American Heart Association published its most recent guidelines regarding antibiotic therapy for infective endocarditis, which has since been endorsed by the Infectious Disease Society of America (IDSA). Therapeutic recommendations are available in the endocarditis chapter of Empiric.
PULMONARY ETIOLOGIES
Pulmonary causes of fever and chest pain are the most likely underlying etiology for the patient’s symptoms and presentation. From as benign as bronchitis to as lethal as pulmonary embolism, a pulmonary etiology should be a strong focus of the investigation. Patients will often have other complaints such as cough or wheezing that direct the physician’s attention to pulmonary pathology. The physical exam may be quite helpful in establishing the diagnosis especially if the presentation is due to pneumonia. However, a diagnosis such as pulmonary embolism can be quite difficult to make since the symptoms as so non-specific. A diligent physician must consider all the possible pulmonary etiologies of chest pain and then work through them in a logical manner.
Pulmonary Embolism (PE)
Pulmonary embolus has emerged as a significant cause of morbidity and mortality with an estimated incidence of at least 600,000 cases with roughly 150,000 deaths. The key is a suspicion to this possible diagnosis, as pulmonary embolism can have a myriad of presentations and often it is overlooked on initial exam. The risk factors for development of deep venous thrombosis (DVT) and pulmonary embolism are listed in Table 4. The classic description of chest pain with pulmonary embolism is a pleuritic type of pain often located in the same area of the embolism (if there is only one pulmonary embolism). Dyspnea is the most frequently associated symptom (70-90%) but the severity of the dyspnea described by the patient can be highly variable. Fever is a relatively infrequent presenting symptom in pulmonary embolism as shown by the Prospective Investigation of Pulmonary Embolism Diagnosis (PIOPED) data. Only 14% of patients with pulmonary embolism and no other source of fever had a documented temperature > 100.0˚F1. Less than 2% of the patients with fever had a temperature > 102. 0˚F. Other clues to the diagnosis of pulmonary embolism include a sudden onset of dyspnea, anxiety, syncope, and right-heart strain on EKG (i.e. S1Q3T3 pattern).
Unfortunately, the CXR in pulmonary embolism is generally not helpful. It is often normal and most abnormalities are non-specific. Blood work is also non-specific except for the d-dimer assay. In patients who are considered low-risk (low clinical suspicion for pulmonary embolism) the d-dimer can be used to rule out pulmonary embolism if the value is within normal limits (nearly 100% negative predictive value if < 500 ng/ml). An abnormal d-dimer essentially mandates an evaluation with a high-resolution spiral CT with IV contrast to evaluate for a thrombus. Sensitivity approaches 95% for large, proximal pulmonary embolism and falls to roughly 75% for sub-segmental pulmonary embolism. In patients who can’t tolerate IV contrast (e.g. anaphylaxis, renal insufficiency), a V/Q scan is an alternative. Unfortunately, most V/Q scan results are low to intermediate probability which does not help exclude or confirm a diagnosis. If the CT and/or V/Q does not yield a diagnosis but there is still clinical suspicion of pulmonary embolism, consider a lower extremity venous duplex to evaluate for DVT. Transthoracic echocardiogram can be used as an adjunctive diagnostic tool to see if a right ventricular strain pattern has developed (large pulmonary embolism).
Treatment of pulmonary embolism involves early anticoagulation with unfractionated or low-molecular weight heparin followed by warfarin therapy. For patients who have a large clot burden or who are hemodynamically unstable, some consideration should be given to use of thrombolysis as the initial treatment modality.
Pneumonia
Pneumonia continues to be the leading cause of infectious death in the United States. For the purposes of this chapter, we will focus on community-acquired pneumonia as it is much more likely to present with fever and chest pain than hospital acquired pneumonia. The most frequent presenting symptoms of community-acquired pneumonia include cough and fever which both occur in roughly 75-80% of patients. Dyspnea, sputum production, and pleuritic chest pain each occur in > 50% of patients. The elderly are more likely to present without fever but more likely than young cohorts to have a change in mental status or tachypnea at the time of presentation. The physical exam may reveal rhonchi, rales, egophony, or wheezing on the affected side but is insensitive and non-specific.
CXR is essential when considering the diagnosis of community-acquired pneumonia. An infiltrate demonstrated by CXR or CT is required for the diagnosis of pneumonia. The CXR may be negative early in course of illness and should be repeated 24-48 hours after admission if initially negative and the concern for pneumonia exists. Patients who are immunocompromised (e.g. AIDS, neutropenic) may not manifest an infiltrate on CXR making CT a more utilized adjunct to make the diagnosis. Laboratory studies are generally not particularly helpful in making the diagnosis of community-acquired pneumonia. The WBC is often elevated but is non-specific. Leukopenia on the other hand, is a poor prognostic indicator in the elderly. Blood cultures are routinely obtained for patients hospitalized for community-acquired pneumonia but are only positive in 5-15% of patients. Sputum collection for gram stain and culture is recommended only if the sample is of good quality. A “quality” sample is defined as one with many polymorphonuclear leukocytes and few squamous epithelial cells. Even with a quality specimen there is no guarantee that the offending organism will be grown out of culture. Urinary antigen testing is available for Legionella and S. pneumoniae but since no organism can be grown on culture, sensitivity testing can’t be obtained.
