Penicillins
Authors: Sandra L. Preston, Pharm.D., George L. Drusano, M.D.
INTRODUCTION
In 1929, Alexander Fleming isolated penicillin from a strain of Penicillium notatum (84). By 1941, benzylpenicillin could be produced in sufficient quantity to treat several infected patients. Clinical trials with the agent, conducted by Florey and colleagues, were successful and during World War II, benzylpenicillin was used to treat patients with streptococcal, gonococcal, and treponemal infections. Shortages of the agent continued until the late 1940s when production of large amounts of drug became possible by a deep-fermentation procedure (85). Since then, many synthetic penicillins have been developed, but resistance to the agents has increased. Despite the emergence of resistance to penicillins and the development of other classes of anti-infective agents, the penicillins remain one of the most important anti-infective classes of drugs well into the nineties. In fact, penicillin G is still the drug of choice for many types of infections, including syphilis and certain types of endocarditis.
CLASS
Chemical Structure (Figure 1)
The basic chemical structure of all penicillins consists of a beta-lactam ring, a thiazolidine ring, and a side chain (6-aminopenicillanic acid). The antibacterial activity of the penicillins lies within the beta-lactam ring. Any alteration in this ring structure forms penicilloic acid and the antibacterial activity of the compound is lost. The side chain varies with each penicillin compound and generally determines the spectrum of activity, as well as the pharmacokinetic properties of the compound. There are several natural penicillins (penicillin dihydro F, X, and K), of which benzylpenicillin (penicillin G) is the most active and is the only natural penicillin used clinically (164).
Structure-Activity Relationships
Manipulations of the side chain have produced compounds that are stable against certain bacteria, such as Staphylococcus aureus, which produce beta-lactamase enzymes (penicillinase). The side chain sterically inhibits the beta-lactamase hydrolysis of the beta-lactam ring. Other penicillin compounds have side chains, which are stable against beta-lactamases produced by gram-negative rods. Side chain changes can also increase the bacterial permeability of the compound and can result in increased oral absorption from the intestinal tract by rendering oral agents more stable to gastric acid breakdown (167, 186).
ANTIMICROBIAL ACTIVITY
Classification of Penicillins and Spectrum of Activity
The penicillin compounds can be divided into categories based upon their spectrum of activity (Table 1). Minimum inhibitory concentration (MIC) data for 50% and 90% of specific organisms are located in Tables 2 and 3 (10, 70, 150, 228, 245, 255, 257, 261). For gram-negative organisms and anaerobes, resistance in up to 15% of strains is possible; therefore MIC90s must be interpreted cautiously.
Natural Penicillins
Penicillin G is a natural penicillin that is produced directly from fermentation of Penicillium crysogenum. Penicillin V is a derivative of penicillin G and because of similarities in spectrum of activity, is considered a natural penicillin. The natural penicillins have activity against non-beta-lactamase producing gram-positive cocci, including viridans streptococci, group A streptococci, Streptococcus pneumoniae, and anaerobic streptococcus (Peptostreptococcus, Peptococcus sp.). Enterococcus sp. is most susceptible to the natural penicillins. Other potential organisms with susceptibility include non-penicillinase producing strains of Staphylococcus aureus and coagulase-negative Staphylococcus, however because of the high likelihood of resistance, it is inappropriate to use natural penicillins as empiric treatment for a suspected Staphylococcal infection unless the organism’s susceptibility is known. The natural penicillins have activity against Clostridium sp. (excluding Clostridium difficile) and Actinomyces sp. Activity against gram-negative cocci is limited and includes Neisseria meningitidis, non- penicillinase producing Neisseria gonorrheae, and Pasteurella multocida. Similar to staphylococcal infection, natural penicillins should not be used for treatment of gonorrhea due to the increased potential of a resistant organism and subsequent treatment failure. The anaerobic coverage of penicillin V is less than that of penicillin G. Natural penicillins also have excellent activity against the spirochete, Treponema pallidum, the causative organism of syphilis.
Penicillinase-Resistant Penicillins
The agents in this group are also known as the antistaphylococcal penicillins. The addition of an isoxazolyl side chain to the penicillin compound protects the beta-lactam ring from acid hydrolysis by penicillinases produced by Staphylococcus sp. (150). Methicillin, the first agent synthesized in this group, is rarely used currently due to a higher incidence of occurrence of interstitial nephritis and is no longer commercially available in the United States. Nafcillin and oxacillin are the agents commonly used parenterally, while dicloxacillin is available for oral use. These agents have activity against Staphylococcus sp (including penicillinase-producing strains). Strains of methicillin-resistant Staphylococcus aureus(MRSA) and methicillin-resistant Staphylococcus epidermidis (MRSE) exist and can be the prevalent Staphylococcal organism in certain areas, such as certain hospitals or wards within the hospital. These organisms are not sensitive to the penicillinase-resistant penicillins.
While less active against streptococcal sp. as compared to the natural penicillins, based on MIC data, use of the penicillinase-resistant penicillins is acceptable (i.e. the MICs are low enough relative to achievable serum concentrations) for use against these organisms. Clinically, in serious, life-threatening infections where a gram-positive organism is suspected, combinations of penicillin G plus a penicillinase-resistant penicillin can be utilized to achieve maximal streptococcal and staphylococcal coverage. A notable exception to the gram-positive coverage of this class of penicillins is the Enterococci. These organisms are not susceptible to this class of penicillins. Anaerobic activity ranges from minimal to none and gram-negative activity is virtually nonexistent.
Aminopenicillins
Because of the need for improved coverage against gram-negative organisms, further manipulation of the side chain was conducted. By adding an amino group to the basic penicillin compound, the aminopenicillins were developed. The spectrum of activity against gram-positive organisms is similar to that of the natural penicillins. These agents retain activity against streptococcal sp. and have slightly greater activity against Enterococcus (ampicillin) and Listeria monocytogenes versus the natural penicillins. The added side chain does not, however, inhibit hydrolysis by Staphylococcal penicillinases or gram-negative beta-lactamases. The enhanced spectrum of these drugs includes activity against gram-negative bacilli, including H. influenzae, E. coli, Proteus mirabilis, Salmonella sp., and Shigella sp. (13, 83). These drugs were developed in the 1960s and were, at that time, very effective against these organisms. Presently, however, many strains of these gram-negative organisms are resistant to ampicillin. Combinations of an aminopenicillin plus a beta-lactamase inhibitor, such as clavulanic acid or sulbactam, are useful for treatment of infections caused by beta-lactamase producing organisms.
Carboxypenicillins
A carboxyl group substitution in place of the amino group yields penicillin compounds that have a greater gram-negative spectrum of action, including activity against Pseudomonas aeruginosa, most likely due to increased bacterial penetration through the cell wall. Carbenicillin and ticarcillin are the two drugs in this class. Their spectrum of activity includes that of ampicillin, while also encompassing Enterobacter, Providencia, Morganella, indole-positive Proteus, and Pseudomonas aeruginosa, with ticarcillin having slightly greater activity against Pseudomonas aeruginosa versus carbenicillin (19). Coverage against Klebsiella and Serratia are less predictable and, unlike ampicillin, these compounds have little activity against Enterococcus. These agents are not effective against beta-lactamase producing organisms unless combined with a beta-lactamase inhibitor (e.g. ticarcillin plus clavulanic acid).
Ureidopenicillins and Piperazine Penicillin
In order to increase gram-negative coverage and particularly coverage against Pseudomonas aeruginosa, a ureido group addition to the penicillin structure produces the compounds azlocillin and mezlocillin. A ureido group plus a piperazine side chain produces piperacillin. The gram-negative coverage of this class of penicillins includes that of the carboxypenicillins, plus coverage against Klebsiella, Serratia, Enterobacter, Enterococcus, and increased anaerobic coverage (228). The activity against Streptococci is slightly less that of the natural penicillins and ampicillin. Of the drugs in this class, piperacillin has the most activity against Pseudomonas aeruginosa (52, 255). As with the carboxypenicillins, drugs in this class are susceptible to inactivation by bacterial beta-lactamase production, unless combined with a beta-lactamase inhibitor (e.g. piperacillin plus tazobactam).
Pharmacodynamic Effects
When choosing an antimicrobial agent and designing appropriate dosing regimens for the drug, it is important to consider spectrum of activity, but also incorporate known pharmacodynamic principles about the drug. In this manner, efficacy can potentially be maximized while toxicity can be minimized. Some excellent reviews on these concepts have been published (71, 76). Such pharmacodynamic variables that are important to consider for the penicillins includes concentration-independent bactericidal activity, the post-antibiotic effect (PAE), and the duration of the dosing interval the drug’s serum concentrations spend above the level of the organism’s MIC (Time > MIC).
Bactericidal Effects
All beta-lactam drugs (including the penicillins) exert relatively concentration-independent bactericidal activity, meaning that the concentration of drug does not appreciably affect its ability to exert an antibacterial effect (25, 209). This assumes, however, that a level that exceeds the organism’s MIC is attained. Theoretically, the bactericidal rate at 2 times above the MIC or 4 times above the MIC would be the same. However, once the drug concentration falls below the level of the MIC and the PAE has ceased, the kill rate diminishes. Time > MIC is therefore the important determinant of outcome for these drugs.
A paradoxical phenomenon of decreased effect with higher drug concentrations, known as the “Eagle effect”, has been described with some strains of streptococci and staphylococci (111). This effect, however, does not appear to be clinically significant, as there is very limited data to support decreased bactericidal activity in vivo due to high serum concentrations.
Another factor that may influence bactericidal activity is bacterial inoculum size. Generally, the more dense the bacterial population (i.e. the older the infection), it is more likely that there will be resistant variants of the organism present. This may be the case with nosocomial gram-negative pneumonias or other serious infections. Treatment with a penicillin as monotherapy may result in a relapse after completion of therapy when the resistant sub-variants are no longer suppressed and begin to regrow. This scenario is not unique to the penicillins, and in fact may occur with other antibiotics when used as monotherapy.
The bactericidal activity of the penicillins does not appear to be affected by changes in pH or oxygen tension. The location of the organism is important, however, as in vitro efficacy may not correspond to in vivo efficacy. Penicillins and other beta-lactams do not penetrate well into phagocytes (104), thus limiting their ability to kill intracellular pathogens. In addition, penicillins only exert their bactericidal effect on bacteria that are actively replicating.
Synergistic Bactericidal Activity
Combinations of a beta-lactam plus another agent, such as an aminoglycoside, kill some organisms most effectively. In these cases, antibacterial synergy occurs. Synergy is defined as an effect, such as bactericidal activity, that is significantly greater with the combination than the sum of the two agents when used alone. The mechanism of this effect with penicillins and aminoglycosides may be due to cell wall disruption by the penicillin, facilitating increased entry of the aminoglycoside into the bacteria (158). Enterococcal endocarditis is such an example, as penicillin monotherapy results in bacteriostatic activity and very high relapse rates after treatment (149), while the combination of penicillin plus an aminoglycoside is bactericidal (157).
Other organisms for which synergy seems to be important with regard to the penicillins includes Pseudomonas aeruginosa. Again, a combination of an antipseudomonal penicillin plus an aminoglycoside may result in increased bactericidal activity. This has been demonstrated in vitro and animal studies (5, 77, 118), but there is limited data in humans to support these findings. In vitro synergy between the extended spectrum penicillins (azlocillin, mezlocillin) and ciprofloxacin has also been demonstrated (153, 178, 225). Immunocompromised patients are a population who may benefit the most from antipseudomonal synergy. There is data to suggest that synergistic combination therapy results in increased survival versus non-synergistic combinations of drugs (124, 130, 204).
Antagonism of Antibacterial Combinations
Antibacterial antagonism is defined as a resulting effect that is significantly less in combination than with either of the two drugs when used as monotherapy. This effect has been demonstrated with the penicillins in combination with chlortetracycline in patients with pneumococcal meningitis, when penicillin monotherapy was more effective that the combination of agents (133). Combinations of penicillin plus chloramphenicol have demonstrated in vitro antagonism against pneumococci (188), however, clinically this may be of little importance since the combination only diminished penicillins bactericidal activity (resulting in bacteriostatic activity) and chloramphenicol retains its antibacterial effect. Also, the use of chloramphenicol has decreased dramatically in the last decade due to the availability of newer agents that are equally efficacious and less toxic.
Antagonism can also occur due to a physical incompatibility with inactivation between two drugs when infused together. This can occur with carbenicillin or ticarcillin with an aminoglycoside. These drugs should therefore not be mixed in the same infusion.
Post-Antibiotic Effect
The PAE is defined as a persistent suppression of bacterial growth after effective exposure to an antimicrobial agent when serum concentrations of the drug have fallen to levels below the MIC. This effect differs between infecting organisms and between drugs. The mechanism of the PAE is not entirely clear, but may be due to persistent binding of the penicillin to penicillin-binding proteins (PBPs) and the time that is necessary for the organism to resynthesize new PBPs (218).
The PAE was first noted with penicillin G and Staphylococcus aureus (179), when it was noted that there was a short period of time where bacterial regrowth did not occur after exposure to the drug. Subsequently, this phenomenon has been described with the penicillins for other gram-positive organisms (42, 108), including Streptococcus pneumoniae andEnterococcus faecalis. The length of the PAE can range from 0-6 hours (Table 4), depending upon the penicillin.
As stated previously, the type of organism can affect the PAE. The penicillins do not exhibit an appreciable PAE against gram-negative organisms. Also, combinations of antimicrobial agents can result in a synergistic PAE. Combinations of penicillins plus various aminoglycosides have resulted in synergistic or additive PAEs for Enterococcus faecalis andEnterococcus faecium (86, 108), along with Staphylococcus aureus (100).
Models of Antibacterial Outcome Determination
Bactericidal activity of penicillins and other beta-lactams appears to be related to the Time > MIC, as demonstrated in several in vitro models. A number of studies of beta-lactam agents demonstrated that increased half-life and not peak concentration influenced bactericidal activity (97, 125, 254, 272). This implies that increased duration of drug exposure above the MIC would be more predictive of positive outcome versus increased drug doses and subsequent increased peak concentrations.
Data from animal models supports Time > MIC as the primary determinant of efficacy for beta-lactam agents (75, 247). In a neutropenic mouse model infected with Pseudomonas aeruginosa, the impact of different dosing intervals of ticarcillin was studied. Equivalent daily doses were administered every hour or every 3 hours. The mice that received drug every hour (a lower dose administered more frequently) had a greater antibacterial effect (88).
These findings were also supported by studies of Klebsiella pneumoniae pneumonia in rats (197), in Klebsiella pneumoniae lung and thigh infections in neutropenic mice (132),Pseudomonas aeruginosa infection in neutropenic rats (159), Staphylococcus aureus in rats recovering from hemorrhagic shock (142), and in Enterococcal endocarditis (231). Additional data (247) demonstrated that for gram-negative infections, a Time > MIC of 100% of the dosing interval was most closely associated with outcome, whereas for gram-positive organisms, a Time > MIC of approximately 50% was all that was needed to be effective. For gram-negative infections, continuous infusion of the penicillin may be most appropriate to maintain serum concentrations above the MIC for the entire dosing interval.
Clinical trials examining the impact of Time > MIC for beta-lactams in relation to outcome is limited. One study examined combinations of carbenicillin plus continuous infusion cefamandole, carbenicillin plus intermittent cefamandole, and carbenicillin plus continuous infusion tobramycin in febrile, neutropenic cancer patients (32). The most effective regimen was the carbenicillin plus continuous infusion cefamandole. Despite the fact that actual Time > MICs were not calculated, the most effective regimen most likely had the greatest Time > MIC, as the carbenicillin was administered every four hours, effectively providing serum concentrations that would remain above the MIC throughout the dosing interval.
A study of cefmenoxime in 14 critically ill patients examined bacterial eradication rates as a function of Time > MIC (203). A relationship between increased Time > MIC and increased eradication rates of gram-negative pathogens was found.
Some recent clinical data has supported time > MIC as an important predictor of outcome. Some data suggests that a time > MIC of > 50% of the dosage interval is most appropriate for cephalosporins (53). The use of cefuroxime as a single drug in the setting of in vitro resistance was associated with an increase in mortality, but this was not seen with discordant therapy when penicillins, ceftriaxone, or cefotaxime were used. This finding may have been attributed to the dosage of 750 mg three times daily which does not provide coverage of time > MIC > 50% of the time for organisms with MICs of 4 g/mL (269) A higher dosage in the order of 1500 mg q8h may be more appropriate versus resistant pneumococci where time > MIC is > 50%.
The Role of Continuous Infusion of Penicillins
In vitro data support more frequent administration of piperacillin in suppression of microbial growth (170). As previously stated, data in humans comparing continuous infusion with intermittent dosing is limited. The study by Bodey et al appears to support such dosing, however some small studies did not demonstrate any differences in response rates (129, 270). The study by Zeisler et al. (270) was not randomized and the study by Lagast et al. (129) only included 45 patients, most likely lacking sufficient power to detect a statistical difference.
The advantage of continuous infusion would be the potential maximization of efficacy and potentially decreased costs (270). Disadvantages, however, include patient inconvenience with a continually infusing solution, lack of knowledge about proper dosing, and compatibility issues with other necessary intravenous drugs (248). Many of these concerns may be addressed by educational efforts. Other concerns include adequate tissue penetration with continuous infusion. Some studies have demonstrated good penetration of continuous infusion beta-lactams into extravascular space (181, 244). Other data appear to support intermittent injections resulting in increased tissue penetration, as seen in models of rabbit fibrin clots (14, 131), however the concentrations achieved with continuous infusion may be adequately above the organism MIC to treat the infection. A limitation of this model, however, is that the clots are implanted in the rabbit’s back and may not be an appropriate representation of physiology.