Treatment of community-acquired pneumonia should begin promptly once the diagnosis has been made or there is strong suspicion for this diagnosis and the patient is ill-appearing. If the patient is going to be hospitalized, the recommended initial treatment (per ATS/IDSA) for community-acquired pneumonia involves a beta-lactam (e.g. ceftriaxone, ampicillin/sulbactam, cefotaxime) and a macrolide. An alternative regimen (especially for penicillin allergy) would involve a anti-pneumococcal fluoroquinolone (e.g. moxifloxacin, levofloxacin). If there is a strong suspicion for MRSA (i.e. recent influenza infection, history of recent skin abscesses), then Vancomycin or Linezolid should be added to the regimen. Current use of an antibiotic (outpatient failure) may also warrant consideration of a more broad-spectrum regimen particularly if the patient is ill. Outpatient treatment of healthy individuals for community-acquired pneumonia can utilize either a macrolides or doxycycline. A fluoroquinolone may also be used for community-acquired pneumonia but should be reserved for patients who have had recent antibiotic use or who have significant co-morbid conditions since fluoroquinolone resistance is rising. Treatment duration for outpatient community-acquired pneumonia is recommended to be 5 days for macrolides and 7 days for doxycycline or fluoroquinolones. Perhaps the most important step in deciding which antibiotic is most suitable is becoming familiar with the resistance patterns in your hospital or practice environment. Since S. pneumoniae is responsible for roughly half of all cases of community-acquired pneumonia it is worthwhile to know the resistance patterns of at leastS. pneumoniae in your patient population.
Septic Pulmonary Embolus
Septic pulmonary embolism is an uncommon entity but one that should not be missed as it carries with it significant morbidity and mortality. There is almost always an associated source for the emboli such as right-sided infective endocarditis, intravenous drug use, indwelling catheters and cardiac devices, Lemierre’s syndrome, and infections of the head and neck. Fever is present in almost 100% of patients and may be associated with night sweats and pleuritic chest pain. The pleuritic chest pain is a manifestation of peripheral, cavitating lesions that may form abscesses. Dyspnea, cough and hemoptysis are also possible clinical manifestations. CXR may reveal peripheral “cannonball” type lesions but is often non-specific. CT of the chest is the diagnostic modality of choice and will reveal bilateral nodules or multifocal infiltrates in the peripheral lung fields. CT should be followed by further investigation in the hospital course to determine the source of the emboli. TTE and TEE may help identify culprit tricuspid valve vegetations consistent with right-sided endocarditis. Neck pain and preceding URI or sinus infection may indicate the need to evaluate for Lemierre’s syndrome with a CT of the neck with IV contrast.
Septic pulmonary emboli represent an infectious emergency and timely administration of antibiotics is important once this diagnosis is considered. In the few case series reviewing microbiologic data, S. aureus is the predominant pathogen. Other bacteria that have been identified include a number of oral flora signifying that periodontal disease may play a role in some cases. With the dramatic rise in MRSA, it would be prudent to incorporate Vancomycin early in the course of this disease. However, these patients are often ill and coverage for gram-negative and anaerobic organisms (oral anaerobes) is recommended. Vancomycin and the anti-pseudomonal penicillin (piperacillin/tazobactam) would be a reasonable choice until cultures direct more targeted therapy.
GASTROINTESTINAL ETIOLOGIES
Fever and chest pain may occasionally be the presenting symptoms of a gastrointestinal disorder (see Table 5). Symptoms such as nausea, vomiting, dysphagia, odynophagia and hematemesis may alert the clinician to the presence of an underlying gastrointestinal illness. Physical examination of the oropharynx may reveal evidence of infection that may extend deeper down the esophagus. Careful examination of the abdomen is also prudent as many sources of fever and chest pain may in fact be referred. In many cases, imaging and direct visualization of the upper digestive tract by endoscopy is necessary to establish a gastrointestinal cause for fever and chest pain.
Infectious Esophagitis
Infectious esophagitis is frequently encountered as an opportunistic infection in the patient with HIV/AIDS or in patients undergoing chemotherapy (immuncompromised status). The most common etiologies for esophageal infection remain Candida albicans, cytomegalovirus (CMV), and herpes simplex virus (HSV) type 1. Considered part of the normal oropharyngeal and gut flora, Candida albicans and other species may cause infection in the setting of impaired cell-mediated immunity, recent antibiotic therapy or preexisting esophageal disease such as achalasia or scleroderma, both of which are associated with severe esophageal luminal stasis. It may also be seen with prolonged use of inhaled, topical glucocorticoids for reactive airway disease and acid-suppressive therapy for gastrointestinal reflux. Infection with CMV and HSV typically occurs by reactivation of the latent virus in the setting of active immunosuppression, and rarely manifests as a primary infection. Although the incidence of these infections in patients with HIV has greatly decreased with highly-active antiretroviral therapy, they remain common even in the patient with a normal CD4 count and low viral load.
Odynophagia and dysphagia are the most common presenting symptoms of infectious esophagitis. Chest pain, better characterized as “heartburn” and fever have been particularly well-described in HSV esophagitis associated with immunocompetent patients. Clinical examination may reveal oropharyngeal candidiasis or herpetic lesions, though neither is required for an esophageal infection with Candida or HSV to be present. Definitive diagnosis requires endoscopy and tissue examination. Molecular techniques such as PCR have revolutionized the identification of CMV and HSV genomes in tissue samples. Oral fluconazole remains the mainstay of therapy for esophageal candidiasis. While HSV esophagitis is generally self-limited in immunocompetent individuals, acyclovir or valacyclovir may be used in severe cases or in immunocompromised patients. CMV esophagitis is usually treated with gancyclovir or foscarnet.