Continuous infusion may be most beneficial in patients with impaired host defenses or in life-threatening infections. In these cases, patient convenience is less of an issue and the potential benefit from maximizing efficacy is greatest. Dosing by continuous infusion can be accomplished by use of nomograms (246) or by monitoring a steady-state serum concentration (after 4-5 half-lives or approximately 4-5 hours into the infusion for most penicillins) and adjusting the dose in relation to the serum concentration and the organism MIC. Use of a bolus dose may be used so that the patient’s serum concentrations will reach a therapeutic level quickly.
mecHANISMS OF ACTION
Penicillins are bactericidal agents that exert their mechanism of action by inhibition of bacterial cell wall synthesis and by inducing a bacterial autolytic effect.
Inhibition of Bacterial Cell Wall Synthesis
Penicillins exert their bactericidal activity primarily by inhibiting bacterial cell wall synthesis. Though the exact mechanism of action is not fully elucidated, it appears that penicillins bind to penicillin-binding proteins (PBPs), which are enzymes (transpeptidases, carboxypeptidases, and endopeptidases) that play an important role in the formation and maintenance of the cell wall structure. The cell wall is made up of peptidoglycan, or murein sacculus, which is a polymeric component consisting of long polysaccharide chains of N-acetylglucosamine and N-acetylmuramic acid cross-linked by shorter peptide chains. The formation of peptidoglycan can be divided into three stages, including precursor formation in the cytoplasm, linkage of precursor products into a long polymer, and finally cross-linking by transpeptidation. It is the final transpeptidation process that is inhibited by penicillins by acting as a structural analog of acyl-D-alanyl-D-alanine (the substrate of the enzyme) and acylating the transpeptidase enzyme. The peptidoglycan structure, and therefore the cell wall structure, is weakened, leading to cell death (234, 236, 266). Other mechanisms of cell death are also possible. Binding to PBPs 1A, 1B, 2, and 3 results in a bactericidal effect (219), however binding to PBPs 4, 5, and 6 is not lethal. Also, there are differences in PBPs between gram-positive and gram-negative bacteria and there are differences in affinity between penicillin compounds to various PBPs. These differences can affect spectrum of activity.
Penicillin-Induced Bacterial Autolytic Effect
There are several PBPs that the penicillins simultaneously inactivate. Inhibition of certain PBPs may be related to the activation of a bacterial autolytic process by inactivation of endogenous inhibitors of these autolysins or murein hydrolases (235). These enzymes cleave parts of the cell wall to make room for peptidoglycan synthesis for cell wall expansion (109). With inhibition of cell wall synthesis, bacterial lysis can occur due to increased osmotic pressure. This autolysis may be cell cycle dependent, that is, most likely to occur while the cell is dividing (147). Certain “tolerant” species of staphylococci and streptococci have been isolated which are autolysin-deficient. These organisms are inhibited, but not killed by penicillins (233).
mecHANISMS OF RESISTANCE
A limitation to the clinical use of penicillins is the emergence of resistant organisms. Antimicrobial resistance can arise during therapy by selective pressure or can be present due to acquisition of a naturally resistant strain. A classic example of penicillin resistance is the case of Staphylococcus aureus, which was susceptible to penicillin G when the compound was first discovered (around 1941). Since that time, resistance occurs in >95% of strains, leading to the necessity of use of alternative compounds such as the antistaphylococcal penicillins or vancomycin. Resistance of other gram-positive and gram-negative organisms also occurs, which can lead to challenges in treatment of active infection. Resistance rates for different organisms vary according to geographic location and are summarized in Table 5 (93, 117, 160, 168, 200, 206). Of particular concern in the United States is the emergence of penicillin-resistant (and multi-drug resistant) pneumococci and methicillin-resistant staphylococci, as treatment options in these scenarios are limited (8, 237).
There are three main mechanisms of resistance to penicillins, including:
• enzymatic degradation of the penicillin,
• inability of the penicillin to penetrate the cell membrane to reach its target site,
• an alteration in the PBP target site.
Enzymatic Degradation
Inactivation by beta-lactamase enzymes is the most common mechanism of resistance to the beta-lactam agents. The beta-lactamase reacts with the beta-lactam bond by hydrolysis forming acidic derivatives and subsequent loss of antibacterial activity. There are several classification schemes for the numerous beta-lactamases, including those of Jack and Richmond (116), Richmond and Sykes (191), and Bush (44, 45). The Bush scheme classifies according to substrate preference and susceptibility to clavulanate inhibition. A limitation of these schemes, however, is that they can be confusing due to numerous codes and abbreviations (140).
Both gram-positive and gram-negative organisms produce beta-lactamases, mediated either by plasmids or chromosomes. An important difference is that gram-positive organisms generally produce more enzyme because it must be excreted into the extracellular space for the antibiotic to be inactivated, while the gram-negative bacteria’s enzymes are located in their periplasmic space.
Gram-positive bacteria that produce beta-lactamases (particularly Staphylococcus) can transfer resistance through plasmids or transposons. Plasmids are extrachromosomal genetic material that are autonomous, self-reproducing and can be conjugating. By conjugation, the genetic information is transferred to other Staphylococcus species, including aureus andepidermidis. Transposons are DNA elements that can move from one part of the bacterial chromosome to another. Genetic information can be transferred to other bacteria through movement of the transposon to a plasmid or from direct transfer to another bacteria’s chromosome, known as conjugative transposons (216). Beta-lactamases of Staphylococcus can be inducible by use of beta-lactam antibiotics, meaning that after exposure to a beta-lactam agent, the organism can greatly increase beta-lactamase production. The inducible production generally ceases after the beta-lactam is removed (172).
As stated previously, gram-negative bacteria secrete beta-lactamases into the periplasmic space and are effective in protecting the PBPs located on the bacterial inner membrane from the antibiotic. These enzymes can be either chromosomally-encoded or plasmid-encoded (227). They are produced either constitutively (production of a constant amount of beta-lactamase regardless of exposure to beta-lactam agents) or are inducible and can affect beta-lactam compounds in different ways. Some agents are quickly destroyed, while others are destroyed at a much slower rate and therefore have increased antibacterial activity.
Production of stably derepressed mutants is a concern during therapy with beta-lactam agents that are weak inducers of beta-lactamase production, such as extended-spectrum and third generation cephalosporins. These mutants produce increased quantities of beta-lactamases (hyperproduction) despite removal of the inducible antibiotic. This is most likely to occur with the chromosomally- mediated Bush Group I enzymes for which the preferred substrate is cephalosporins. Rapid emergence of resistance can occur in this circumstance, particularly in infections caused by Pseudomonas aeruginosa or Enterobacter cloacae (50, 141), due to selection of the mutants after the more susceptible organisms are killed during treatment. In this instance, the mutants can proliferate and can become the predominant infecting organism. The only effective beta-lactam would be a carbapenem, as Class I beta-lactamases can hydrolyze all other types of beta-lactams agents.
Extended-spectrum beta-lactamases (ESBLs) are plasmid mediated with a wide substrate profile. These enzymes are a relatively recent problem, affecting some strains of Klebsiellasp. as well as some strains of Enterobacter and E. coli. The emergence of ESBL-producing organisms has been linked with the widespread use of extended-spectrum cephalosporins (154,190). A carbapenem is a drug of choice against these organisms, while beta-lactamase inhibitor combinations may also be effective (93).
Video: Mechanism of Resistance -- Destruction
Reduced Penetration of the Penicillin
It is easier for penicillins to acetylate the PBPs in gram-positive bacteria because these bacteria have only a thick cell wall layer protecting the PBPs on the inner membrane. Gram-negative bacteria, however, have an outer membrane composed of a lipopolysaccharide and phospholipid bilayer and between the layers is a periplasmic space. An inner membrane is composed of peptidoglycan. Another space separates the inner membrane with the cytoplasmic membrane. PBPs are located in the cytoplasmic membrane and are protected by beta-lactamases. In the outer membrane there are proteins, known as porins, which act as channels for nutrients and waste products into and out of the bacteria. Penicillins may enter the gram-negative bacteria by this route. Porin permeability to penicillins depends upon size of the molecule, hydrophilicity, and electrical charge (267). Decreases in the number of porin channels have been reported to be a mechanism of resistance to beta-lactam agents (105). Most research has been conducted with the outer-membrane proteins (Omp) of E. Coli. Omp F and Omp C are the two main porins, with Omp F being most permeable to beta-lactam agents. Some mutants which lack Omp F porins can be resistant to beta-lactams due to decreased and slower penetration through the remaining porins (Omp C) and subsequent increased beta-lactamase degradation (66).
Alteration of the PBP Target Site
Binding to the PBP is necessary for the penicillin to exert its antibacterial effect. There are natural differences in the affinity for penicillin to a PBP. For instance, the affinity of the Enterococcal PBP to the antistaphylococcal penicillins is very low versus a high affinity to penicillin G or ampicillin. This accounts for the resistance seen in the case of oxacillin and Enterococcus.
Mutations can occur which can result in changes in susceptibility of an organism, which is “normally” susceptible to a particular penicillin, usually by producing PBPs with a decreased affinity to the penicillin. An alteration in PBP2 by Staphylococcus to PBP2a results in methicillin resistance, as PBP2a exhibits a decreased affinity for methicillin and most other beta-lactam agents (102). With Staphylococcus aureus (241) this type of production of PBPs with decreased affinity for the penicillin is inducible by exposure to the agent, resulting in decreased susceptibility to low concentrations of the drug.
An important example of bacteria that can develop such mutations that confer resistance is Streptococcus pneumoniae that is penicillin-resistant. The resistance mutation is genetically coded with "mosaics" that are made up of native pneumococcal DNA and DNA that is presumably from another streptococcal species, such as viridans streptococci, more resistant to penicillin (93,127). The genes that appear to be most affected are PBP 2b and 2x.
The current interpretive MIC breakpoints for penicillin as determined by the National Committee for Clinical Laboratory Standards (NCCLS) are <0.06 ≤g/mL (susceptible), 0.12-1.0 g/mL (intermediate), and ≥2.0 g/mL (resistant) (165). In the United States, the prevalence of penicillin-non-susceptibility has risen from 4% in the 1980s to approximately 35% in 1997-1998 (8). Because of resistance, penicillin may not achieve adequate concentrations in the cerebrospinal fluid to treat meningitis if the infecting organism is intermediate or highly resistant to the drug. The clinical impact of penicillin resistant Streptococcus pneumoniae outside the setting of the central nervous system has been uncertain, however one large prospective study of 844 hospitalized patients with positive blood cultures for Streptococcus pneumoniae examined the impact of resistance, antibiotics administered, and clinical outcome. Of the isolates, 15% were intermediate and 9.6% were resistant to penicillin, but penicillin resistance was not a risk factor for mortality and NCCLS breakpoints were not predictive of clinical outcome for penicillin or ceftriaxone (but were for cefuroxime), suggesting that penicillin-resistant pneumococcus is not clinically relevant for parenteral therapy of pneumococcal pneumonia (269)
Video: Mechanism of Resistance -- Mutation
Methods to Overcome and Prevent Resistance
Because of concerns over infectious organisms that are emerging resistant to our standard therapies, there is a need for prevention. Infection control practices should be followed, which include hand washing and changing gloves between examination of patients. These methods can limit the dissemination of a resistant organism in a hospital environment (95). Unfortunately, such practices are not routinely followed by health-care providers despite educational efforts (94, 68).
Optimization of antimicrobial use in hospitals is desirable as it is has been demonstrated that use (and overuse) of broad-spectrum antimicrobials is associated with emergence of resistant organisms (50, 249), particularly with ESBL-producing organisms (154, 190) and it is suspected with penicillin and vancomycin resistant enterococcus. Antibiotic control programs have been implemented in many institutions with some success (79, 264). Successful policies, however, can be time and labor intensive and require a full institutional commitment in the form of adequate personnel for implementation and medical staff support for the program.
Pharmacologically, there are strategies to overcome and prevent resistance. The use of combination antimicrobial therapy is a method to provide adequate coverage against suspected organisms (14). There is animal model data to suggest that combination chemotherapy that is synergistic may have a benefit in prevention of emergence of resistance (89, 118), however clinical data is limited.
PHARMACOKINETICS
The pharmacokinetics of the penicillins varies between compounds. Absorption between oral agents varies greatly, with amoxicillin and dicloxacillin producing adequate serum concentrations and penicillin G and carbenicillin producing very poor serum concentrations. The penicillins are widely distributed in the body, with adequate levels achieved in serum, tissues, bile, and synovial fluid. Penetration into the cerebrospinal fluid (CSF) in patients with uninflamed meninges is relatively poor with only 0.5-2% of serum concentrations attained (173). When the meninges are inflamed, however, penetration increases to approximately 5-20% of serum concentrations, as demonstrated in rabbit models (202, 229). The primary route of elimination for most penicillins is renal, with some hepatic metabolism. Some compounds, however, are primarily eliminated by the hepatic route.
The absorption, distribution, metabolism, and excretion will be described for each class of penicillins. Pharmacokinetic properties for the penicillins are summarized in Table 6.
Natural Penicillins
Aqueous crystalline penicillin G, or benzylpenicillin, administered intravenously is the most commonly utilized formulation for this class of penicillins. This route of administration is preferred in ill patients due to increased serum concentrations achieved versus oral or intramuscular (IM) routes of administration with penicillin G or other natural penicillins. The drug is widely distributed with an apparent volume of distribution (Vd) of 0.35 L/kg. Distribution into the CSF is minimal with uninflamed meninges, but increases with inflammation. CSF concentrations can be approximately 5% of serum concentrations (205). Elimination is primarily (90%) renal (10% by glomerular filtration, 90% by tubular secretion) (40,73). There is, however, some hepatic elimination. The pharmacokinetic advantage to this drug is that high serum concentrations are achieved rapidly, but the half-life is approximately 30 minutes, necessitating redoing every 4-6 hours.
Penicillin G is poorly absorbed orally, with a bioavailability of 15-30%. The environment of the stomach decreases its absorption due to gastric acid breakdown. In hypochlorhydric patients, such as the elderly, oral penicillin G has an increased absorption due to an increasing gastric pH. Penicillin V, administered orally, has an increased absorption compared to penicillin G due to its increased acid stability (nearly double the peak serum concentrations). Low concentrations are attained in tissues. Concurrent administration of food can decrease the absorption of the oral natural penicillins, most likely due to binding of the penicillin onto the food particles. Because of poor absorption and limited clinical utility, oral penicillin G is no longer available in the United States.
Procaine penicillin G (PPG) and benzathine penicillin G (BPG) are repository forms of penicillin administered intramuscularly (IM), with prolonged absorption and subsequent extended serum concentrations of penicillin G. PPG serum concentration can last for up to 24 hours, while low level BPG serum concentrations (0.10 - 0.15 units/mL) can last for up to 3-4 weeks. The advantage of these long acting agents is that dosing can be less frequent if the organism is susceptible to the lower levels achieved, such as in the case of BPG and Treponema pallidum, the causative agent of syphilis, where MICs are usually 0.03 units/mL. PPB contains 120 mg procaine with every 300,000 units penicillin G. Patients who are hypersensitive to procaine may experience adverse reactions, particularly when high doses (e.g. 4.8 million units PPG) are used.
Penicillinase-Resistant Penicillins
Methicillin is not orally absorbed and is therefore only given by the intravenous route. Nafcillin has poor oral absorption and its use is generally limited to intravenous or intramuscular routes. Oxacillin, cloxacillin, and dicloxacillin can be administered orally, however oral oxacillin produces a peak serum concentration nearly ¼ that of dicloxacillin.
Penetration into the CSF with inflamed meninges is variable, due to increased protein binding (50-92%). Penetration into the bile is highest with nafcillin of approximately 4000% of serum concentrations (164). Methicillin is eliminated primarily through the kidney by glomerular filtration or tubular secretion. Oxacillin is both renally eliminated and hepatically metabolized. Nafcillin, however, is primarily hepatically metabolized; therefore a dosage adjustment in renally impaired patients is not necessary.
Aminopenicillins
Unlike the natural penicillins, these agents exhibit increased stability to gastric acid hydrolysis. Amoxicillin’s bioavailability is greater than that seen with ampicillin (75-90% with amoxicillin versus 30-50% with ampicillin). Because of this difference, oral ampicillin has been favored for treatment of a localized Shigella infection when lack of absorption is desirable. The incidence of diarrhea is increased, however, with this agent due it’s effect on normal flora of the gastrointestinal tract. Food delays the absorption of ampicillin and amoxicillin, however the extent of absorption is decreased only for ampicillin.
Penetration of ampicillin into the CSF in patients with inflamed meninges occurs with CSF concentrations of approximately 1.5 µg/mL being attained after a single 1-g dose. Elimination of the drug is primarily renal, but about 10% of the drug is metabolized hepatically. Both drugs are removed by hemodialysis (30-40%).
Bacampicillin is a prodrug of ampicillin and is hydrolyzed to ampicillin by esterases during absorption and distribution. Increased serum concentrations of ampicillin are seen due to increased absorption (bioavailability of 80-95%).
Extended-Spectrum Penicillins
The absorption of carbenicillin (the only orally available extended-spectrum penicillin) is poor with a bioavailability of 30-40%. Serum concentrations achieved are inadequate to appropriately treat systemic infection; therefore, it’s clinical use is limited to urinary tract infection and in some instances, prostatitis. The use of this drug, however, has decreased since the availability of orally administered quinolones for these indications.