Esophageal Rupture
Perforation or rupture of the esophagus may be iatrogenic, spontaneous or traumatic in origin. Endoscopies account for the majority of iatrogenic causes. Rapid rise in intraluminal pressures during vomiting and straining is the usual etiology of spontaneous esophageal rupture, also known as Boerhaave’s syndrome. Weakening of the esophageal wall by severe reflux, infection, and malignancy can all increase the risk of perforation, which commonly occurs in the left posterior aspect of the distal esophagus. Traumatic perforation may be either blunt or penetrating. Perforation by an ingested foreign body is also possible. As the esophagus lacks a serosal layer, perforation allows direct drainage of gastric contents into the mediastinum and pleural space which may lead to chemical and infectious mediastinitis, abscess, empyema, pneumomediastinum, pneumothorax, pleural effusion and pneumohydrothorax.
Acute esophageal leakage from a thoracic rupture or perforation may present with chest pain, dyspnea and fever. The chest pain may be either retrosternal or localized to the side of the injury. Vomiting and dysphagia may or may not be present. The patient is frequently tachycardic and tachypneic. Physical examination may be notable for subcutaneous emphysema. A rasping sound auscultated over the precordium with each heartbeat, known as Hamman’s sign, signifies mediastinal emphysema. Diminished breath sounds are often noted. The complete triad of vomiting followed by chest pain and subcutaneous emphysema thought to be pathognomonic for spontaneous esophageal rupture is rarely seen in clinical practice. For this reason, the nonspecific presentation of esophageal rupture may be easily mistaken for myocardial infarction, pneumothorax, aortic aneurysm, pancreatitis and peptic ulcer. Patients with large perforations however may present acutely ill with hypotension and hypoxia, indicative of sepsis. Mortality and morbidity remain high for esophageal rupture because it is a frequently overlooked diagnosis.
An upright chest radiograph is abnormal in most patients presenting with esophageal rupture, although it may be normal immediately following the event. Pneumomediastinum, widened mediastinum, hydropneumothorax and pleural effusion are the most frequent findings. A water-soluble contrast esophagram may assist in the identification of an esophageal tear and as the contrast extravasates. In some cases, diagnosis of a rupture may require multiple esophagrams if extravasation is not initially seen and clinical suspicion remains high. CT of the chest allows better assessment of involvement of structures adjacent to the esophagus in the hemithoraces and mediastinum, but may not localize the site of the perforation as well as an esophagram. In some cases, endoscopy may be employed to directly visualize the point of rupture. In the setting of a pleural effusion, thoracentesis and analysis of pleural fluid may reveal a low pH and a high amylase level, both of which would support the diagnosis of an esophageal rupture.
Medical intervention consists of prompt broad-spectrum antibiotics (anaerobes, gram negatives, and gram positives should be covered), H2 receptor blockers, fluid resuscitation, nasogastric decompression and pleural drainage. Conservative medical management is possible in cases of well-contained esophageal perforation without pleural involvement or signs of sepsis. As most thoracic ruptures are uncontained and risk ongoing contamination and infection, early surgical consultation is crucial for adequate drainage of the mediastinum and thoracic cavity. In the absence of underlying esophageal disease, primary repair of the perforated esophagus within twenty-four hours remains the gold standard. Esophageal resection may be preferable in cases of underlying esophageal malignancy or severe esophagitis, scleroderma or achalasia.
Intra-Abdominal Etiologies
While the most common etiologies of fever and chest pain lie above the diaphragm, it is important to mention that intra-abdominal disease may also inadvertently manifest with similar presentations. Historically, subphrenic abscesses have been attributed to complications of surgery involving the stomach, duodenum and biliary tract. In many instances, patients may present with chest pain and pain referred to the shoulder in addition to abdominal tenderness. Such abscesses may even extend into the thoracic cavity causing empyema or lung abscess and are typically polymicrobial in nature. Fever and pleuritic chest pain may also be seen in liver and splenic abscesses. Liver abscesses may arise from infections of the biliary tree such as cholecystitis and cholangitis, portal bacteremia or through bacteremia from an abdominal infection. Infection with Entamoeba histolytica may result in amebic liver abscesses. Splenic abscesses frequently arise as a complication of bacteremia from infective endocarditis, trauma, or infarction in the setting of sickle cell disease. CT and ultrasound remain the primary tools for evaluating for an abdominal abscess. While broad-spectrum antibiotic therapy may be employed, surgical drainage of a subphrenic abscess is often necessary. The same may be said for pyogenic liver abscesses with the caveat that percutaneous drainage may also be an option. Amebic liver abscesses may be treated with metronidazole and rarely require drainage. Splenic abscesses may be an indication for splenectomy.
HEMATOLOGIC AND ONCOLOGIC ETIOLOGIES
Hematologic and oncologic etiologies of fever and chest pain are usually more readily diagnosed as the patient will often carry a diagnosis of malignancy or sickle cell disease at the time of presentation. However, not all malignancy related diagnoses will be made upon presentation and the physician must take into account age and risk factors for malignancy when initiating a work up for fever and chest pain.
Acute Chest Syndrome
Acute chest syndrome is one of the true emergencies that can occur in patients with sickle cell disease. It is thought to arise as a result of an exaggerated immune response to either an infectious (e.g. pneumonia) or non-infectious etiology (e.g. fat embolus) subsequently resulting in acute lung injury distinct from the underlying cause. Occurring in almost half of patients with sickle cell disease at some point in their lifetime, acute chest syndrome carries a mortality rate of 3-5% and significant morbidity (mean hospital stay 10-14 days). Patients with sickle cell disease at any age are susceptible to acute chest syndrome although the mortality rate appears to be slightly higher in adults. Importantly, patients with either Hb SS or Hb SC are equally as likely to present with acute chest syndrome.