Ticarcillin, mezlocillin, and piperacillin penetrate fairly well into the CSF in patients with inflamed meninges. Continuous infusion piperacillin in 4 meningitis patients receiving 325-425 mg/kg/day attained a mean CSF level of 23 µg/mL (68). They also distribute well into bile, with concentrations of piperacillin nearly 50 times higher than that seen in the serum (92,199). Penetration into diseased biliary tracts (e.g. cholecystitis) is limited, and adequate concentrations may not be achieved in this setting. Elimination of these compounds is by both renal and nonrenal routes.
Effects of Renal Impairment and Dialysis on the Clearance of Penicillins
Because many of the penicillins are renally excreted, impairment in renal function can result in prolonged half-lives and subsequent increased serum concentrations of drug (18) and can increase the propensity for adverse effects. It is therefore important to adjust doses or dosing intervals for many of the penicillins in these patients (Table 7). Penicillin G is a renally excreted drug and it’s clearance can be related to creatinine clearance (CrCL) using the equation, penicillin clearance in mL/min = 35.5 + 3.35 * CrCL in mL/min. The daily dose of penicillin G can be calculated using the equation dose (in million units per day) = 3.2 + CrCL/7 (40). Other penicillins, including mezlocillin and piperacillin (24, 64) should also have their dosing regimen adjusted in renal impairment. With these drugs, however, biliary excretion also occurs, resulting in serum concentrations that are not in proportion to the degree of renal impairment. Oxacillin, cloxacillin, and dicloxacillin, while partially renally excreted, have only moderate increases in half-life (1.5-2-fold) in anuric patients (22, 41, 258), therefore dosage adjustment is most likely only necessary in severe renal impairment. Carbenicillin should be avoided in patients with renal impairment and renal failure as urinary concentrations are inadequate for treatment of urinary tract infection (the drug’s only clinical indication).
Many of the penicillins are cleared during hemodialysis (50-90% of serum concentrations) (22, 77), therefore a supplemental dose after dialysis is recommended for penicillin G, ampicillin, piperacillin, ticarcillin, and mezlocillin. Nafcillin and oxacillin are not appreciably cleared by hemodialysis, so supplemental dosing is not necessary.
Peritoneal dialysis does not significantly remove any of the penicillins; therefore supplemental dosing is not necessary.
Effects of Hepatic Insufficiency on the Pharmacokinetics of the Penicillins
Most penicillins are primarily renally eliminated and do not require a dosage adjustment on hepatic impairment. Because of the nonspecific nature of liver function tests (ALT, AST, GGT, etc.) and the fact that increases in these markers may not correlate with intrinsic hepatocellular activity, it is difficult to recommend precise dosage adjustments for hepatically eliminated drugs. Some penicillins that may warrant a dosage adjustment in hepatic impairment include nafcillin and mezlocillin. An up to 50% dose reduction of nafcillin may be appropriate in patients with a combination of renal and hepatic insufficiency. Mezlocillin’s dose may be reduced by 50% or the dosing interval doubled in patients with severe hepatic impairment (43, 155).
Effects of Pregnancy on the Pharmacokinetics of the Penicillins
Pregnant patients have increased volumes of distribution and may result in decreased serum concentrations of drugs. This has been demonstrated with piperacillin (106) and ampicillin (126), but may occur with other penicillins as well. In a comparative study with piperacillin and mezlocillin, a shorter half-life and increased clearance was seen in post-partum patients receiving piperacillin versus non-pregnant patients, but was not seen in post-partum women receiving mezlocillin (152). This data implies that dosage increases may be more important in post-partum patients when using piperacillin, as this drug may be more affected by the physiologic changes induced by pregnancy.
DOSAGE
A summary of common adult and pediatric dosage regimens for the penicillins are shown in Table 7 (156). Many pediatric dosages are specified on a per kilogram basis. It is important to keep in mind that the pediatric dosage should not exceed the usual adult dose when using this method of dosing, particularly in a large child. Formulations available are listed in Table 1 and costs for typical courses of therapy are shown in Table 8.
Natural Penicillins
Penicillin G dosages are usually described in units. One unit is defined as a concentration of drug that produces a certain size zone of growth inhibition around an Oxford strain of Staphylococcus aureus. One unit is equivalent to 0.6 µg of crystalline sodium salt of penicillin G, therefore 250 mg equals 400,000 units. Aqueous crystalline penicillin G is administered intravenously at dosages of 6-20 million units daily, either in 4-6 divided doses or by continuous infusion.
Benzylpenicillin is available as a sodium or potassium salt, either providing 20 meq of sodium or 1.7 meq potassium per 1 million unit. The potassium salt is most often used clinically, except in patients with severe renal failure, where the sodium salt may be more appropriate.
BPG and PPG should not be administered subcutaneously due to severe pain and induration at injection site. In adults, the injection should be made into the gluteus maximus or midlateral thigh. Intravenous injection may result in severe neurotoxicity and should be avoided.
Penicillinase-Resistant Penicillins and Aminopenicillins
The penicillinase-resistant penicillins are available as sodium salts of the drugs. Dosages for methicillin are expressed as methicillin sodium, while the other agents in this class have their dosages expressed as the base compound (e.g. 1g methicillin sodium = 900mg methicillin). Of the aminopenicillins, bacampicillin 400 mg is equivalent to 280 mg of ampicillin.
Extended-Spectrum Penicillins
These compounds are available as disodium salts and contain a significant amount of sodium with each dose. Ticarcillin sodium has the most sodium load with 5.2 mEq/gram (120 mg/gram). Carbenicillin (intravenous form), carbenicillin indanyl (oral form), mezlocillin, and piperacillin contain 4.7, 2.6, 1.85, and 1.85 mEq/gram, respectively. This increased sodium load can be problematic in patients with congestive heart failure and renal impairment.
For patients on continuous renal replacement, dosages should be modified (Table 9).
PENICILLIN HYPERSENSITIVITY
Incidence
Allergy to penicillin is estimated to occur in 1-10% of patients receiving the drug (66). Manifestations can range from a maculopapular rash to an anaphylactic reaction. While anaphylaxis is relatively rare (0.004- 0.015% of patients (113), the reaction can be potentially fatal with rates of approximately 3-9% in such patients (113, 201).
Mechanism and Types of Allergic Reactions
Penicillin is degraded into several products, including benzylpenicilloyl, or major determinant, which makes up 95% of the breakdown products. The remaining 5% is termed the minor determinants and include a mixture of benzylpenicillin, benzylpenicilloate, and benzylpenilloate. Benzylpenicilloylamine may also be included as a minor determinant, though its clinical relevance is questionable (20). Antibodies to the major and minor determinants can exist and an immune response can be elicited upon binding of these determinants to tissue proteins, forming a hapten-protein complex and hence, a complete antigen (135). Antibodies to the major determinant can include IgE, IgG, and IgA. Only IgE antibodies have been demonstrated to the minor determinants. A sensitivity to the beta-lactam ring or to the side chain of semisynthetic penicillins may also be mechanisms of eliciting an immune response.
A Type I, or immediate anaphylactic reaction can occur , usually within 2-20 minutes of drug administration. When contact is made with the antigen, IgE antibodies present on mast cells and basophils degranulate releasing various mediators, including histamine, prostaglandins, leukotrienes and others. Histamine release increases capillary permeability, and stimulates bronchial smooth muscle and nerve endings. Bronchoconstriction, laryngeal edema, and urticaria occur, along with hypotension (31, 177). While sensitivity to the major determinant can cause an anaphylactic reaction, sensitivity to the minor determinants are more closely associated with that allergic manifestation (135). This may be explained by the high binding affinity of the minor determinant to IgE. With exposure to the major determinant, IgG is also produced, along with IgE, and may compete with IgG for binding to the antigen. Minor determinants do not elicit IgG and therefore there is no competition for antigen binding (135, 136).
Type II reactions are cytotoxic reactions that can result from exposure to major determinant and are mediated by IgG, reacting with penicillin adsorbed on red cells. Manifestations include a Coombs-positive non-acute hemolytic anemia and usually occur in a small percentage of patients receiving increased doses in intravenous penicillin for a prolonged period of time (192). The anemia is reversible upon drug discontinuation.
A Type III hypersensitivity to penicillin can result due to circulating antigen-antibody complexes that can deposit in the skin, kidneys, and blood vessels and cause tissue damage through activation of complement. This type of reaction is usually due to IgG or IgM antibodies, though IgE may play a role in enhancing complex deposition (180). A serum sickness-like syndrome can occur 1-3 weeks after the start of penicillin therapy or even after drug discontinuation and can manifest as rash, fever, arthralgia, and lymphadenopathy (210). The syndrome will diminish when the drug is completely cleared from the body.
Delayed hypersensitivity, or Type IV, reactions can also occur with exposure to penicillin. Lymphocytes and macrophages are believed to mediate these reactions, which can manifest a number of ways. Contact dermatitis can occur secondary to skin exposure. Acute interstitial nephritis can occur with any penicillin but is most commonly associated with methicillin and it is believed to be caused by a Type IV reaction. Renal insufficiency can occur, along with hematuria, eosinophilia, eosinophiluria, and proteinuria. This effect is usually reversible upon drug discontinuation (139).
Risk Factors
Though allergy can occur at any age, patients between 20-49 years are at increased risk for anaphylaxis (113). Reactions may be more frequent and severe with parenteral formulations of drug. In addition, it is believed that as many as 85% of patients who reacted to penicillin may not react upon a second exposure if the time interval from the last exposure is prolonged (110, 210). It may, however, be difficult to discern the 15% of patient who will indeed have an allergic reaction upon rechallenge without skin testing. Traditionally, atopic individuals were believed to be predisposed to development of a penicillin allergy. The data suggests, however, that there is no relationship (98). Family history of allergy is also not a risk factor.
Diagnosis
There are many indications where a penicillin is a drug of choice or the drug of choice. Patients with a label of “penicillin allergy” will have an alternative therapy prescribed or will need to be desensitized to the agent. Alternative therapies can be less effective (e.g. enterococcal endocarditis) and desensitization can be a time-consuming process. Therefore, accurate diagnosis is important. Two methods of diagnosis include patient history and skin testing.
A detailed history about the allergic reaction is important is discerning between a true allergy and a simple gastrointestinal (GI) intolerance. Nearly 20% of patients with a penicillin allergy label described symptoms of a GI intolerance when a detailed history was obtained (185). Those patients could potentially receive a penicillin if necessary, despite the allergy label. Patient histories can be unreliable, however, and some may have been too young to fully remember the reaction. Reliance on history alone can result in overdiagnosis of allergy.
Skin testing for allergy may also be performed and can be used to detect propensity for a Type I reaction. Positive skin tests have been observed in 7-19% of patients with a positive history for allergy (48, 87, 211). Approximately 0.02-0.03 mL is injected intradermally and wheal size is measured 10-20 minutes after injection. A positive test is considered a wheal size of3-5mm greater than a saline control at 10-20 minutes. The major determinant, benzylpenicilloyl poly-L-lysine is commercially available as Pre-Pen® at a concentration of 10-6 M. Alone, this will detect 90% of allergic patients. To increase this percentage, the minor determinant mixture (MDM) must also be used, as up to 10% of patients react only to these determinants (87,242), and patients sensitive to the MDM are at highest risk of a Type I reaction (136). Even the combination of the major determinant mixture and benzylpenicillin (a minor determinant) can result in false-negatives in 3% of patients (98). In fact, it may be that side-chain specific reagents are necessary to truly exclude the possibility of allergy in patients with a clinical history (211).
There are several disadvantages and limitations to routine skin testing of all patients with a history of penicillin allergy. First, the MDM must be compounded freshly, as a commercial preparation is not available (189), which can be time-consuming and costly. Second, skin testing can be associated with precipitation of an anaphylactic reaction in sensitized individuals, however this is rare and may be avoided by performing a scratch test and observing for a wheal and flare reaction. Recent data has suggested that the likelihood of sensitization by skin testing is small (175). Third, skin testing does not identify patients at risk for Type II-IV reactions, though these are generally not immediately life-threatening effects in the way anaphylaxis is. Lastly, a negative skin test is only valid for 48 hours prior to administration of the penicillin. In patients where an acceptable therapeutic alternative is available, such a substitution may be more appropriate that skin testing. Skin testing would be an alternative in patients with a positive history of an allergy and with an infection that a penicillin would be a drug of choice. If a patient has a positive skin test there is a 67% chance of an allergic reaction occurring upon penicillin exposure (37), therefore a therapeutic alternative should be used or desensitization to the penicillin compound should be instituted before treatment with the drug. In patients with a positive history of penicillin allergy with a negative skin test, penicillin use appears to be safe (145), but caution is recommended.
Desensitization
In instances such as Enterococcal endocarditis, neurosyphilis, and in infections with organisms resistant to other antibiotics, desensitization should be considered in a patient with a likelihood of a Type I allergic reaction occurring (desensitization is not effective in preventing Type II-IV reactions). A protocol of administration of gradually increasing doses of the agent every 15 minutes can increase the threshold of IgE induced mast cell degranulation (162). The procedure should be continuously supervised (intensive care setting preferred) and epinephrine should be available. Intravenous, subcutaneous, or oral routes may be used for the procedure. An advantage to the oral route is that it is shorter and can possibly be safer, though in one study 5 of 25 patients receiving oral penicillin desensitization acutely developed urticaria, pruritus, and angioedema (220). Once the desensitization protocol has been completed, treatment doses may be initiated. Interruption of penicillin treatment by > 48 hours is an indication for repeat desensitization (210).
Cross-Reactivity with Other Beta-Lactams and Related Compounds
There is a concern over the potential for allergy to other beta-lactam compounds, such as cephalosporins, aztreonam, and the carbapenems, in patients allergic to penicillin. Estimates of cross-reactivity with cephalosporins range from 2-10% (182, 195), though there is data to suggest that the incidence is much lower (6). No major or minor determinants exist with cephalosporins, which could account for the low cross-reaction potential. Cross reaction with the carbapenems may also occur, however the monobactams (aztreonam) appear the have a low propensity for eliciting an immune response and have not shown a cross-reaction with penicillin antibodies when tested in vitro (1). The bulky side chain, rather than the beta-lactam ring may be the site of immunologic reactivity. The in vitro studies (1, 201) also demonstrated that cross-reaction between aztreonam and ceftazidime occurred, which is expected since the two compounds have identical side chains. Though the risk of cross-reactivity appears to be low, in patients with a history of severe allergy it may be prudent to avoid the use of cephalosporins as good therapeutic alternatives are available.
The potential for a cross-reaction with penicillamine has also been explored, as penicillamine is a metabolite of penicillin degradation. A study examined 40 patients with a positive history of penicillin allergy. Sixteen patients skin tested positive for sensitivity to penicillin only and 1 patient had a positive penicillamine skin test (21). This data suggests that the incidence of cross-reaction is low, but that penicillamine should be administered with caution to these patients.
AdvERSE EFFECTS
The penicillins are associated with several adverse effects. These adverse effects will be discussed according to body system affected.
Gastrointestinal Effects
Perhaps the most common adverse reaction to orally administered penicillins is gastrointestinal effects. Diarrhea can occur in up to 20% and 5% of patients receiving oral ampicillin and amoxicillin, respectively. The incidence may be increased to up to 40% in children. Other effects, such as nausea, vomiting, and epigastric distress may also occur. Diarrhea has also been reported in patients receiving intravenous penicillins (approximately 3%).
Antibiotic-associated pseudomembranous colitis caused by Clostridium difficile, may occur during or immediately after therapy with a penicillin due to changes in normal bowel flora from the broad spectrum coverage and overgrowth of this organism. While Clostridium difficile is “sensitive” to ampicillin and amoxicillin, it produces spore form, which survives. In the scenario of diarrhea associated with presence of Clostridium difficile and depending upon the severity of illness, appropriate treatment with metronidazole or oral vancomycin should be considered.
Skin Effects
Rash may occur with administration of any penicillin. Patients are over twice as likely to develop a rash while receiving ampicillin or amoxicillin (5-10%) versus the other penicillins (2%) (138). The ampicillin rash is maculopapular and is often self-limited. Patients who have infectious mononucleosis, cytomegalovirus infection, chronic lymphocytic leukemia, or are on concurrent allopurinol are at increased risk of development of such a rash.
Hematologic Effects
Neutropenia can occur with administration of any penicillin at an estimated incidence of 3-8%. Risk factors include high doses for a prolonged (> 10 day) period of time (166, 176) and hepatic impairment (212). The mechanism may be due to immune complex deposition on the neutrophil cell membranes (198). Patients should be monitored for this adverse effect if prolonged treatment courses are used.
Inhibition of platelet aggregation can occur due to alterations in adenosine diphosphate responses, particularly with ticarcillin and carbenicillin. Prolonged bleeding times can result, along with actual bleeding (2, 4, 82, 226). Patients with thrombocytopenia and/or azotemia appear to be at increased risk. Increased bleeding times were seen in a study of 156 patients receiving ticarcillin (73%), piperacillin (43%), mezlocillin (25%), or cefotaxime (17%). Though some patients were receiving chemotherapy, which could confound results, the trend remained after those patients were removed from the analysis. Bleeding occurred in 34%, 17%, and 2% of patients receiving ticarcillin, piperacillin, and mezlocillin, respectively (82). This effect generally reverses upon drug discontinuation.
CNS Effects
Increased doses and resultant serum concentrations of penicillin G have been associated with encephalopathy, particularly in patients with severe renal impairment (30). Seizures can also be induced with elevated CSF concentrations of any penicillin (208). Predisposing factors include renal impairment, a history of a seizure disorder, meningitis, or intraventricular antibiotic administration (15). If neurologic symptoms develop, the dose of penicillin should be reduced or discontinued. If seizures develop, benzodiazepines may be effective as treatment.
Metabolic Effects
Hypokalemia has been reported with the penicillins (39), possibly due to effects on renal tubules and subsequent potassium loss. This effect is more common with the carboxypenicillins. Hyperkalemia can result from use of penicillin G potassium, and reports of death have occurred (240). Hypernatremia may also occur with the carboxypenicillins due to the increased sodium content in their formulations. Patients with renal impairment should be monitored for potential electrolyte disturbances.
Hepatic Effects
Transient increases in transaminases can occur. Hepatitis or cholestasis can occur with high dose oxacillin and is generally reversible upon drug discontinuation (38).
Thrombophlebitis
Intravenous administration of penicillin G, nafcillin, oxacillin, and methicillin can cause thrombophlebitis. Tissue necrosis can occur with extravasation of nafcillin. If extravasation occurs, hyaluronidase can be used as a local antidote at the site of injury.
Jarisch-Herxheimer Reaction
This reaction occurs in patients being treated with a penicillin (usually penicillin G) for a spirochetal infection (usually syphilis, but can include leptospirosis, Lyme disease, and others) and is a result of release of pyrogens from infecting organisms (268). In patients with syphilis, the incidence is 50% in primary syphilis and rises to 75% in patients with secondary syphilis. The reaction usually begins within 2 hours of initiating syphilis treatment and it consists of fever, chills, sweating, tachycardia, hyperventilation, flushing, and myalgia. The duration is about 1 day and it can be treated with aspirin or prednisone (238).
Miscellaneous
When procaine penicillin G is used intramuscularly, <1% of patients will experience a procaine reaction consisting of dizziness, auditory, visual, and/or taste disturbances, neuromuscular twitching, and a fear of imminent death. This reaction has been associated with doses of 4.8 million units and typically lasts up to 10 minutes (99).
DRUG INTERACTIONS
The penicillins are associated with relatively few drug interactions as compared to other drugs, such as some quinolones and protease inhibitors. Notable interactions are listed below.
Aminoglycosides
Inactivation of the aminoglycosides by the penicillins has been documented in vitro (184, 193) and can particularly be a problem if the penicillin and aminoglycoside are mixed in the same infusion solution and are allowed to sit for 30 minutes or greater. Clinically, this interaction can occur in patients with severe renal impairment where drug elimination and serum concentrations are prolonged, increasing the time that the drugs are in contact with one another (28, 79, 103). It appears that amikacin is the most stable aminoglycoside to penicillin-induced inactivation (120), therefore this aminoglycoside may be preferred in patients with end-stage renal disease who require a combination of a penicillin and aminoglycoside for treatment.
Probenecid
Probenecid competitively inhibits renal tubular secretion of penicillins and therefore increases serum concentrations of the penicillins (91, 252). Studies with piperacillin demonstrated that probenecid increases the peak concentration by 30% and decreases the volume of distribution by 20% (232). This interaction has been used clinically in patients receiving procaine penicillin G for treatment of gonorrhea to increase the serum concentrations of the penicillin.
Mezlocillin and Oxacillin
Oxacillin clearance was decreased by 38% when administered concomitantly with mezlocillin (121), therefore it is recommended to decrease the dose of oxacillin by 50% in severely renally impaired patients receiving this combination.
Penicillin VK and Neomycin
The absorption of penicillin VK is decreased by nearly 50% when administered with neomycin (49). Concomitant use of these drugs should be avoided.
CLINICAL INDICATIONS
The penicillins are utilized for treatment of many different indications.
Bone and Joint Infections
The most common pathogen causing infectious arthritis is Staphylococcus aureus. Other causative organisms include Neisseria gonorrhoeae, Streptococci, and gram-negative bacilli. It is recommended that empiric therapy be based upon synovial fluid Gram stain results, patient age, and sexual activity (213, 214). A penicillinase-resistant penicillin (e.g. nafcillin 2g IV q6h) can be used to treat a Staphylococcal arthritis, however if Neisseria gonorrhoeae is suspected, ceftriaxone or other third generation cephalosporin would be recommended. Streptococcal arthritis does not respond well to the penicillinase-resistant penicillins, therefore penicillin G (2mu IV q4h) or clindamycin should be used (213, 214). Length of therapy ranges from 1-4 weeks, with the longer duration for Staphylococcal disease.
Osteomyelitis may be caused by a number of different organisms, including Staphylococcus aureus (most common), gram-negative rods, group A streptococci, Pseudomonas aeruginosa, and anaerobes (particularly with direct extension osteomyelitis). Penicillins are recommended as treatments of choice for several types of osteomyelitis, including penicillin G (4 mu q6h) for penicillin-sensitive Staphylococcus aureus, nafcillin or oxacillin (2g q6h) for penicillin-resistant Staphylococcus aureus, and penicillin G (4 mu q6h) for streptococcal infection (137). Duration of therapy should be 4-6 weeks. Children with Staphylococcal osteomyelitis have been treated successfully with oral antibiotics and may be switched to oral therapy (with dicloxacillin or cephalexin) after two weeks of a positive response to intravenous therapy (230). For Staphylococcal osteomyelitis, rifampin may be used in combination with the penicillin to enhance the antimicrobial response (171).
Central Nervous System Infections
Acute bacterial meningitis is caused by a number of different organisms, usually depending upon the age of the patient. In young adults and children, Neisseria meningitidis is a common pathogen for which intravenous penicillin G is the drug of choice. Reduced susceptibility (MICs of 0.1-1.0 µg/mL), however, has been reported in certain areas (119, 243, 263), but penicillin G may still be effective against these organisms (119).
Another common pathogen causing meningitis is Streptococcus pneumoniae. Traditionally, intravenous penicillin G or ampicillin have been drugs of choice for penicillin-susceptible strains. Strains with intermediate resistance (MIC 0.1-1.0 µg/mL) and high-level resistance (MIC 1.0 µg/mL) are becoming more common and CSF fluid levels achieved with penicillin G may not exceed the MIC or MBC of these organisms (187). In other body sites of infection, penicillin-resistance to the pneumococcus can be overcome by increasing the penicillin dose, however in meningitis, potential neurotoxicity may result. Empirically, vancomycin plus a cephalosporin is recommended as treatment for a gram-positive cocci meningitis or a pneumococcal meningitis until susceptibility to penicillin G is determined (187).
Haemophilus influenzae produces beta-lactamase in approximately 32% of cases of acute meningitis where it is the causative pathogen (253). The utility of the penicillins is therefore limited in these infections and other alternatives, such as the third generation cephalosporins should be chosen for treatment empirically. If beta-lactamase negative, therapy can be changed to ampicillin.
Other pathogens that can cause meningitis for which penicillin G or ampicillin are drugs of choice include Listeria monocytogenes and Streptococcus agalactiae. When treating Listeria meningitis, gentamicin is often used in combination with ampicillin because of in vitro synergy, though adequate evidence of this in humans has not been demonstrated (194).
Brain abscesses may be caused by streptococci, microaerophilic streptococci (Streptococcus milleri), or anaerobes, such as Bacteroides sp, as well as other organisms. High dose penicillin G (4mu IV q4h) in combination with metronidazole if often used empirically for treatment (61, 262) for at least 4-6 weeks. Penicillin G’s penetration into brain abscess fluid is acceptable, however degradation of penicillin G may occur due to enzymes present in the abscess (60, 265).
Endocarditis
Endocarditis is a serious infection of the endocardial surface of the heart. The most common organisms causing endocarditis include viridans Streptococci, Enterococcus, and Staphylococcus sp. Intravenous penicillin G is the drug of choice for treatment of viridans Streptococci and Streptococcus bovis endocarditis. Several regimens can be used for treatment of organisms with an MIC of 0.1 µg/mL, including a 4 week course of penicillin G 10-20 mu/day continuously or in 6 divided doses (122, 148) or a 4 week course of penicillin G plus a 2 week course of an aminoglycoside (streptomycin or gentamicin). A two-week course of the combination of penicillin G at the above doses plus an aminoglycoside may also be used and there is data using PPG 1.2mu IM q6h with streptomycin successfully for treatment for two weeks in uncomplicated cases (259, 260). In patients with organisms with MICs between 0.1 µg/mL and 0.5 µg/mL, or having nutritionally-deficient streptococci, the combination of 4 weeks of penicillin G at the higher dose with a 2 week course of an aminoglycoside is recommended (26), as animal models suggest that the combination is superior to penicillin alone in reducing nutritionally-deficient streptococci colony counts (107).
Enterococcal infections should always be treated with a combination of a penicillin plus an aminoglycoside, as neither agent alone is bactericidal against this organism and the combination is synergistic (158, 250). A 4-6 week course of intravenous penicillin G (20-30 mu/day continuously or in 6 divided doses) or ampicillin (12g/day continuously or in 6 divided doses) plus gentamicin or streptomycin is the recommended treatment regimen (26).
To appropriately treat Staphylococcal endocarditis, it must be determined whether prosthetic material is involved and if the organism is methicillin-susceptible. If methicillin resistant, vancomycin with rifampin and gentamicin should be used. For those patients with methicillin susceptible Staphylococci without the presence of prosthetic material, an antistaphylococcal penicillin (intravenous nafcillin or oxacillin) can be used. The dosage is 1.5-2g every 4 hours for 4-6 weeks. Gentamicin may be added for the first 3-5 days of therapy.
If prosthetic material is involved, the causative organism is more likely to be a coagulase-negative staphylococcus (usually methicillin-resistant). Treatment is more prolonged (6 weeks), gentamicin is utilized for the first 2 weeks of the regimen, and rifampin 300mg every 8h orally is used for the entire treatment course. If methicillin susceptible, nafcillin or oxacillin can be used; if methicillin resistant, vancomycin must be used (26).
Penicillins are often used for prophylaxis of infective endocarditis in certain at-risk patients (e.g. prosthetic valve in place or congenital heart anomaly) undergoing dental procedures/surgery, or minor gastrointestinal or genitourinary procedures. The prophylaxis is believed to treat the bacteremia that occurs during these procedures which could cause endocarditis. While no prospective study has proved the effectiveness of such prophylaxis, oral amoxicillin 3.0 g given 1 hour prior to the procedure and 1.5g 6 hours later had traditionally been a standard regimen recommended (72). A 2.0 g dose demonstrated adequate serum concentrations in a normal volunteer study and may result in decreased gastrointestinal adverse effects (55) and recent recommendations therefore include an initial 2.0 g dose with no follow-up antibiotic dose (54). In penicillin-allergic patients, clindamycin, cefadroxil, or azithromycin may be substituted.
Intra-Abdominal Infection
Infections in the abdomen are often caused by mixed flora, including anaerobes and facultative aerobes. Bacteroides fragilis must be adequately covered empirically and is typically resistant to the penicillins except for beta-lactam/beta-lactamase inhibitor combinations. It is recommended that mild to moderate community-acquired infections a combination of beta-lactam/beta-lactamase inhibitor or a cephalosporin with activity against Bacteroides fragilis be utilized. Imipenem monotherapy or combinations of aztreonam,metronidazole, and aminoglycoside may be used for severe infections (33).
Obstetrics and Gynecological Infections
Penicillin has been studied in women as prophylaxis for infectious complications of premature rupture of the membranes. Patients received either intravenous penicillin G 1mu every 4 hours with oral penicillin VK as followup or placebo. Significantly fewer infections occurred in the patients receiving penicillin (78).
Penicillin and ampicillin have also been studied as prophylaxis of group B streptococcal infection in infants of mothers with birth canal colonization when administered intrapartum. Bactericidal concentrations of ampicillin are achieved in the amniotic fluid within 5 minutes of a 2g infusion (29). A meta-analysis demonstrated that there appears to be a benefit of such prophylaxis, but appropriate timing of therapy and methods to determine vaginal colonization are not yet known (3). Oral ampicillin has also been studied (1000mg every 8 hours for 7 days) with positive results (163). In women who are colonized with group B streptococci at weeks 35 to 36 of the pregnancy, the CDC recommends intrapartum antibiotic use, with penicillin G as the drug of choice at a dose of 5 million units IV, then 2.5 million units every 4 hours until delivery (9).
Postpartum endomyometritis, often caused by anaerobes, can be effectively treated with ampicillin or mezlocillin, unless the causative organism is Bacteroides fragilis. Response rates of 80-90% were seen in one prospective study of these agents (217).
Respiratory Tract Infections
Pharyngitis is commonly caused by Streptococcus pyogenes and should be treated in order to prevent rheumatic fever and complications such as sinusitis and otitis media (101). Penicillin is treatment of choice since it is cost-effective, has a narrow spectrum of activity, and resistance is not currently a widespread problem (56). Adults can be treated with oral penicillin VK 250-500mg four times daily for 10 days or 500 mg twice daily. Once a day penicillin is not effective (90). Alternatively, benzathine penicillin can be utilized in patients if compliance is considered to be a problem (63).
Acute otitis media, an infection of the middle ear, commonly occurs in children and can be caused by a number of organisms, including S. pneumoniae (40%), H. influenzae (30%), and M. catarrhalis (10%). Treatment is complicated by the fact that H. influenzae and M. catarrhalis produce beta-lactamase 30-90% of the time and culture of ear fluid by needle aspiration is not often done, therefore a culture and sensitivity report is rare. Amoxicillin is considered a drug of choice for treatment of this infection, particularly in children with their first episode of otitis media, because there is an increased likelihood of response to this agent due to activity against the likely infecting organisms and the fact that the cost of the agent is relatively low. Since amoxicillin would not be effective against beta-lactamase producing organisms, patients should be monitored for improvement in signs and symptoms of infection within 48-72 hours. If treatment failure occurs, alternative therapy with activity against beta-lactamase producing organism, such as the combination of amoxicillin-clavulanic acid or a cephalosporin should be instituted.
Sinusitis caused by S. pneumoniae and beta-lactamase producing H. influenzae. If there is suspicion of penicillin-resistant pneumococcus, the dose of amoxicillin should be increased to a maximum of 3g/day to attempt to overcome this resistance. H. influenzae has a high prevalence of resistance to the penicillins due to beta-lactamase production. Use of penicillins should be limited to penicillin/beta-lactamase inhibitor combinations.
Treatment of acute exacerbation of chronic bronchitis can be accomplished with amoxicillin/clavulanic acid or a cephalosporin, as beta-lactamase producing organisms are prevalent. While more expensive, these therapies may be more cost-effective than amoxicillin (65).
Pneumonia can be divided into community-acquired pneumonia (CAP) and nosocomial pneumonia. Community-acquired pneumonia is often treated empirically to cover the most likely organisms, including Streptococcus pneumoniae, Hemophilus influenzae, and atypical pneumonia (Mycoplasma pneumoniae and Legionella pneumophila) (17). The penicillins that was included in recent guidelines for empiric oral treatment of CAP include high-dose amoxicillin (1g po q8h) or amoxicillin/clavulanate 875 mg po twice daily (256, 169). Other choices include cefuroxime or cefpodoxime, or a macrolide or doxycycline) for uncomplicated pneumonia. Some data suggests that azithromycin is more effective clinically and radiologically than intravenous penicillin G for suspected pneumococcal community-acquired pneumonia (34) perhaps due to its activity against atypical pathogens, however optimal therapy in patients with pneumococcal bacteremia is not known. In hospitalized patients, second or third generation cephalosporins or beta lactam/beta-lactamase inhibitor combinations are among the recommended agents (16, 17, 36, 169).
In patients with documented pneumococcal pneumonia that requires hospitalization, intravenous penicillin G may be used for 7-14 days (35). Nosocomial pneumonia is often due to gram-negative rods and treatment with an extended spectrum penicillin plus an aminoglycoside may be used in certain circumstances. Resistance patterns may vary between institutions, therefore treatment strategies should be individualized.
Sexually Transmitted Diseases
Neisseria gonorrhoeae, the causative organism of gonorrhea, was at one time universally susceptible to penicillin. Now, penicillinase-producing gonococci are prevalent world-wide (207). Because of difficulties in determining susceptibility and the need to have a quick and effective method of treatment available, ceftriaxone,cefixime, or an oral quinolone are now the recommended treatments.
Penicillin G is considered the drug of choice for treatment of syphilis, caused by Treponema pallidum. In patients with primary or secondary syphilis, BPG 2.4 mu IM q week for 2-3 weeks is an effective form of therapy. Oral amoxicillin 3g bid in combination with probenecid 1g, for 14 days is another alternative (161). In patients with tertiary or neurosyphilis, or in patients with HIV infection, penicillin G 2-4 mu IV q4h for 10 days should be utilized. Penicillin is most effective against T. pallidum if there are serum concentrations > 0.03 µg/mL for at least 7 days (114). If serum concentrations fall to below the MIC for 18-24 hours, spirochetal regrowth will occur (74). There is concern that BPG does not achieve adequate concentrations in the CSF to eradicate T. pallidum, therefore the CNS would not be treated appropriately (144, 239). It may be, however, that the treponemal burden is low early in the disease process in immunocompetent patients and treatment with BPG may be sufficient (114, 271). Some clinicians may wish to use more aggressive therapy, however. Patients with HIV infection and early syphilis may fail treatment with BPG and develop neurosyphilis (96) therefore these patients should be treated as if they had neurosyphilis, particularly in the later stages of HIV infection. In patients with neurosyphilis, failure of high-dose penicillin may occur (96).