The standard definition of acute chest syndrome includes the presence of fever > 101.3˚F, new infiltrate on CXR involving at least one complete lung segment, chest pain, and either tachypnea, wheezing, or cough. Fever is the most frequent symptom present in roughly 80% of patients. Chest pain has been reported in 40-60% of patients with acute chest syndrome and is often described as severe and sharp in nature. The physical exam can be variable (normal lung examination in up to 30%) and the patient may complain of pain at other sites on their body that are more typical of their “sickle cell pain” when they have a crisis.
Acute Chest Syndrome is often considered a multifactorial disease process and the clinician should consider the possibility of bacterial infection, viral infection, or non-infectious etiology as the inciting event (see Table 6 for a more complete list of etiologies). Bacteria involved in acute chest syndrome are frequently those also seen in community-acquired pneumonia such as Chlamydophila pneumoniae and Mycoplasma pneumoniae. Viruses such as RSV have also been implicated especially in younger hosts. Fat embolism has received a great deal of interest as lipid-laden macrophages have been recovered on bronchoscopy in anywhere from 10-20% of patients with ACS. However, the etiology often remains unknown making prudent empiric therapy a key to successful patient recovery.
When diagnosing acute chest syndrome the key for the physician is maintaining a high level of suspicion for this entity. It is challenging to differentiate a specific pulmonary process (e.g. PE, pneumonia) from the syndrome that comprises acute chest syndrome. The CXR invariably will have an infiltrate although there seems to be preferential involvement of the lower lobes. Leukocytosis, thrombocytosis, and acute fall in hemoglobin level are usually seen on laboratory studies. Blood cultures should be obtained but will only be positive in 1-5% of cases of acute chest syndrome.
Initial management should focus on correction of hypoxia, fluid resuscitation, pain control, and empiric antibiotics. Since part of the disease presentation is pain resulting from sickled hemoglobin in the microvasculature, oxygen therapy and fluid resuscitation are essential. However, overly aggressive fluid administration may result in pulmonary edema so repeat examinations after fluid boluses are recommended. Pain management generally requires utility of narcotics while exercising caution to avoid respiratory depression and need for mechanical ventilation. The choice of empiric antibiotics should follow the ATS/IDSA guidelines for inpatient management of community-acquired pneumonia. This involves a beta-lactam and a macrolide or a fluoroquinolone for those with a penicillin allergy. If the patient does not improve with the standard measures, then consideration can be given to exchange transfusion.
Malignancy
A number of different malignant processes can present with fever, but only a few present with the combination of both fever and chest pain. Lymphoma (Hodgkin’s and Non-Hodgkin’s), multiple myeloma, carcinoid, and post-obstructive pneumonia secondary to a primary malignancy can all present with fever and chest pain. While certainly lymphoma is the most likely malignancy to present with fever and chest pain, it is important to keep other etiologies on the differential.
Hodgkin’s and Non-Hodgkin’s lymphoma are both characterized by mediastinal involvement in roughly 20-60% (much more likely in Hodgkin’s) of patients at presentation as well as fever in 10-20% of patients as well. Mediastinal lymphadenopathy can certainly cause chest pain while other possible sources of chest pain from lymphoma include pleural effusion, pericardial effusion, superior vena cavae syndrome (rare), and potential malignant involvement of the thoracic vertebrae. Fever in both Hodgkin’s and non-Hodgkin's lymphoma is part of a constellation of symptoms possible at presentation termed “B” symptoms. Fever, weight loss, and night sweats make up this constellation of symptoms seen in 25-50% of patients with either disease entity. The fever is classically more prevalent in the evening and increases in severity and duration with the length of time prior to diagnosis. The “B” symptoms can have prognostic significance with either Hodgkin’s or non-Hodgkins lymphoma. Initial investigation of patients who may have either of these two diagnoses should involve a CXR to search for hilar lymphadenopathy and a CT of the chest/abdomen/pelvis looking for lymphadenopathy and solid organ involvement.
Multiple myeloma frequently presents with bony pain from metastatic disease and this occasionally manifests as chest pain from thoracic rib or more often vertebral metastatic disease. The bone pain orginating from multiple myeloma is distinctive from bony pain caused by metastatic disease in that multiple myeloma causes pain that is induced by movement and is not worse at night. Pathologic fractures are not uncommon and may be subacute in nature at the time of presentation. Since multiple myeloma is considered an immunocompromised state, the fever associated with presentation is more likely due to an associated infection rather than from tumor associated inflammatory markers. The most prevalent infections are due to gram-negative organisms involving the pulmonary or urinary systems. Fevers attributed solely to the malignancy are infrequent and a thorough infectious workup should be performed before ascribing the fever to multiple myeloma itself. An initial workup when suspicious of multiple myeloma should involve a skeletal survey to evaluate for lytic lesions. Laboratory studies are usually of benefit and may reflect acute renal failure, hypercalcemia (from bony lytic lesions), anemia, and a decreased anion gap from the cationic M proteins in the serum. An SPEP and UPEP should be included in the workup to evaluate for monoclonal immunoglobulins. Initial management involves aggressive management of any infectious process since infection is the leading cause of death in multiple myeloma patients. Primary lung tumors do not often incite fever and chest pain unless associated with an infection (The exception being the rare carcinoid tumor accounting for 1-2% of pulmonary tumors). Chest pain is a common finding associated with the presentation of a primary lung tumor. Estimated to occur in 25-50% of patients who present prior to diagnosis, the description of the chest pain is variable. Dull, intermittent pain is more common and is generally located on the same side as the tumor. Severe or persistent pain may signify malignant invasion of the chest wall or mediastinum and can easily be confused for pain associated with acute coronary syndrome especially since the patient population is similar. The most likely presentation of fever and chest pain involving a primary lung tumor would involve a post-obstructive pneumonia. Squamous cell carcinoma is the most likely type of lung cancer to present with a post-obstructive pneumonia. Because of its tendency to involve proximal tracheobronchial tree, obstruction and subsequent pneumonia can develop in 10-20% of patients at some point in their disease course. Post-obstructive pneumonia should be treated aggressively with broad spectrum antimicrobials that includes coverage for anaerobes (e.g. metronidazole). A proposed regimen could include ceftriaxone, a macrolide, and metronidazole or single agent coverage with the anti-pseudomonal penicillin (piperacillin/tazobactam) since it has anaerobic activity. Diagnosis of any type of lung cancer should initially involve a CXR followed by a CT of the chest for further evaluation.
DERMATOLOGIC ETIOLOGIES
Diseases of the skin are usually recognized by patients and often prompt early evaluation since patients’ often have anxiety about diseases they can visualize. The skin should always be examined in the evaluation of fever and chest pain, as infectious rashes and eruptions may be identified. And while the differential diagnosis may not be as broad when rash is combined with fever and chest pain, serious pathology may nonetheless exist. Cellulitis, necrotizing soft tissue infection and herpes zoster represent the most common skin-related causes of fever and chest pain (see Table 7).
Cellulitis
Cellulitis is a diffuse, spreading infection of the dermis and subcutaneous tissue. While more frequently seen in the lower extremities, cellulitis of the chest wall is not uncommon in the setting of certain risk factors. Any type of skin trauma may create a portal of entry for infection. Lacerations, burns, and surgical wounds are common sites for cellulitis. Devices such as cardiac pacemakers, central lines, and implantable defibrillators positioned in the subcutaneous tissue of the chest wall may likewise serve as a nidus for infection. Disruption of lymphatic drainage following axillary node dissection as part of a radical mastectomy or lumpectomy for breast cancer may result in lymphedema, which is a prominent risk factor for cellulitis. Increasing age, obesity, immunosuppression, underlying diabetes mellitus and intravenous drug abuse are all widely recognized risk factors as well. Streptococcus pyogenes and Staphylococcus aureus represent the primary organisms responsible for most cellulitis. Infection with S. aureus may often be complicated by an underlying abscess. In many parts of the United States, community-acquired methicillin-resistant S. aureus (MRSA) is responsible for the majority of skin infections presenting to the emergency department.
Cellulitis typically manifests with poorly-demarcated erythema, edema, induration, and pain. Erysipelas is a specific form of cellulitis that presents with well-circumscribed borders and lymphatic inflammation. In other forms of cellulitis, vesicles, bullae, or pustules (especially Staph aureus) may be present. Likewise, petechiae and ecchymosis may also be noted. The clinical presentation may range anywhere from localized discomfort at the site of infection to a picture of systemic toxicity with fever, tachycardia and hypotension.
Blood cultures are rarely positive in most uncomplicated cases of cellulitis in the general population. Clinical evidence of bacteremia such as high-grade fever, chills and leukocytosis should however warrant blood cultures, especially in the setting of cellulitis superimposed upon preexisting lymphedema.
Antimicrobial therapy should be tailored towards coverage of streptococci and S. aureus with oral penicillinase-resistant penicillin or a first-generation cephalosporin. It is important to consider community-acquired MRSA as well and give some consideration to usage of trimethoprim-sulfamethoxazole, doxycycline, or clindamycin in those patients. In cases of antibiotic-resistant infection requiring hospital admission or allergy to penicillin, treatment with vancomycin or clindamycin may be necessary. Most cases of uncomplicated cellulitis are treated for 7 to 10 days, although evidence suggests that a 5-day course may be equally effective. Cellulitis associated with implanted medical devices may ultimately require removal of the hardware in order for the infection to resolve.
Necrotizing Soft Tissue Infection
In contrast to cellulitis, necrotizing soft tissue infections attack the fascia and subcutaneous tissue. Rapidly progressive and frequently fatal, they often develop as secondary infections in the setting of trauma, surgery, peripheral vascular disease, skin ulcers and diabetes mellitus. Necrotizing fasciitis is a rare infection that spreads along the superficial fascia, compromising the subcutaneous tissue between the skin and underlying muscle. Polymicrobial necrotizing infections (type 1 necrotizing fasciitis) may be caused by a mix of streptococci, staphylococci, enterococci, enteric gram-negative bacteria and anaerobes. Monomicrobial necrotizing infections (type II necrotizing fasciitis) may be caused by group A β- hemolytic streptococcior S. aureus. As in cellulitis, an increasing number of cases of necrotizing fasciitis have been attributed to community-acquired MRSA. Necrotizing fasciitis involving the chest wall is extremely uncommon but bears an especially high mortality. Tube thoracostomy for empyema has been identified as the most common procedure preceding infection in many cases. In at least one case, pneumonia with empyema without prior surgical intervention has also been associated with necrotizing fasciitis.