Infants with congenital syphilis may be treated with either intravenous penicillin G or PPG. The data suggests, however that CSF concentrations achieved with intravenous penicillin G are significantly higher than that achieved with PPG (12), therefore intravenous penicillin G may be preferred.
Skin and Soft Tissue Infections
Penicillins are commonly used are treatment for skin and soft tissue infections. Impetigo is commonly caused by either group A streptococci orStaphylococcus aureus and can occasionally result in acute glomerulonephritis. BPG (1.2 mu in adults), penicillin VK, or amoxicillin have been used as treatment, but treatment failure may occur if the causative organism is Staphylococcus aureus (62). Use of a penicillinase-resistant oral penicillin (e.g. dicloxacillin) is an effective alternative (27). Bullous impetigo, usually caused by Staphylococcus aureus, may be treated with an oral penicillinase-resistant penicillin. Staphylococcal scalded skin syndrome should be treated with an intravenous penicillinase-resistant penicillin.
Erysipelas,commonly caused by group A streptococci, is a type of superficial cellulitis which may be treated successfully with oral penicillin VK (250-500mg q6h) (27). If the infection is more severe, intravenous penicillin G may be used. Cellulitis is a more extensive infection, which may be due to a number of different streptococci or Staphylococcus aureus. A penicillinase-resistant penicillin should be used empirically, either orally or intravenously, depending upon the severity of infection.
More severe skin infections, including necrotizing fasciitis, can be caused by group A streptococcus (streptococcal gangrene), along with other organisms, such as anaerobes and gram-negative rods. In skin infections caused by streptococcus, aggressive therapy with penicillin G has been utilized yet is still associated with morbidity and mortality (27). It is thought that the high organism inoculum seen in these infections is indicative of an increased number of organisms that are not actively replicating, against which penicillin is less effective (224). There is experimental data to suggest that penicillin in combination with clindamycin may be more effective, since clindamycin’s mechanism of action is cell cycle independent (223, 224).
Gas gangrene is a complication of a surgical or traumatic wound that can result in severe pain, skin discoloration, and edema. Clostridium perfringens and other Clostridial species are the common causative organisms. While surgical debridement is a cornerstone of therapy, antibiotic treatment is essential. The drug of choice is penicillin G intravenously 10-24 mu daily in divided doses (57). Decreased susceptibility of Clostridium perfringens has been seen in vitro however (151), and some animal model studies have demonstrated poorer results in the penicillin treated animals versus animals that received other antibiotics such as metronidazole or clindamycin (222). The clinical significance of these findings is unclear, but some clinicians are using combinations of penicillin G and another agent for treatment of this serious infection.
Naturally occurring cutaneous anthrax, caused by Bacillus anthracis spores, can occur in patients exposed to wool (128). Intravenous penicillin G (1MU q 4-6 hours) has been demonstrated to effectively result in negative cultures of previously positive blister fluid (196) and is considered the treatment of choice.
Oral penicillin VK is often used in patients after receiving an animal bite, particularly a cat bite. Antimicrobial prophylaxis is often indicated after such bites as the infection rate is high and is often caused by Pasteurella multocida (143). Penicillin VK’s coverage against this organism is good and a dosage of 250-500 mg every 6 hours for 10 days is often utilized. The oral flora of a cat or dog can also include Staphylococcus aureus and anaerobes; therefore some clinicians may choose amoxicillin/clavulanic acid to cover other possible organisms adequately. Human oral flora includes Staphylococcus aureus, Eikenella corrodens, and anaerobes, therefore these bites should be treated with amoxicillin/clavulanic acid.
Other traumatic wounds may require antimicrobial prophylaxis, as well. In a study of 599 patients with traumatic wounds of the hands or feet with underlying bone, tendon, or joint lesions, a penicillin G injection was compared with 6 days of oral penicillin VK or no treatment. Patients receiving intravenous penicillin G were significantly less likely to develop infection versus no treatment and patients receiving oral therapy had more gastrointestinal complaints. A single dose of penicillin G may be a useful alternative as prophylaxis in these patients (146).
Urinary Tract Infections
Treatment of acute pyelonephritis depends upon urine gram-stain and culture results. Amoxicillin may be used for treatment of enterococcal infection, but cephalosporins are probably a better choice for coverage of Staphylococcus (e.g. Staphylococcus saprophyticus). In the past, ampicillin and amoxicillin were drugs of choice for treatment ofE. coli infection, however it is reported that 25-35% of isolates are now resistant (112). If use of a penicillin compound is desired, a combination beta-lactam/beta-lactamase inhibitor can be used (either orally or intravenously).
Lower urinary tract infection can be treated with single dose therapy and amoxicillin 3g is a regimen that has been used (173), but because of increasing resistance, other agents may be more desirable. Some experts suggest that with single dose or a 3-day course of therapy, the beta-lactams are inferior to other agents such as trimethoprim-sulfamethoxazole at early and late follow-up (215).
Anthrax Resulting from Bioterrorism
Penicillin G can be used as an adjunct to therapy for the treatment of inhalational, gastrointestinal, or oropharyngeal anthrax resulting from bioterrorism, however it should not be used as a sole agent (115). Data from an outbreak in late 2001 resulting from intentional anthrax infection noted that the isolates of Bacillus anthraciswere producers of constitutive and inducible beta-lactamases and non betalactamase stable penicillins, and are not likely to be clinically effective (46). Penicillin G continues to be indicated for naturally occurring infections (10). Amoxicillin may also be used as an option for completion of therapy for cutaneous anthrax after treatment with ciprofloxacin or doxycycline and after clinical improvement is documented (115).
Amoxicillin may be used for completion of a 60-day course of anthrax post exposure prophylaxis at a dosage of 500 mg three times daily in patients who have received a fluoroquinolone or doxycycline for 14-21 days and if there are contraindications to use of those drugs, e.g. children and pregnant women (47).
Miscellaneous
In infants and young children with sickle cell disease, oral penicillin VK is recommended as prophylaxis against pneumococcal infection at a dose of 125mg twice daily, starting before the child is 4 months of age. Oral penicillin VK is also recommended for children with functional or anatomical asplenia (7).
Yaws, caused by Treponema pallidum, subspecies pertenue effects persons in tropical or subtropical areas and is characterized by skin and bone lesions. Treatment of choice is a single dose of BPG 1.2 mu for individuals aged 10 or over and 600,000u for younger children. Other treponemal diseases include pinta, which manifests as unsightly skin lesions, and bejel, or endemic syphilis, which causes lesions of skin and bone and is not transmitted by sexual contact. BPG 1.2MU as a single dose is effective for both these diseases (51).
Leptospirosis, transmitted from animals and caused by the spirochete Leptospira interrogans, can manifest as a self-limited form (anicteric) or a more severe form of multi-organ dysfunction (icteric). Mild disease can be treated with oral ampicillin (500-750mg q6h), amoxicillin (500mg q6h), or doxycycline (81). In moderate to severe disease, intravenous penicillin G (1.5mu q6h) has been shown to improve symptoms and shorten hospital stay (251). For prophylaxis, doxycycline, but not penicillin, is recommended (81).
Lyme disease, caused by Borrelia burgdorferi, is treated with amoxicillin 500 mg qid or doxycycline (59). Length of therapy is usually 10 days for mild (localized) infection and 20-30 days for disseminated disease (e.g. arthritis). In patients with objective neurologic abnormalities or high degree atrioventricular heart block, intravenous therapy with penicillin G (5 mu qid), ceftriaxone, or cefotaxime may be used (58, 183, 221). Doses of intravenous penicillin G 3g every 6 hours were studied in patients with neuroborreliosis and were found to attain concentrations of 0.5 µg/mL in the CSF 2-3 hours after dosing. This concentration was determined to be appropriate to sufficiently treat neuroborreliosis (123).
Enteritis necroticans is caused by Clostridium perfringens type C and manifests as severe abdominal pain and bloody stools. Transmural necrosis of the small bowel occurs with this infection and surgery may be indicated. Penicillin G is the drug of choice and alternative antibiotics include metronidazole, clindamycin, or chloramphenicol.
Actinomycosis can manifest as a disease of the oral-cervicofacial area, thoracic area, or as abdominal disease. Actinomyces species are causative organisms, particularlyActinomyces israelii. It is important to treat this infection with high doses for a prolonged period of time due to decreased antimicrobial penetration into scarred areas. Penicillin G intravenously at doses of 18-24 mu daily for 2-6 weeks is the treatment of choice for most actinomycosis infections, followed up with oral penicillin VK or amoxicillin for 6-12 months. In vitro susceptibility of Actinomyces to oxacillin and dicloxacillin is poor; therefore these drugs should be avoided (134).
SuMMARY
Penicillins are important agents in the therapeutic armamentarium of antimicrobial agents, being efficacious with relatively limited toxicity profiles. While penicillins remain the drug of choice for many infections, resistance in certain organisms is increasing; therefore their utility in the treatment of certain infections may change.
REFERENCES
1. Adkinson NF Jr., Swabb EA, Sugerman AA. Immunology of the monobactam aztreonam. Antimicrob Agents Chemother 1984;25:93-7. [PubMed]
2. Alexander DP, Russo ME, Fohrman DE, Rothstein G. Nafcillin-induced platelet dysfunction and bleeding. Antimicrob Agents Chemother 1983;23:59-62. [PubMed]
3. Allen UD, Navas L, King SM. Effectiveness of intrapartum penicillin prophylaxis in preventing early-onset group B streptococcal infection: results of a meta-analysis. Can Med Assoc J 1993;149:1659-65. [PubMed]
4. Andrassy K, Weischedel E, Ritz E, Andrassy T. Bleeding in uremic patients after carbenicillin. Thromb Haemos 1976;36:115-26. [PubMed]
5. Andriole VT. Antibiotic synergy in experimental infection with Pseudomonas. II. The effect of carbenicillin, cephalothin, or cephanone combined with tobramycin or gentamicin. J Infect Dis 1974;129:124-33. [PubMed]
6. Anne S, Reisman RE. Risk of administering cephalosporin antibiotics to patients with histories of penicillin allergy. Ann All Asthma Immunol 1995;74:167-70. [PubMed]
7. Anonymous. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb Mortal Week Rep 1997;46:1-24. [PubMed]
8. Appelbaum PC. Resistance among Streptococcus pneumoniae: implications for drug selection. Clin Infect Dis 2002;34:1613-20. [PubMed]
9. Anonymous. Prevention of Perinatal Group B Streptococcal disease: a public health perspective. Morb Mortal Week Rep 1996;45(RR-7):15-20. [PubMed]
10. Anonymous. The choice of antibacterial drugs. Med Lett Drugs Ther 1996;38:25-34. [PubMed]
11. Ayliffe GA. The progressive intercontinental spread of methicillin-resistant Staphylococcus aureus. Clin Infect Dis 1997;24(Suppl 1):S74-9. [PubMed]
12. Azimi PH, Janner D, Berne P, Fulroth R, Lvoff V, Franklin L, Berman SM. Concentrations of procaine and aqueous penicillin in the cerebrospinal fluid of infants treated for congenital syphilis. J Pediatr 1994;124:649-53. [PubMed]
13. Barber M, Waterworth PM. Antibacterial activity of the penicillins. Br Med J 1962;1:1159-64. [PubMed]
14. Barriere SL. Bacterial resistance to beta-lactams, and its prevention with combination antimicrobial therapy. Pharmacotherapy 1992;12:397-402. [PubMed]
15. Barrons RW, Murray KM, Richey RM. Populations at risk for penicillin-induced seizures. Ann Pharmacother 1992;26:26-9. [PubMed]
16. Bartlett JG, Dowell SF, Mandell LA, File Jr TM, Musher DM, Fine MJ. Practice guidelines for the mamagement of community-acquired pneumonia in adults. Clin Infect is 2000;31:347-82. [PubMed]
17. Bartlett JG, Mundy LM. Community-acquired pneumonia. N Engl J Med 1995;333:1618-24. [PubMed]
18. Barza M, Weinstein L. Pharmacokinetics of the penicillins in man. Clin Pharmacokinet 1976;1:297-308. [PubMed]
19. Basker MJ, Edmondson RA, Sutherland R. Comparative antibacterial activity of azlocillin, mezlocillin, carbenicillin, and ticarcillin and relative stability to beta-lactamases ofPseudomonas aeruginosa and Klebsiella aerogenes. Infection 1979;7:67-73. [PubMed]
20. Beall GN. Penicillins, pp 205-9. In: Saxon A, moderator. Immediate hypersensitivity reactions to beta-lactam antibiotics. Ann Intern Med 1987;107:204-15. [PubMed]
21. Bell CL, Graziano FM. The safety of administration of penicillamine to penicillin-sensitive individuals. Arthritis Rheum 1983;26:801-3. [PubMed]
22. Bennett JV, Gravenkemper CF, Brodie JL, Kirby WMM. Dicloxacillin, a new antibiotic: clinical studies and laboratory comparisons with oxacillin and cloxacillin. Antimicrob Agents Chemother 1964;257-62. [PubMed]
23. Bennett WM, Aronoff GR, Morrison G, Golper TA, Pulliam J, Wolfson M, Singer I. Drug prescribing in renal failure: dosing guidelines for adults. Am J Kidney Dis 1983, 3:155-93. [PubMed]
24. Bergan T, Brodwall EK, Wiik-Larsen E. Mezlocillin pharmacokinetics in patients with normal and impaired renal functions. Antimicrob Agents Chemother 1979;16:651-4. [PubMed]
25. Bergen T, Carlsen IB. Bacterial kill rates of amoxicillin and ampicillin at exponentially diminishing concentrations simulation in vivo conditions. Infection 1980;8:S103-8.
26. Bisno AL, Dismukes WE, Durack DT, Kaplan EL, Karchmer AW, Kaye D, Rahimtoola SH, Sande MA, Sanford JP, Watanakunakorn C, Wilson WR. Antimicrobial treatment of infective endocarditis due to Viridans Streptococci, Enterococci, and Staphylococci. JAMA 1989;261:1471-7. [PubMed]
27. Bisno AL, Stevens DL. Streptococcal infections of skin and soft tissues. N Engl J Med 1996;334:240-5. [PubMed]
28. Blair DC, Duggan DO, Schroeder ET. Inactivation of amikacin and gentamicin by carbenicillin in patients with end-stage renal failure. Antimicrob Agents Chemother 1982;22:376-9. [PubMed]
29. Bloom SL, Cox SM, Bawdon RE, Gilstrap LC. Ampicillin for neonatal group B streptococcal prophylaxis: how rapidly can bactericidal concentrations be achieved? Am J Obstet Gynecol 1996;175:974-6. [PubMed]
30. Bloomer HA, Barton LJ, Maddock RK Jr. Penicillin-induced encephalopathy in uremic patients. JAMA 1967;200:121-3. [PubMed]
31. Bochner BS, Lichtenstein LM. Anaphylaxis. N Engl J Med 1991;324:1785-90. [PubMed]
32. Bodey GP, Ketchel SJ, Rodriguez V. A randomized study of carbenicillin plus cefamandole or tobramycin in the treatment of febrile episodes in cancer patients. Am J Med 1979;67:608-16. [PubMed]
33. Bohnen JM, Solomkin JS, Dellinger EP, Bjornson HS, Page CP. Guidelines for clinical care: anti-infective agents for intra-abdominal infection. A surgical infection society policy statement. Arch Surg 1992;127:83-9. [PubMed]
34. Bohte R, van’t Wout JW, Lobatto S, Blusse van Oud, Alblas A, Boekhout M, Nauta EH, Hermans J, van den Broek PJ. Efficacy and safety of azithromycin versus benzylpenicillin or erythromycin in community-acquired pneumonia. Eur J Clin Microbiol Infect Dis 1995;14:182-7. [PubMed]
35. Brewin A, Arango L, Hadley WK, Murray JF. High-dose penicillin therapy and pneumococcal pneumonia. JAMA 1974;230:409-13. [PubMed]
36. The British Thoracic Society, Public Health Laboratory Service. Community-acquired pneumonia in adults in British hospitals in 1982-1983: a survey of aetiology, mortality, prognostic factors, and outcome. Q J Med 1987;62:195-220. [PubMed]
37. Brown BC, Price EV, Moore MB Jr. Penicilloyl-polylysine as an intradermal test of penicillin sensitivity. JAMA 1964;189:599-604. [PubMed]
38. Bruckstein AH, Attia AA. Oxacillin hepatitis. Am J Med 1978;64:519-22. [PubMed]
39. Brunner FP, Frick PG. Hypokalemia, metabolic acidosis, and hypernatremia due to massive sodium penicillin therapy. Br Med J 1968;4:550-2. [PubMed]
40. Bryan CS, Stone WJ. “Comparably massive” penicillin G therapy in renal failure. Ann Intern Med 1975;82:189-95. [PubMed]
41. Bulger RJ, Lindholm DD, Murray JS, Kirby WM. Effect of uremia on methicillin and oxacillin blood levels. JAMA 1964;187:319-22. [PubMed]
42. Bundtzen RW, Gerber AU, Cohn DL, Craig WA. Postantibiotic suppression of bacterial growth. Rev Infect Dis 1981;3:28-37. [PubMed]
43. Bunke CM, Aronoff GR, Brier ME, Sloan RS, Luft FC. Mezlocillin kinetics in hepatic insufficiency. Clin Pharmacol Ther 1983;33:73-6. [PubMed]
44. Bush K. Characterization of beta-lactamases. Antimicrob Agents Chemother 1989;33:259-76. [PubMed]
45. Bush K, Jacoby GA, Medeiros AA. A functional classification shceme for β-lactamases and its correlation with molecular structures. Antimicrob Agents Chemother 1995;39:1211-33. [PubMed]
46. Center for Disease Control. Update: Investigation of bioterrorism-related anthrax and interim guidelines for exposure management and antimicrobial therapy. October 2001. morb Mortal Weekly Rep 2001;50:909-19. [PubMed]
47. Center for Disease Control. Update: Interim recommendations for antimicrobial prophylaxis for children and breastfeeding mothers and treatment of children with anthrax. Morb Mortal Weekly Rep 2001c;50:1014-6. [PubMed]
48. Chandra RK, Joglekar SA, Tomas E. Penicillin allergy: anti-penicillin IgE antibodies and immediate hypersensitivity skin reactions employing major and minor determinants of penicillin. Arch Dis Child 1980;55:857-60. [PubMed]
49. Cheng SH, White A. Effect of orally administered neomycin on the absorption of penicillin V. N Engl J Med 1962;267:1296-7. [PubMed]
50. Chow JW, Fine MJ, Shlaes DM, Quinn JP, Hooper DC, Johnson MP, Pamphal R, Wagener MM, Miyashiro DK, Yu VL. Enterobacter bacteremia: clinical features and emergence of antibiotic resistance during therapy. Ann Intern Med 1991;115:650-1. [PubMed]
51. Chulay JD. Treponema species (Yaws, Pinta, Bejel). In: Mandell GL, Bennett JE, Dolin R. (eds.) Principles and Practice of Infectious Diseases. Chruchill Livingstone, New York, 1995.