Necrotizing fasciitis is often mistaken for cellulitis in its early stages. In fact, almost two-thirds of cases of necrotizing fasciitis are initially misdiagnosed as either cellulitis or abscess. Skin discoloration, warmth, induration, and edema are common findings, but fail to resolve with antibiotic therapy. The affected area may be firm, with a wooden consistency, in contrast to the fleshy consistency of cellulitis. A paucity of skin findings in the early stages of infection is equally likely. In these instances, pain disproportionate to the physical findings may serve as a helpful clue to a deeper infection. As the illness progresses, development of violaceous bullae, diffuse ecchymosis, cyanosis, and skin sloughing signify tissue necrosis below the skin. The hemorrhagic fluid in the bullae may evolve into a gray, foul-smelling fluid, frequently referred to as “dishwater pus.” Skin anesthesia may result from cutaneous nerve destruction. Crepitus may be palpated in polymicrobial infections. Fever, hypotension and mental status change herald bacteremia and sepsis.
The diagnosis of necrotizing fasciitis remains one that is heavily based upon history and clinical examination. Surgical exploration with direct inspection of the fascia is the most expeditious means to confirm the diagnosis. Fascial planes are easily separated using a blunt probe as a result of extensive tissue necrosis. Laboratory evaluation may reveal leukocytosis and elevated sedimentation rate, though both may be nonspecific. Hypocalcemia may arise from extensive fat necrosis. Worsening renal and hepatic indices may signal multiorgan failure from sepsis. Blood cultures are warranted and deep incisional tissue biopsy with aerobic and anaerobic cultures may aid in identifying causative organisms. Conventional radiography may be notable for subcutaneous gas, though CT is more sensitive. Magnetic resonance imaging (MRI) has proven useful in identifying the extent of deep fascial involvement in necrotizing infection, though its sensitivity exceeds its specificity and may result in overestimation. Given the rapid nature of necrotizing fasciitis, imaging should never delay prompt surgical evaluation.
Extensive surgical debridement coupled with fasciotomy is crucial to survival. In many cases, multiple debridements in the operating theater may be necessary. Polymicrobial infections should be treated with a combination of ampicillin-sulbactam, clindamycin and ciprofloxacin for broad aerobic and anaerobic coverage. Monomicrobial infection from group A streptococci should be treated with clindamycin and penicillin. The addition of vancomycin or newer antibiotics such aslinezolid may be necessary for infections involving MRSA with resistance to clindamycin. The role for hyperbaric oxygen therapy in the treatment of necrotizing fasciitis is not well established, though it is frequently employed as an adjunct to surgical and antimicrobial therapy at institutions where it is available.
Herpes Zoster
In those who have had a primary infection with varicella-zoster virus in the past, reactivation of latent virus in the dorsal root ganglia clinically manifests itself as a painful vesicular rash known as herpes zoster. More than 90% of adults in the United States have been exposed to varicella-zoster virus and, as a consequence, more than 500,000 cases of herpes zoster are estimated to occur annually. Naturally waning cell-mediated immunity with increasing age is one of the primary risk factors for developing herpes zoster. Patients on immunosuppressive therapy, including corticosteroids and chemotherapy, are likewise at increased risk, as are those with immune systems compromised by lymphoma or HIV.
Herpes zoster frequently occurs along the distribution of a thoracic nerve, resulting in a rash with a unilateral dermatomal distribution across one side of the chest and back that does not cross the midline. A prodrome of pain and paresthesia in the affected sensory nerve may precede the rash by two to three days along with fever, headache and malaise. The rash progresses from macular to vesicular to pustular and finally ulcerates and then crusts after three to five days. Recurrent pain along the course of the affected nerve more than a month after the rash has healed is classified as post-herpetic neuralgia. Affected areas of skin may be at higher risk for cellulitis by superinfection with staphylococci and streptococci.
Although herpes zoster is a clinical diagnosis for the most part, it may be confirmed by direct immunofluorescence assay of fluid from a cutaneous lesion. The important aspect of the physical examination is a thorough skin examination to ensure no herpetic lesions exist outside of the affected dermatome. Immunosuppressed patients (e.g. HIV, chronic steroids, neutropenic) are at risk of disseminated herpes zoster with a significantly increased morbidity and mortality.
Treatment for herpes zoster begins with antiviral therapy. Oral antiviral medications such as acyclovir, valacyclovir and famciclovir may reduce the severity and duration of herpes zoster-related pain if initiated within seventy-two hours of onset of the rash, while viral replication is still ongoing. Concomitant administration of corticosteroids may further accelerate healing and attenuate pain. Immunosuppressed patients or those with any indications of disseminated herpes zoster should be given strong consideration for intravenous therapy and admission to the hospital.
RHEUMATOLOGIC ETIOLOGIES
Rheumatologic etiologies are infrequent causes of the combination of both fever and chest pain. The most likely rheumatologic causes of fever and chest pain include rheumatoid arthritis and systemic lupus erythematosus (SLE) which can each have a variety of manifestations in the thorax. However, most patients will already carry this diagnosis when they present with a subsequent cardiothoracic process resulting in fever and chest pain. Thus, making the diagnosis of the underlying rheumatologic disease is not as imperative as keeping in mind the various possible presentations of these disease entities.
Rheumatoid Arthritis and Systemic Lupus Erythematous
Rheumatoid arthritis can manifest as a myriad of disease processes in the thorax. A pericardial effusion is seen in almost 50% of patients with the diagnosis of rheumatoid arthritis However, symptomatic pericarditis (please see cardiac etiology section for further detail) is uncommon and generally only occurs when a disease flare is occurring elsewhere in the body. In a worst case scenario, pericarditis can progress to chronic constrictive pericarditis and symptoms of heart failure. Rheumatoid nodules may develop in the pulmonary parenchyma, in the myocardium, or even on the valve leaflets. Symptoms vary from valvular insufficiency to asymptomatic depending on the size and location of the nodule. The most likely pulmonary manifestation of RA to present with fever and chest pain is pleurisy. Pleurisy results in irritation of the pleural lining, pleuritic type chest pain, occasionally pleural effusion, and sometimes an associated fever. This diagnosis is largely clinical since a CXR or CT will not show inflammation of the pleural lining. If there is a pleural effusion seen, it should be tapped to rule out an associated infectious etiology.
Systemic lupus erythematous may cause many of the same manifestations as rheumatoid arthritis that may present with fever and chest pain. Pericarditis occurs in roughly 25% of patients with systemic lupus erythematous during the course of their disease but fortunately it does not have the possibility of developing into chronic constrictive pericarditis as can patients with pericarditis from rheumatoid arthritis. A more serious manifestation includes myocarditis which can present with fevers, chest pain, dyspnea on exertion, and CHF. Similarly to rheumatoid arthritis, valvular involvement may occur most classically through “Libman-Sachs” endocarditis. Verrucous vegetations are found on the valve in this condition which prefers the mitral and tricuspid locations. Pleurisy with or without an effusion is a relatively common event for patients with SLE as one study demonstrated that half of all systemic lupus erythematous patients will have at least one episode of pleural inflammation. Pleurisy can easily be confused with the presentation of pneumonia or pulmonary embolus and each should be entertained in the differential especially if no pleural effusion is seen on CXR. Patients with systemic lupus erythematous are at higher risk for PE/DVT so any pleuritic chest pain in these patients should raise concern for this diagnosis.
READING LIST
1. Lange RA, Hillis LD. Acute pericarditis. N Engl J Med. 2004;351:2195-2202. [PubMed]
2. Maisch B, Seferović PM, Ristić AD, Erbel R, Rienmüller R, Adler Y, et al. Guidelines on the diagnosis and management of pericardial diseases executive summary. Eur Heart J. 2004;25:587-610. [PubMed]
3. Ellis CR, Di Salvo T. Myocarditis: basic and clinical aspects. Cardiology in Review. 2007;15:170-177. [PubMed]
4. Magnani JW, Dec GW. Myocarditis: current trends in diagnosis and treatment. Circulation. 2006;113:876-890. [PubMed]
5. Feldman AM, McNamara D. Myocarditis. N Engl J Med. 2000;343:1388-1398. [PubMed]
6. Mylonakis E, Calderwood SB. Infective endocarditis in adults. New Engl J Med. 2001;345:1318-1330. [PubMed]
7. Baddour LM, Wilson WR, et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Health Association. Circulation. 2005;111:e394-e433. [PubMed]
8. Fedullo P, Tapson V. The Evaluation of Suspected Pulmonary Embolism. New Engl J Med. 2003;349:1247-1256. [PubMed]
9. Mandell L, Wunderink R, et al. Infectious Disease Society of America/American Thoracic Society Consensus Guidelines on the Management of Community-Acquired Pneumonia in Adults. Clin Infect Dis. 2007;44:Supplement 2. [PubMed]
10. Wilcox CM, Schwartz DA, Clark WS. Esophageal ulceration in human immunodeficiency virus infection: causes, response to therapy, and long-term outcome. Ann Intern Med. 1995;123:143-149. [PubMed]
11. Vichinsky E, Neumayr L, et al. Causes and Outcomes of the Acute Chest Syndrome in Sickle Cell Disease. New Engl J Med. 2000;342:1855-1865. [PubMed]
12. Stevens DL, Bisno AL, Chambers HF, Everett ED, Dellinger P, Goldstein EJC et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373-1406. [PubMed]
13. Urschel JD, Takita H, Antkowiak JG. Necrotizing soft tissue infections of the chest wall. Ann Thorac Surg. 1997;64:276-279. [PubMed]
14. Dworkin RH, Johnson RW, Breuer J, Gnann JW, Levin MJ, Backonja M et al. Recommendations for the management of herpes zoster. Clin Infect Dis. 2007;44:S1-S26. [PubMed]