52. Coppens L, Klastersky J. Comparative study of anti-Pseudomonas activity of azlocillin, mezlocillin, piperacillin, and ticarcillin, alone and in combination with an aminoglycoside. Antimicrob Agents Chemother 1979;15:396-9. [PubMed]
53. Dagan R, Klugman KP, Craig WA, Baquero R. Evidence to support the rationale that bacterial eradication in respiratory tract infection is an important aim of antibacterial therapy. J Antimicrob Chemother 2001;47:129-40. [PubMed]
54. Dajani AS, Taubert KA, Wilson W, Bolger AF, Bayer A, Ferrieri P, Gewitz MH, Shulman ST, Nouri S, Newburger JW, Pallasch TJ, Gage TW, Levison ME, Peter G, Zuccaro G Jr. Prevention of bacterial endocarditis. Recommendations by the American Heart Association. JAMA 1997;277:1794-801. [PubMed]
55. Dajani AS, Bawdon RE, Berry MC. Oral amoxicillin as prophylaxis for endocarditis: what is the optimal dose? Clin Infect Dis 1994;18:157-60. [PubMed]
56. Dajani A, Taubert K, Ferrieri P, Peter G, Shulman S. Treatment of acute streptococcal pharyngitis and prevention of rheumatic fever: a statement for health professionals. Pediatrics 1995;96:758-64. [PubMed]
57. Darke SG, King AM, Slack WK. Gas gangrene and related infection: classification. Clinical features and etiology, management and mortality. A report of 88 cases. Br J Surg 1977;64:104-12. [PubMed]
58. Dattwyler RJ, Halperin JJ, Volkman DJ, Luft BJ. Treatment of late Lyme borreliosis - randomized comparison of ceftriaxone and penicillin. Lancet 1988;1:1191-4. [PubMed]
59. Dattwyler RJ, Volkman DJ, Conaty SM, Platkin SP, Luft BJ. Amoxycillin plus probenecid versus doxycycline for treatment of erythema migrans borreliosis. Lancet 1990;336:1404-6. [PubMed]
60. de Louvois J, Hurley R. Inactivation of penicillin by purulent exudates. Br Med J 1977;1:998-1000. [PubMed]
61. de Louvois J. The bacteriology and chemotherapy of brain abscess. J Antimicrob Chemother 1978;4:395-413. [PubMed]
62. Demidovich CW, Wittler RR, Ruff ME, Bass JW, Browning WC. Impetigo: current etiology and comparision of penicillin, erythromycin, and cephalexin therapies. Am J Dis Child 1990;144:1313-5. [PubMed]
63. Denny FW. Current management of streptococcal pharyngitis. J Fam Pract 1992;35:619-20. [PubMed]
64. De Schepper PJ, Tjandramaga TB, Mullie A, Verbesselt R, Van Hecken A, Verberckmoes R, Verbist L. Comparative pharmacokinetics of piperacillin in normals and in patients with renal failure. J Antimicrob Chemother 1982;9(Suppl B):49-57. [PubMed]
65. Destache CJ, Dewan N, O'Donohue WJ, Campbell JC, Angelillo VA. Clinical and economic considerations in the treatment of acute exacerbations of chronic bronchitis. J Antimicrob Chemother 1999;43(Suppl A):107-13. [PubMed]
66. Deswarte RD. Drug allergy-problems and strategies. J Allergy Clin Immunol 1984;74:209-21. [PubMed]
67. Dever LA, Dermody TS. Mechanisms of bacterial resistance to antibiotics. Arch Intern Med 1991;151:886-95. [PubMed]
68. Dickinson GM, Droller DG, Greenman RL, Hoffman TA. Clinical evaluation of piperacillin with observations on penetrability into cerebrospinal fluid. Antimicrob Agents Chemother 1981;20:481-6. [PubMed]
69. Doebbeling BN, Stanley GL, Sheetz CT, Pfaller MA, Houston AK, Annis L, Li N, Wenzel RP. Comparative efficacy of alternative hand-washing agents in reducing nosocomial infections in intensive care units. N Engl J Med 1992;327:88-93. [PubMed]
70. Donowitz GR, Mandell GL. Beta-lactam antibiotics. N Engl J Med 1988;318:419-26. [PubMed]
71. Drusano GL. Role of pharmacokinetics in the outcome of infections. Antimicrob Agents Chemother 1988;32:289-97. [PubMed]
72. Durack DT. Prevention of infective endocarditis. N Engl J Med 1995;332:38-44. [PubMed]
73. Eagle H, Newman E. Renal clearance of penicillin F, G, K, and X in rabbits and man. J Clin Invest 1947;26:903-18. [PubMed]
74. Eagle H. Therapeutic significance of penicillin blood levels. Ann Intern Med. 1948;28:260.
75. Eagle H, Fleischman R, Musselman AD. Effect of schedule of administration on the therapeutic efficacy of penicillin. Am J Med 1950;9:280-99. [PubMed]
76. Ebert SC, Craig WA. Pharmacodynamic properties of antibiotics: application to drug monitoring and dosage regimen design. Infect Control Hosp Epidemiol 1990;11:319-26. [PubMed]
77. Eliopoulos GM, Moellering RC Jr. Azlocillin, mezlocillin, and piperacillin: new broad-spectrum penicillins. Ann Intern Med 1982;97:755-60. [PubMed]
78. Ernest JM, Givner LB. A prospective, randomized, placebo-controlled trial of penicillin in preterm premature rupture of membranes. Am J Obstet Gyn 1994;170:516-21. [PubMed]
79. Ervin FR, Bullock WE Jr, Nuttall CE. Inactivation of gentamicin by penicillins in patients with renal failure. Antimicrob Agents Chemother 1976;9:1004-1011. [PubMed]
80. Evans RS, Pestotnik SL, Burke JP, Gardner RM, Larsen RA, Classen DC. Reducing the duration of prophylactic antibiotic use through computer monitoring of surgical patients. DICP 1990;24:351-4. [PubMed]
81. Farr RW. Leptospirosis. Clin Infect Dis 1995;21:1-8. [PubMed]
82. Fass RJ, Copelan EA, Brandt JT, Moeschberger ML, Ashton JJ. Platelet-mediated bleeding caused by broad-spectrum penicillins. J Infect Dis 1987;155:1242-8. [PubMed]
83. Finland M, Garner C, Wilcox C, Sabath LD. Susceptibility of pneumococci and Haemophilus influenzae to antibacterial agents. Antimicrob Agents Chemother 1976;9:274-87. [PubMed]
84. Fleming A. On the antibacterial action of cultures of a penicillium, with special reference to their use in the isolation of B. influenzae. Br J Exp Pathol 1929;10:226. [PubMed]
85. Fleming, A. History and development of penicillin. In: Penicillin: Its Practical Application. (Fleming, A., ed.) The Blakiston Co., Philadelphia, 1946, pp. 1-33.
86. Fuursted K. Comparative killing activity and postantibiotic effect of streptomycin combined with ampicillin, ciprofloxacin, imipenem, piperacillin, or vancomycin against strains of Streptococcus faecalis and Streptococcus faecium. Chemotherapy 1988;34:229-34. [PubMed]
87. Gadde J, Spence M, Wheeler B, Adkinson NF Jr. Clinical experience with penicillin skin testing in a large inner-city STD clinic. JAMA 1993;270:2456-63. [PubMed]
88. Gerber AU, Craig WA, Brugger HP, Feller C, Vastola AP, Brandel J, Impact of dosing intervals on activity of gentamicin and ticarcillin against Pseudomonas aeruginosa in granulocytopenic mice. J Infect Dis 1983;147:910-7. [PubMed]
89. Gerber AU, Vastola AP, Brandel J, Craig WA. Selection of aminoglycoside-resistant variants of Pseudomonas aeruginosa in an in vivo model. J Infect Dis 1982;146:691-7. [PubMed]
90. Gerber MA, Randolph MF, Demeo K, Feder HM Jr, Kaplan EL. Failure of once-daily penicillin V therapy for streptoccal pharyngitis. Am J Dis Child 1989;143:153-5. [PubMed]
91. Gibaldi M, Schwartz MA. Apparent effect of probenecid on the distribution of penicillin in man. Clin Pharmacol Ther 1968;9:345-9. [PubMed]
92. Giron JA, Meyers BR, Hirschman SZ. Biliary concentration of piperacillin in patients undergoing cholecyctectomy. Antimicrob Agents Chemother 1981;19:309-11. [PubMed]
93. Gold HS, Moellering RC Jr.Antimicrobial drug resistance. N Engl J Med 1996;335:1445-53. [PubMed]
94. Goldmann D, Larson E. Hand-washing and nosocomial infections. N Engl J Med 1992;327:120-2. [PubMed]
95. Goldmann DA, Weinstein RA, Wenzel RP, Tablan OC, Duma RJ, Gaynes RP, Schlosser J, Martone WJ. Strategies to prevent and control the emergence and spread of antimicrobial-resistant microorganisms in hospitals. A challenge to hospital leadership. JAMA 1996;275:234-40. [PubMed]
96. Gordon SM, Eaton ME, George R, Larsen S, Lukehart SA, Kuypers J, Marra CM, Thompson S. The response of symptomatic neurosyphilis to high-dose intravenous penicillin G in patients with human immunodeficiency virus infection. N Engl J Med 1994;331:1469-73. [PubMed]
97. Grasso S, Meinardi G, de Carneri I, Tamassia V. New in vitro model to study the effect of antibiotic concentration and rate of elimination on antibacterial activity. Antimicrob Agents Chemother 1978;13:570-6. [PubMed]
98. Green GR, Rosenblum A. Report of the penicillin study group-American Academy of Allergy. J Allergy Clin Immunol 1971;48:331-43. [PubMed]
99. Green RL, Lewis JE, Kraus SJ, Fredrickson EL. Elevated plasma procaine concentrations after administration of procaine penicillin G. N Engl J Med 1974;291:223-6. [PubMed]
100. Gudmundsson S, Erlendsdottir H, Gottfredsson M, Gudmundsson A. The postantibiotic effect induced by antimicrobial combinations. Scand J Infect Dis 1991;74(Suppl):80-93. [PubMed]
101. Gwaltney JM Jr. Pharyngitis. In: Mandell GL, Bennett JE, Dolin R. (eds.) Principles and Practice of Infectious Diseases. Chruchill Livingstone, New York, 1995.
102. Hackbarth CJ, Chambers HF. Methicillin-resistant staphylococci: genetics and mechanisms of resistance. Antimicrob Agents Chemother 1989;33:991-4. [PubMed]
103. Halstenson CE, Wong MO, Herman CS, Heim-Duthoy KL, Teal MA, Affrime MB, Kelloway JH, Keane WF, Awni WM. Effect of concomitant administration of piperacillin on the dispositions of insepamicin and gentamicin in patients with end-stage renal disease. Antimicrob Agents Chemother 1992;36:1832-6. [PubMed]
104. Hand WL, King-Thompson N, Holman JW. Entry of roxithromycin (RU 965), imipenem, cefotaxime, trimethoprim, and metronidazole into human polymorphonuclear leukocytes. Antimicrob Agents Chemother 1987;31:1553-7. [PubMed]
105. Harder KJ, Nikaido H, Matsuhashi M. Mutants of Escherichia coli that are resistant to certain beta-lactam compounds that lack ompF porin. Antimicrob Agents Chemother 1981;20:549-52. [PubMed]
106. Heikkila A, Erkkola R. Pharmacokinetics of piperacillin during pregnancy. J Antimicrob Chemother 1991;28:419-23. [PubMed]
107. Henry NK, Wilson WR, Roberts RB, Acar JF, Geraci JE. Antimicrobial therapy of experimental endocarditis caused by nutritionally variant viridans group streptococci. Antimicrob Agents Chemother 1986;30:465-7. [PubMed]
108. Hessen MT, Pitsakis PG, Levison ME. Postantibiotic effect of penicillin plus gentamicin versus Enterococcus faecalis in vitro and in vivo. Antimicrob Agents Chemother 1989;33:608-11. [PubMed]
109. Higgins ML, Shockman GD. Procaryotic cell division with respect to wall and membranes. CRC Crit Rev Microbiol 1971;1:29-72. [PubMed]
110. Holgate ST. Penicillin allergy: how to diagnose and when to treat [letter]. Br Med J 1988;296:1213-4. [PubMed]
111. Holm SE, Tornqvist IO, Cars O. Paradoxical effects of antibiotics. Scand J Infect Dis 1991;74(Suppl):113-7. [PubMed]
112. Hooton TM, Stam WE. Management of acute uncomplicated urinary tract infection in adults. Med Clin North Am 1991;75:339-57. [PubMed]
113. Idsoe O, Guthe T, Willcox RR, Weck AL. Nature and extent of penicillin side reactions with particular reference to fatalities from anaphylactic shock. Bull WHO 1968;38:159-88. [PubMed]
114. Idsoe O, Guthe T, Willcox RR. Penicillin in the treatment of syphilis. The experience of three decades. Bull WHO 1972;47(Suppl):1-68. [PubMed]
115. Inglesby TV, O'Toole T, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Friedlander AM, Gerberding J, Hauer J, Hughes J, McDade J, Osterholm MT, Parker G, Perl TM, Russell PK, Tonat K; Working Group on Civilian Biodefense. Consensus statement: Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA 2002;287:2236-52. [PubMed]
116. Jack GW, Richmond MH. A comparative study of eight distinct beta-lactamases synthesized by gram-negative bacteria. J Gen Microbiol 1970;61:43-61. [PubMed]
117. James PA, Lewis DA, Jordens JZ, Cribb J, Dawson SJ, Murray SA. The incidence and epidemiology of beta-lactam resistance in Haemophilus influenzae. J Antimicrob Chemother 1996;37:737-46. [PubMed]
118. Johnson DE, Thompson B, Calia FM. Comparative activities of piperacillin, ceftazidime, and amikacin, alone and in all possible combinations, against experimental Pseudomonas aeruginosa infections in neutropenic rats. Antimicrob Agents Chemother 1985;27:735-9. [PubMed]
119. Jones DM, Sutcliffe EM. Meningococci with reduced susceptibility to penicillin. Lancet 1990;335:863-4. [PubMed]
120. Jorgenson JH, Crawford SA. Selective inactivation of aminoglycosides by newer beta-lactam agents. Curr Ther Res Clin Exp 1982;32:25-35.
121. Kampf D. Effects of mezlocillin on the pharmacokinetics of oxacillin and dicloxacillin. J Antimcrob Chemother 1983;11(Suppl C):25-32. [PubMed]
122. Karchmer AW, Moellering RC Jr, Maki DG, Swartz MN. Single-antibiotic therapy for streptococcal endocarditis. JAMA 1979;241:1801-6. [PubMed]
123. Karlsson M, Hammers S, Nilsson-Ehle I, Malmborg AS, Wretlind B. Concentrations of doxycycline and penicillin G in sera and cerebrospinal fluid of patients treated for neuroborreliosis. Antimicrob Agents Chemother 1996;40:1104-7. [PubMed]
124. Klastersky J, Hensgens C, Meunier-Carpentier F. Comparative effectiveness of combinations of amikacin with penicillin G and amikacin with carbenicillin in gram-negative septicemia: double-blind clinical trial. J Infect Dis 1976;134(Suppl):S433-40. [PubMed]
125. Klaus U, Henninger W, Jacobi P, Wiedemann B. Bacterial elimination and therapeutic effectiveness under different schedules of amoxicillin administration. Chemotherapy 1981;27:200-8. [PubMed]
126. Kubacka RT, Johnstone HE, Tan HS, Reeme PD, Myre SA. Intravenous ampicillin pharmacokinetics in the third trimester of pregnancy. Ther Drug Monitor 1983;5:55-60. [PubMed]
127. Laible G, Spratt BG, Hakenbeck R. Interspecies recombinational events during the evolution of altered PBP 2x genes in penicillin-resistant clinical isolates of Streptococcus pneumoniae. Mol Microbiol 1991;5:1993-2002. [PubMed]
128. LaForce FM. Anthrax. Clin Infect Dis 1994;19:1009-14. [PubMed]
129. Lagast H, Meunier-Carpentier F, Klastersky J. Treatment of gram-negative bacillary septicemia with cefoperazone. Eur J Clin Microbiol 1983;2:554-8. [PubMed]
130. Lau WK, Young LS, Black RE, Winston DJ, Linne SR, Weinstein RJ, Hewitt WL. Comparative efficacy and toxicity of amikacin/carbenicillin versus gentamicin/carbenicillin in leukopenic patients. Am J Med 1977;62:959-66. [PubMed]
131. Lavoie GY, Bergeron MG. Influence of four modes of administration on penetration of aztreonam, cefuroxime, and ampicillin into interstitial fibrin clots and on in vivo efficacy against Haemophilus influenzae. Antimicrob Agents Chemother 1985;28:404-12. [PubMed]
132. Leggett JE, Fantin B, Ebert S, Totsuka K, Vogelman B, Calame W, Mattie H, Craig WA. Comparative antibiotic dose-effect relationship at several dosing intervals in murine pneumonitis and thigh-infection models. J Infect Dis 1989;159:281-92. [PubMed]
133. Lepper MH, Dowling HF. Treatment of pneumococcic meningitis with penicillin compared with penicillin plus aureomycin. Arch Intern Med 1951;88:489-94. [PubMed]
134. Lerner PI. Susceptibility of pathogenic Actinomycetes to antimicrobial compounds. Antimicrob Agents Chemother 1974;5:302-9. [PubMed]
135. Levine BB. Immunologic mechanisms of penicillin allergy. A haptenic model system for the study of allergic diseases in man. N Engl J Med 1966;275:1115-25. [PubMed]
136. Levine BB, Zolov DM. Prediction of penicillin allergy by immunological tests. J Allergy 1969;43:231-44. [PubMed]
137. Lew DP, Waldvogel FA. Osteomyelitis. N Engl J Med 1997;336:999-1007. [PubMed]
138. Lieberman R, Erffmeyer JE, Treadwell G. Drug reactions. In: Lockey RF, Buknatz SC, eds., Principles of Immunology and Allergy. Philadelphia: WB Saunders, 1987:111-37.
139. Linton AL, Clark WF, Driedger AA, Turnbull DI, Lindsay RM. Acute interstitial nephritis due to drugs. Ann Intern Med 1980;93:735. [PubMed]
140. Livermore DM. beta-lactamases in laboratory and clinical resistance. Clin Microbiol Rev 1995;8:557-84. [PubMed]
141. Livermore DM. Clinical significance of beta-lactamase induction and stable derepression in gram-negative rods. Eur J Clin Microbiol 1987;6:439-45. [PubMed]
142. Livingston DH, Wang MT. Continuous infusion of cefazolin is superior to intermittent dosing in decreasing infection after hemorrhagic shock. Am J Surg 1993;165:203-7. [PubMed]
143. Lucas GL, Bartlett DH. Pasteurella multocida infection in the hand. Plast Reconstr Surg. 1981;67:49-53. [PubMed]
144. Lukehart S, Hook EW, Baker-Zander SA, Collier AC, Critchlow CW, Hansfield HH. Invasion of the central nervous system by Treponema pallidum. Implications for diagnosis and therapy. Ann Intern Med 1988;109:855-62. [PubMed]
145. Macy E, Mangal R, Burchette RJ. Penicillin skin testing in advance of need: multiyear follow-up in 568 test result-negative subjects exposed to oral penicillins. J Allergy Clin Immunol 2003;111:1111-5. [PubMed]
146. Madsen MS, Neumann L, Andersen JA. Penicillin prophylaxis in complicated wounds of hands and feet: a randomized, double-blind trial. Injury 1996;27:275-8. [PubMed]
147. Maidhof H, Johannsen L, Labischinski H, Giesbrecht P. Onset of penicillin-induced bacteriolysis in Staphylococci is cell cycle dependent. J Bacteriol 1989;171:2252-7. [PubMed]
148. Malacoff RF, Frank E, Andriole VT. Streptococcal endocarditis (nonenterococcal, non-group A): single vs. combination therapy. JAMA 1979;241:1807-10. [PubMed]
149. Mandell GL, Kaye D, Levison ME, Hook EW. Enterococcal endocarditis. An analysis of 38 patients observed at the New York Hospital-Cornell Medical Center. Arch Intern Med 1970;125:258-64. [PubMed]
150. Marcy SM, Klein JO. The isoxazolyl penicillins: oxacillin, cloxacillin, and dicloxacillin. Med Clin N Amer 1970;54:1127-43. [PubMed]
151. Marrie TJ, Haldane EV, Swantee CA, Kerr EA. Susceptibiltiy of anaerobic bacteria to nine antimicrobial agents and demonstration of decreased susceptibility of Clostridium perfringensto penicillin. Antimicrob Agents Chemother 1981;19:51-5. [PubMed]
152. Martens MG, Faro S, Feldman S, cotton DB, Dorman K, Riddle GD. Pharmacokinetics of the acylureidopenicillins piperacillin and mezlocillin in the postpartum patient. Antimicrob Agents Chemother 1987;31:2015-7. [PubMed]
153. Meyer RD, Liu S. In vitro synergy with ciprofloxacin and selected beta-lactam agents and aminoglycosides against multi-drug resistant Pseudomonas aeruginosa. Diag Microbiol Infect Dis 1988;11:151-7. [PubMed]
154. Meyer KS, Urban C, Eagan JA, Berger BJ, Rahal JJ. Nosocomial outbreak of Klebsiella infection resistant to late-generation cephalosporins. Ann Intern Med 1993;119:353-8. [PubMed]
155. Meyers BR, Srulevitch ES, Sacks HS, Hirschman SZ, Worner TM, Wormser GP, Jacobson J. Pharmacokinetics of mezlocillin in patients with hepatobiliary dysfunction. J Antimicrob Chemother 1986;18:709-13. [PubMed]
156. McEvoy GK. (Ed.) AHFS Drug Information 1997. American Society of Health-System Pharmacists, Bethesda , 1997.
157. Moellering RC Jr, Wennersten C, Weinberg AN. Studies on antbiotic synergism against enterococci: I. Bacteriologic studies. J Lab Clin Med 1971;77:821-8. [PubMed]
158. Moellering RC JR, Weinberg AN. Studies on antibiotic synergism against enterococci. II. Effect of various antibiotics on the uptake of 14C-labelled streptomycin by enterococci. J Clin Invest 1971;50:2580-4. [PubMed]
159. Mordenti JJ, Quintiliani R, Nightingale CH. Combination antibiotic therapy: comparison of constant infusion and intermittent bolus dosing in an experimental animal model. J Antimicrob Chemother 1985;15(Suppl A):313-21. [PubMed]
160. Moreira BM, Daum RS. Antimicrobial resistance in staphylococci. Ped Clin North Amer 1995;42:619-48. [PubMed] (Schreiber JR, Jacobs MR. Antibiotic-resistant pneumococci. Ped Clin North Amer 1995;42:519-37. [PubMed] )
161. Morrison E, Harrison S, Tramont EC. Oral amoxicillin, an alternative treatment of neurosyphilis. Genitourin Med 1985;61:359-62. [PubMed]
162. Naclerio R, Mizrahi EA, Adkinson NF Jr. Immunologic observations during desensitization and maintenance of clinical tolerance to pencillin. J Allergy Clin Immunol 1983;71:294-301. [PubMed]
163. Nadisauskiene R, Bergstrom S, Kilda A. Gynecol Obstetr Invest 1996;41:89-92. [PubMed]
164. Nathwani D, Wood MJ. Penicillins. A current review of their clinical pharmacology and therapeutic use. Drugs 1993;45:866-94. [PubMed]
165. National Committee for Clinical Laboratory Standards (NCCLS). Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard. 6th ed. NCCLS documents M7-A5. Wayne, PA: NCCLS, 2001. [PubMed]
166. Neftel KA, Hauser SP, Muller MR. Inhibition of granulopoiesis in vivo and in vitro by beta-lactam antibiotics. J Infect Dis 1985;152:90-8. [PubMed]
167. Neu HC. Relation of structural properties of beta-lactam antibiotics to antibacterial activity. Am J Med 1985;79(Suppl 2A):2-13. [PubMed]
168. Neu HC. The crisis in antibiotic resistance. Science 1992;257:1064-73. [PubMed]
169. Niederman MS, Mandell LA, Anzueto A, Bass JB, Broughton WA, Campbell GD, Dean N, File T, Fine MJ, Gross PA, Martinez F, Marrie TJ, Plouffe JF, Ramirez J, Sarosi GA, Torres A, Wilson R, Yu VL; American Thoracic Society. Guidelines for the initial management of adults with community acquired pneumonia: diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care 2001;163:1730-54. [PubMed]
170. Nolting A, Dalla Costa T, Rand KH, Derendorf H. Pharmacokinetic-pharmacodynamic modeling of the antibiotic effect of piperacillin in vitro. Pharm Res 1996;13:91-6. [PubMed]
171. Norden CW, Bryant R, Palmer D, Montgomerie JZ, Wheat J. Chronic osteomyelitis caused by Staphylococcus aureus: Controlled clinical trial of nafcillin therapy and nafcillin-rifampin therapy. South Med J 1986;79:947-51. [PubMed]
172. Nordstrom K, Sykes RB. Induction kinetics of beta-lactamase biosynthesis in Pseudomonas aeruginosa. Antimicrob Agents Chemother 1974;6:734-40. [PubMed]
173. Norrby SR. Short-term treatment of uncomplicated lower urinary tract infections in women. Rev Infect Dis 1990;12:458-67. [PubMed]
174. Norrby R. A review of the penetration of antibiotics into CSF and its clinical significance. Scand J Infect Dis 1978;14(Suppl):296-309. [PubMed]
175. Nugent JS, Quinn JM, McGrath CM, Hrncir DE, Boleman WT, Freeman TM. Determination of the incidence of sensitization after penicillin skin testing. Ann Alergy Asthma Immunol 2003;90:398-403. [PubMed]
176. Olaison L, Alestig K. A prospective study of neutropenia induced by high doses of beta-lactam antibiotics. J Antimicrob Chemother 1990;25:449-53. [PubMed]
177. O’Leary MR, Smith MS. Penicillin anaphylaxis. Am J Emerg Med 1986;4:241-7. [PubMed]
178. Orlando PL, Barriere SL, Hindler JA, Frost RW. Serum bactericidal activity from intravenous ciprofloxacin and azlocillin given alone and in combination to healthy subjects. Diag Microbiol Infect Dis 1990;13:93-7. [PubMed]
179. Parker RF, Marsh HC. The action of penicillin on staphylococcus. J Bacteriol 1946;51:181-6. [PubMed]
180. Patterson R, Anderson J. Allergic reactions to drugs and biologic agents. JAMA 1982;248:2637-45. [PubMed]
181. Peterson LR, Gerding DN, Fasching CE. Effects of antibiotic administration on extravascular penetration: cross-over study of cefazolin given by intermittent injection or constant infusion. J Antimicrob Chemother 1981;7:71-9. [PubMed]
182. Petz LD. Immunologic cross-reactivity between penicillins and cephalosporins: a review. J Infect Dis 1978;137(Suppl):74-9. [PubMed]
183. Pfister HW, Preac-Mursic V, Wilske B, Schielke E, Sorgel F, Einhaupl KM. Randomized comparison of ceftriaxone and cefotaxime in Lyme neuroborreliosis. J Infect Dis 1991;163:311-8. [PubMed]
184. Pickering LK, Gearhart P. Effect of time and concentration upon interaction between gentamicin, tobramycin, netilmicin, or amikacin and carbenicillin or ticarcillin. Antimicrob Agents Cehmother 1979;15:592-6. [PubMed]
185. Preston SL, Briceland LL, Lesar TS. Accuracy of penicillin allergy reporting. Am J Hosp Pharm 1994;51:79-84. [PubMed]
186. Price KE, Gourevitch A, Cheney LC. Biological properties of semisynthetic penicillins: structure-activity relationships. Antimicrob Agents Chemother 1966;13:670-698. [PubMed]
187. Quagliarello VJ, Scheld WM. Treatment of bacterial meningitis. N Engl J Med 1997;336:708-716. [PubMed]
188. Rahal JJ Jr. Antibiotic combinations: The clinical relevance of synergy and antagonism. Medicine (Baltimore) 1978;57:179-95. [PubMed]
189. Ressler C, Neag PM, Mendelson LM. A liquid chromatographic study of the minor determinants of penicillin allergy: a stable minor determinant mixture skin test preparation. J Pharm Sci 1985;74:448-54. [PubMed]
190. Rice LB, Willey SH, Papanicolaou GA, Medeiros AA, Eliopoulous GM, Moellering RCJr, Jacoby GA. Outbreak of ceftazidime resistance caused by extended-spectrum beta-lactamases at a Massachusetts chronic care facility. Antimicrob Agents Chemother 1990;34:2193-9. [PubMed]
191. Richmond MH, Sykes RB. The beta-lactamases of gram-negative bacteria and their possible physiological role. Adv Microb Physiol 1973;9:31-88. [PubMed]
192. Ries CA, Rosenbaum TJ, Garratty G, Petz LD, Fudenberg HH. Penicillin-induced immune hemolytic anemia. JAMA 1975;233:432-5. [PubMed]
193. Riff LJ, Jackson GG. Laboratory and clinical conditions for gentamicin inactivation by carbenicillin. Arch Intern Med 1972;130:887-91. [PubMed]
194. Rockowitz J, Tunkel AR. Bacterial meningitis. Practical guidelines and management. Drugs 1995;50:838-53. [PubMed]
195. Rohr ES. Cephalosporins. In: Saxon A, Beall GN, Rohr AS, Adelman DC. Immediate hypersensitivity reactions to beta lactam antibiotics. Ann Intern Med 1987;107:204-15. [PubMed]
196. Ronaghy HA, Azadeh B, Kohout E, Dutz W. Penicillin therapy of human cutaneous anthrax. Curr Ther Res Clin Exp 1972;14:721-5. [PubMed]
197. Roosendaal R, Bakker-Woudenberg IA, van den Berghe JC, Michel MF. Therapeutic efficacy of continuous versus intermittent administration of ceftazidime in an experimentalKlebsiella pneumoniae pneumonia in rats. J Infect Dis 1985;152:373-8. [PubMed]
198. Rouveix B, Lassoued K, Vittecoq D, Regnier B. Neutropenia due to beta-lactamine antibodies. Br Med J 1983;287:1832-4. [PubMed]
199. Saito T, Yamada Y. Excretion in bile and clinical study of T-1220 (piperacillin) in biliary tract diseases. Jpn J Antibiot 1977;30:835-9. [PubMed]
200. Sanders CC, Sanders WE Jr. beta-lactam resistance in gram-negative bacteria: global trends and clinical impact. Clin Infect Dis 1992;15:824-29. [PubMed]
201. Saxon A, Swabb E, Adkinson NF Jr., Investigation into the immunologic cross-reactivity of aztreonam with other beta-lactam antibodies. Am J Med 1985;78:19-26. [PubMed]
202. Scheld WM, Brodeur JP, Sande MA, Alliegro GM. Comparison of cefoperazone with penicillin, ampicillin, gentamicin, and chloramphenicol in the therapy of experimental meningitis. Antimicrob Agents Chemother 1982;22:652-6. [PubMed]
203. Schentag JJ, Smith IL, Swanson DJ, DeAngelis C, Fracasso JE, Vari A, Vance JW. Role for dual individualization with cefmenoxime. Am J Med 1984;77(Suppl 6A):43-50. [PubMed]
204. Schimpff S, Satterlee W, Young VM, Serpick A. Empiric therapy with carbenicillin and gentamicin for febrile patients with cancer and granulocytopenia. N Engl J Med 1971;284:1061-5. [PubMed]
205. Schoth PE, Wolters EC. Penicillin concentrations in serum and CSF during high dose intravenous treatment for neurosyphilis. Neurology 1987;37:1214-6. [PubMed]
206. Schreiber JR, Jacobs MR. Antibiotic-resistant pneumococci. Ped Clin North Amer 1995;42:519-37. [PubMed]
207. Schwarcz SK, Zenilmann JM, Schnell D, Knapp JS, Hook EW, Thompson S, Judson FN, Holmes KK, amd the Gonococcal Isolate Surveillance Project. National surveillance of antimicrobial resistance in Neisseria gonorrhoeae. JAMA 1990;264:1413-7. [PubMed]
208. Seamans KB, Gloor P, Dobell RA, Wyant JD. Penicillin-induced seizures during cardiopulmonary bypass - a clinical and electroencephalographic study. N Engl J Med 1968;278:861-8. [PubMed]
209. Shah PM, Ghahremani M, Gorres FJ, Stille W. Bactericidal activity of antimicrobials in the dynamic kill-curve model. J Drug Dev 1988;1(Suppl 3):35-47. [PubMed]
210. Sher TH. Penicillin hypersensitivity - a review. Pediatr Clin North Amer 1983;30:161-76. [PubMed]
211. Silviu-Dan F, McPhillips S, Warrington RJ. The frequency of skin test reactions to side-chain penicillin determinants. J Allergy Clin Immunol 1993;91:694-701. [PubMed]
212. Singh N, Yu VL, Mieles LA, Wagener MM. Beta-lactam antibiotic-induced leukopenia in severe hepatic dysfunction: risk factors and implications for dosing in patients with liver disease. Am J Med 1993;94:251-6. [PubMed]
213. Smith JW, Piercy EA. Infectious arthritis. Clin Infect Dis 1995;20:225-31. [PubMed]
214. Smith JW, Piercy EA. Infectious arthritis. In: Mandell GL, Bennett JE, Dolin R. (eds.) Principles and Practice of Infectious Diseases. Chruchill Livingstone, New York, 1995.