15. Isaac B, Kernbaum S, Burke M. Unexplained Fever. CRC Press, Boca Raton, FL, 1991.
Tables
Table 1: Complete Differential Diagnosis for Fever and Chest Pain
Organ System |
Differential Diagnosis |
|---|---|
Cardiac |
Pericarditis Endocarditis (Infective or sterile) Myocarditis Aortic dissection Myocardial infarction |
Pulmonary |
Pneumonia(CAP, HAP, viral, etc…) Pulmonary embolus Bronchitis Empyema PCP pneumonia Idiopathic pulmonary fibrosis Abscess Fungal infection (e.g. Histoplasmosis) Interstitial lung disease Crack lung Septic pulmonary embolus |
Hematologic/Oncologic |
Acute chest syndrome(sickle cell disease) Primary lung malignancy Metastatic lung disease Lymphoma (Hodgkin’s and Non-Hodgkin’s) Post-obstructive pneumonia (due to malignancy) Multiple myeloma Thymoma Metastatic disease to ribs or sternum(rare) Radiation pneumonitis |
Rheumatologic |
Systemic Lupus Erythematosus Rheumatoid arthritis Sarcoidosis Systemic sclerosis Sjogren’s syndrome Dermatomyositis Takayasu’s arteritis Polymyositis Wegener’s granulomatosis Goodpasture’s syndrome |
Gastrointestinal |
Esophagitis (Infectious or non-infectious) Esophageal rupture Cholecystitis (Chest pain is unusual) Pancreatitis (Chest pain is unusual) Perforated viscu (peptic ulcer) Subphrenic abscess |
Dermatologic |
Herpes zoster |
Musculoskeletal |
Osteomyelitis (Sternum) Costochondritis (fever extremely rare) |
Miscellaneous |
Mediastinitis |
Rare Diagnoses are Listed in Italics
Table 2: Cardiac Etiologies of Chest Pain and Fever
| Risk factors | Presentation | Management | |
|---|---|---|---|
| Pericarditis | Idiopathic
Infection Uremia Myocardial infarction Malignancy Trauma Autoimmune disease |
Sharp, retrosternal pain Positional Pericardial friction rub Signs of tamponade ± Cardiac biomarkers ECG: diffuse ST elevations |
± Echocardiogram
NSAIDs |
| Myocarditis | Infection Drugs/toxins Autoimmune disease |
Variable, Asymptomatic à Chest pain à Failure ± S3 or S4 + Cardiac biomarkers if acute ECG: ± ST-T changes |
Echocardiogram Endomyocardial biopsy Diuretics, ACE-inhibitors, Beta blockers ± inotropes, assist device or heart transplant |
| Infective endocarditis | Valvular disease Intravenous drug use Prosthetic valve Surgical procedures Implanted devices Hemodialysis |
Variable Heart murmur Peripheral stigmata Leukocytosis ECG: ± conduction abnormalities |
Blood cultures Echocardiogram Antibiotic therapy Surgical intervention |
Table 3: Modified Duke Criteria for the Diagnosis of Infective Endocarditis
Major criteria Blood culture positive for IE Typical microorganisms for IE isolated from 2 separate blood cultures (Staphylococcus aureus, Viridans streptococci, Streptococcus bovis, HACEK group, or community-acquired enterococci without a primary focus) or Micoorganisms consistent with IE from persistently positive blood cultures or Single positive blood culture for Coxiella burnetti or phase I IgG antibody titer >1:800
Evidence of endocardial involvement Echocardiogram positive for IE (oscillating intracardiac mass on a valve or supporting structures, paravalvular abscess or new dehiscence of a prosthetic valve) or New valvular regurgitation (worsening or change in pre-existing murmur is not sufficient) |
Minor criteria Predisposing factors for IE (e.g. valvular disease, injection drug use) Fever > 38º C Vascular phenomena (major arterial emboli, septic pulmonary emboli, mycotic aneurysm, intracranial hemorrhage, conjunctival hemorrhages, and Janeway lesions) Immunologic phenomena (glomerulonephritis, Osler’s nodes, Roth’s spots and rheumatoid factor) Microbiological evidence (positive blood culture not meeting major criteria or serologic evidence of active infection with an organism consistent with IE) |
Definite infective endocarditis 2 major criteria 1 major criteria and 3 minor criteria 5 minor criteria |
Possible infective endocarditis 1 major criteria and 1 minor criteria 3 minor criteria |
Table 4: Risks Factors for Deep Venous Thrombosis and Pulmonary Embolism
Primary Hypercoagulable State
|
Secondary Hypercoagulable State
|
Table 5: Gastrointestinal Etiologies of Chest Pain and Fever
| Risk factors | Presentation | Management | |
|---|---|---|---|
| Infectious esophagitis | HIV
Chemotherapy Transplant immunosuppression Luminal stasis Inhaled glucocorticoids Acid-suppressive therapy |
Retrosternal/epigastric pain Odynophagia Dysphagia ± oropharyngeal lesions
|
Endoscopy
Antifungal vs. antiviral therapy |
| Esophageal rupture | Severe vomiting
Underlying infection Malignancy Foreign body Trauma |
Retrosternal/epigastric pain ± Hypotension, tachycardia Subcutaneous emphysema ± Pleural effusion |
Upright chest film Esophagram CT Surgical consultation |
| Intra-abdominal infection | Abdominal surgery | Variable, ± pleuritic pain
Referred pain to the shoulder |
Antibiotic therapy
Surgical drainage |
Table 6: Most Common Inciting Events Precipitating Acute Chest Syndrome (ACS)
•Fat embolism
•Viral etiology
•Legionella pneumophila
•Pulmonary infarction
•Unknown cause (roughly 50% of cases)
Table 7: Dermatologic Etiologies of Chest Pain and Fever
Risk factors |
Presentation | Management | |
|---|---|---|---|
| Cellulitis | Skin trauma Implanted devices Lymphema Intravenous drug use Diabetes mellitus |
Diffusely tender area of erythema, edema & induration ± Abscess |
Empiric antibiotic therapy Abscess drainage ± Blood cultures ± Removal of implanted device |
| Necrotizing fasciitis | Trauma Surgical procedure (e.g. thoracostomy) Diabetes mellitus |
Skin findings initially similar to cellulitis with disproportionate pain Bullae, ecchymosis, cyanosis, skin sloughing Cutaneous anesthesia ± Hypotension, tachycardia Leukocytosis Hypocalcemia |
Surgical débridement Empiric antibiotic therapy Wound culture Blood culture ± Hyperbaric oxygen
|
| Herpes zoster | Increasing age HIV Lymphoma Chemotherapy Transplant immunosuppression |
Dermatomal distribution Initially, vesicular or pustular Ulcerated or crusted after 3-5 days ± Superimposed cellulitis |
Empiric antiviral therapy Corticosteroids ± direct immuno- fluoresence vs. PCR to confirm etiology |
Algorithm 1. Clinical Approach to Fever and Chest Pain
Guided Medline Search For:
Reviews
Mandell LA. Infectious Diseases Society of America/American Thoracic Society Consensus Guidelines on the Management of Community-Acquired Pneumonia in Adults. Clin Infect Dis 2007;44:S27-S72.