215. Sobel JD, Kaye D. Urinary tract infections. In: Mandell GL, Bennett JE, Dolin R. (eds.) Principles and Practice of Infectious Diseases. Chruchill Livingstone, New York, 1995.
216. Solh N, Allignet J, Bismuth R, Buret B, Fouace JM. Conjugative transfer of staphylococcal antibiotic resistance markers in the absence of detectable plasmid DNA. Antimicrob Agents Chemother 1986;30:161-9. [PubMed]
217. Sorrell TC, Marshall JR, Yoshimori R, Chow AW. Antimicrobial therapy of postpartum endomyometritis. II. Prospective, randomized trial of mezlocillin vs. ampicillin. Am J Obstet Gynecol 1981;141:246-51. [PubMed]
218. Spivey JM. The postantibiotic effect. Clin Pharm 1992;11:865-75. [PubMed]
219. Spratt BG. Biochemical and genetical approaches to the mechanism of action of penicillin. Philos Trans R Soc Lond 1980;289:27-283. [PubMed]
220. Stark BJ, Earl HS, Gross GN, Lumry WR, goodman EL, Sullivan TJ. Acute and chronic desensitization of penicillin-allergic patients using oral penicillin. J Allergy Clin Immunol 1987;79:523-32. [PubMed]
221. Steere AC, Pachner AR, Malawista SE. Neurologic abnormalities of Lyme disease: Successful treatment with high-dose intravenous penicillin. Ann Intern Med 1983;99:767. [PubMed]
222. Stevens DL, Maier KA, Laine BM, Mitten JE. Comparison of clindamycin, rifampin, tetracycline, metronidazole, and penicillin for efficacy in prevention of experimental gas gangrene due to Clostridium perfringens. J Infect Dis 1987;155:220-8. [PubMed]
223. Stevens DL, Gibbons AE, Bergstrom R, Winn V. The Eagle effect revisited: efficacy of clindamycin, erythromycin, and penicillin in the treatment of streptococcal myositis. J Infect Dis 1988;158:23-8. [PubMed]
224. Stevens DL, Yan S, Bryant AE. Penicillin-binding protein expression at different growth stages determines penicillin efficacy in vitro and in vivo: an explanation for the inoculum effect. J Infect Dis 1993;167:1401-5. [PubMed]
225. Stratton CW, Franke JJ, Weeks LS, Manion FA. Comparison of the bactericidal activity of ciprofloxacin alone and in combination with selected antipseudomonal beta-lactam agents against clinical isolates of Pseudomonas aeruginosa. Diag Microbiol Infect Dis 1988;11:41-52. [PubMed]
226. Stuart JJ. Ticarcillin-induced hemorrhage in a patient with thrombocytosis. South Med J 1980;73:1084-5. [PubMed]
227. Sykes RB, Matthew M. The β-lactamases of gram-negative bacteria and their role in resistance to β-lactam agents. J Antimicrob Chemother 1976;2:115-57. [PubMed]
228. Sutter VL, Finegold SM. Susceptibility of anaerobic bacterial to 23 antimicrobial agents. Antimicrob Agents Chemother 1976;10:736-52. [PubMed]
229. Tauber MG, Doroshow CA, Hackbarth CJ, Rusnak MG, Drake TA, Sande MA. Antibacterial activity of beta-lactam antibiotics in experimental meningitis due to Streptococcus pneumoniae. J Infect Dis 1984;149:568-74. [PubMed]
230. Tetzlaff TR, McCracken GH Jr, Nelson JD. Oral antibiotic therapy for skeletal infections in children. II. Therapy of osteomyelitis and suppurative arthritis. J Pediatr 1978;92:485-90. [PubMed]
231. Thauvin C, Eliopoulos GM, Willey S, Wennersten C, Moellering RC. Continuous-infusion ampicillin therapy of enterococcal endocarditis in rats. Antimicrob Agents Chemother 1987;31:139-43. [PubMed]
232. Tjandramaga TB, Mullie A, Verbesselt R, De Schepper PJ, Verbist L. Piperacillin: human pharmacokinetics after intravenous and intramuscular administration. Antimicrob Agents Chemother 1978;14:829-37. [PubMed]
233. Tomasz A, Albino A, Zanati E. Multiple anitbiotic resistance in a bacterium with suppressed autolytic system. Nature 1970;227:138-50. [PubMed]
234. Tomasz A. The mechanism of the irreversible antimicrobial effects of penicillin: how the beta-lactam antibiotics kill and lyse bacteria. Ann Rev Microbiol 1979a;33:113-37. [PubMed]
235. Tomasz A. From penicillin-binding proteins to the lysis and death of bacteria: a 1979 view. Rev Infect Dis 1979b;1:434-67. [PubMed]
236. Tomasz A. Penicillin-binding proteins and the antibacterial effectiveness of the beta-lactam antibiotics. Rev Infect Dis 1986;8(Suppl 3):S260-78. [PubMed]
237. Tomasz A. Antibiotic resistance in Streptococcus pneumoniae. Clin Infect Dis 1997;24(Suppl 1):S85-8. [PubMed]
238. Tramont EC. Treponema pallidum (syphilis). In: Mandell GL, Bennett JE, Dolin R. (eds.). Principles and Practice of Infectious Diseases. Chruchill Livingstone, New York, 1995, 2117-32.
239. Tramont EC. Persistence of Treponema pallidum following penicillin G therapy. JAMA 1976;236:2206-7. [PubMed]
240. Tullett GL. Sudden death occurring during “massive-dose” potassium penicillin G therapy. Wisconsin Med J 1970;69:216-7. [PubMed]
241. Ubukata K, Yamashita N, Konno M. Occurrence of a beta-lactam inducible penicillin-binding protein in methicillin-resistant staphylococci. Antimicrob Agents Chemother 1985;27:851-7. [PubMed]
242. VanArsdel PP Jr, Martonick GJ, Johnson LE, Sprenger JD, Altman LC, Henderson WR Jr. The value of skin testing for penicillin allergy diagnosis. West J Med 1986;144:311-4. [PubMed]
243. Van Esso D, Fontanals D, Uriz S, Morera MA, Juncosa T, Latorre C, Duran M. Neisseria meningitidis with reduced susceptibility to penicillin. Pediatr Infect Dis 1987;6:438-9. [PubMed]
244. Van Etta LL, Kravitz GR, Russ TE, Fasching CE, Gerding DN, Peterson LR. Effect of method of administration on extravascular penetration of four antibiotics. Antimicrob Agents Chemother 1982;21:873-80. [PubMed]
245. Verbist L. Comparison of the activities of the new ureidopenicillins piperacillin, mezlocillin, azlocillin, and Bay k 4999 against gram-negative organisms. Antimicrob Agents Chemother 1979;16:115-9. [PubMed]
246. Visser LG, Arnouts P, van Furth R, Mattie H, van den Broek PJ. Clinical pharmacokinetics of continuous intravenous administration of penicillins. Clin Infect Dis 1993;17:491-5. [PubMed]
247. Vogelman B, Gudmundsson S, Leggett J, Turnidge J, Ebert S, Craig WA. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J Infect Dis 1988;158:831-47. [PubMed]
248. Vondracek TG. Beta-lactam antibiotics: is continuous infusion the preferred method of administration? Ann Pharmacother 1995;29:415-24. [PubMed]
249. Walder M, Haeggman S, Tullus K, Burman LG. A hospital outbreak of high-level beta-lactam resistant Enterobacter spp.: association more with ampicillin and cephalosporin therpay than with nosocomial transmission. Scand J Infect Dis 1996;28:293-6. [PubMed]
250. Watanakunakorn C, Glotzbecker C. Synergism with aminoglycosides of penicillin, ampicillin, and vancomycin against nonenterococcal group D streptococci and viridans streptococci. J Med Microbiol 1977;10:133-8. [PubMed]
251. Watt G, Padre LP, Tuazon ML, Calubaquib C, Santiago E, Ranoa CP, Laughlin LW. Placebo-controlled trial of intravenous penicillin for severe and late leptospirosis. Lancet 1988;1:433-5. [PubMed]
252. Weiner IM, Washington JA, Mudge GH. On the mechanism of action of probenecid on renal tubular secretion. Bull Johns Hopkins Hosp 1960;106:333-46. [PubMed]
253. Wenger JD, Hightower AW, Facklam RR, Gaventa S, Broome CV, Bacterial Meningitis Study Group. Bacterial meningitis in the United States 1986: report of a multistate surveillance study. J Infect Dis 1990;162:1316-23. [PubMed]
254. White CA, Toothaker RD. Influence of ampicillin elimination half-life on in vitro bactericidal effect. J Antimicrob Chemother 1985;15(Suppl A):257-60. [PubMed]
255. White GW, Malow JB, Zimelis VM, Pahlavanzadeh H, Panwalker AP, Jackson GG. Comparative in vitro activity of azlocillin, ampicillin, mezlocillin, piperacillin, and ticarcillin, alone and in combination with an aminoglycoside. Antimicrob Agents Chemother 1979;15:540-3. [PubMed]
256. Whitney CG, Farley MM, Hadler J, Harrison LH, Lexau C, Reingold A, Lefkowitz L, Cieslak PR, Cetron M, Zell ER, Jorgensen JH, Schuchat A; Active Bacterial Core Surveillance Program of the Emerging Infections Program Network. Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States. N Engl J Med 2000;343:1917-24. [PubMed]
257. Wiggins GL, Albritton WL, Feeley JC. Antibiotic susceptibility of clinical isolates of Listeria monocytogenes. Antimicrob Agents Chemother 1978;13:854-60. [PubMed]
258. Williams TW Jr, Lawson SA, Brook MI, Ory EM, Morgen RO. Effect of hemodialysis on dicloxacillin concentration in plasma. Antimicrob Agents Chemother 1967;7:767-9. [PubMed]
259. Wilson WR, Geraci JE, Wilkowske CJ, Washington JA 2d. Short-term intramuscular therapy with procaine penicillin plus streptomycin for infective endocarditis due to viridans streptococci. Circulation 1978;57:1158-61. [PubMed]
260. Wilson WR, Thompson RL, Wilkowske CJ, Washington JA 2d, Giuliani ER, Geraci JE. Short-term therapy for streptococcal infective endocarditis: combined intramuscular administration of penicillin and streptomycin. JAMA 1981;245:360-3. [PubMed]
261. Wise R, Gillett AP, Andrews JM, Bedford KA. Activity of azlocillin and mezlocillin against gram-negative organisms: comparison to other penicillins. Antimicrob Agents Chemother 1978;13:559-65. [PubMed]
262. Wispelwey B, Scheld WM. Brain abscess. Clin Neuropharmacol 1987;10:483-510. [PubMed]
263. Woods CR, Smith AL, Wasilauskas BL, Campos J, Givner LB. Invasive disease caused by Neisseria meningitidis relatively resistant to penicillin in North Carolina. J Infect Dis 1994;170:453-6. [PubMed]
264. Woodward RS, Medoff G, Smith MD, Gray JL. Antibiotic cost savings from formulary resitrictions and physician monitoring in a medical-school affiliated hospital. Am J Med 1987;83:817-23. [PubMed]
265. Yamamoto M, Jimbo M, Ide M, Tanaka N, Umebara Y, Hagiwara S. Penetration of intravenous antibiotics into brain abscesses. Neurosurgery 1993;33:44-9. [PubMed]
266. Yocum RR, Rasmussen JR, Strominger JL. The mechanism of action of penicillin. J Biol Chem 1980;255:3977-86. [PubMed]
267. Yoshimura F, Nikaido H. Diffusion of β-lactam antibiotics through the porin channels of Escherichia coli K-12. Antimicrob Agents Chemother 1985;27:84-92. [PubMed]
268. Young EJ, Weingarten NM, Baughn RE, Duncan WC. Studies on the pathogenesis of the Jarisch-Herxheimer reaction. J Infect Dis 1982;146:606-15. [PubMed]
269. Yu VL, Chiou CC, Feldman C, Ortqvist A, Rello J, Morris AJ, Baddour LM, Luna CM, Snydman DR, Ip M, Ko WC, Chedid MB, Andremont A, Klugman KP; International Pneumococcal Study Group. An international prospective study of pneumococcal bacteremia: correlation with in vitro resistance antibiotics administered and clinical outcome. Clin Infect Dis 2003;37:230-7. [PubMed]
270. Zeisler JA, McCarty JD, Richelieu WA, Nichol MB. Cefuroxime by continuous infusion: a new standard of care? Infect Med 1992;9:54-60.
271. Zenker PN, Rolfs RT. Treatment of syphilis, 1989. Rev Infect Dis 1990;Suppl 6:590-609. [PubMed]
272. Zinner SH, Dudley MN, Gilbert D, Bassignani M. Effect of dose and schedule on cefoperazone pharmacodynamics in an in vitro model of infection in a neutropenic host. Am J Med 1988;85(Suppl 1A):56-8. [PubMed]
Tables
Figure 1. Penicillin Structure.
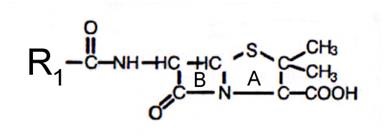
Table 1. Classification of Penicillins
| Class and compounds | Trade names1 | Availability |
|---|---|---|
| Natural penicillins | ||
| penicillin G potassium | Pfizerpen | Parenteral: 5, 20 mu vials |
| phenoxymethyl penicillin | various generics | Tablets: 250, 500mg |
| Solution: 125mg/5 mL, 250mg/5 mL | ||
| penicillin G procaine | Wycillin | Parenteral: 600,000 u/mL |
| penicillin G benzathine | Bicillin L-A, Permapen | Parenteral: 300,000u/mL, 600,000u/mL |
| penicillin G procaine/ penicillin G benzathine combination | Bicillin C-R | Parenteral: 150,000u/150,000 u/mL, 150,000/450,000 u/mL, 300,000/30,000 u/mL |
| Penicillinase-Resistant Penicillins | ||
| methicillin | Staphcillin | no longer available |
| nafcillin | various generics | Parenteral: 20 mg/mL vials |
| oxacillin | Bactocill | Capsules: 250, 500mg |
| Solution: 250mg/5mL | ||
| Parenteral: 500mg, 1, 2, 4 g vials | ||
| cloxacillin | various generics | Capsules: 250, 500mg |
| Solution: 125mg/5 mL | ||
| dicloxacillin | Dynapen | Capsules: 250, 500mg |
| Suspension: 62.5 mg/5 mL | ||
| Aminopenicillins | ||
| ampicillin | Principen | Capsules: 250, 500 mg |
| Suspension: 125 mg/5 mL, 250 mg/5 mL | ||
| Parenteral: 125, 250, 500 mg, 1, 2 g vials | ||
| amoxicillin | Amoxil, Trimox | Capsules: 250, 500 mg |
| Chewable tablets: 125, 250, 400 mg | ||
| Film-coated tablets: 500, 875 mg | ||
| Suspension: 50mg/mL, 125 mg/5 mL, 200 mg/5 mL, 250 mg/5 mL, 400 mg/5 mL | ||
| bacampicillin | Spectrobid | no longer available |
| Carboxypenicillins | ||
| carbenicillin | Geocillin | Tablets: 382 mg |
| ticarcillin | Ticar | Parenteral: 1, 3, 6 g vials |
| Ureidopenicillins and piperazine penicillin | ||
| azlocillin | Azlin | no longer available in U.S. market |
| mezlocillin | Mezlin | no longer available in U.S. market |
| piperacillin | Pipracil | Parenteral: 2, 3, 4g vials |
1 = not all formulations are available with every brand
Table 2. Minimal Inhibitory Concentrations (MIC50) of Specific Organisms
| MIC-50 (µg/mL) | ||||||
|---|---|---|---|---|---|---|
| Organism | Penicillin G | Penicillin V | Oxacillin | Ampicillin/Amoxicillin | Ticarcillin | Mezlocillin Piperacillin |
| Gram-Positive aerobes | ||||||
| Enterococcus sp. | 2.0 | 4.0 | >32 | 0.5 | 64 | 2 |
| Staphylococcus aureus | ||||||
| (Non Penicillinase producing) | 0.03 | 0.03 | 0.4 | 0.06 | 1.0 | 0.06 |
| (Penicillinase-producing) | >32 | >32 | 1.6 | >32 | 32 | 32 |
| Staphlyococcus epidermidis | 0.02 | 0.02 | 0.2 | 0.05 | 1.0 | 1.0 |
| Streptococcus pneumoniae | 0.01 | 0.01 | 0.1 | 0.02 | 0.4 | 0.02 |
| Viridans streptococci | 0.01 | 0.01 | 0.2 | 0.05 | 0.5 | 0.25 |
| Streptococcus pyogenes | 0.015 | 0.015 | 0.04 | 0.03 | 0.25 | 0.125 |
| Listeria monocytogenes | 0.25 | 0.25 | >4 | 0.25 | 4 | 0.5 |
| Gram-negative aerobes | ||||||
| Neisseria gonorrhoeaea | 0.25 | 1.0 | 0.06 | 0.03 | 0.015 | |
| Neisseria meningitidis | 0.03 | 0.06 | 0.06 | 0.03 | 0.03 | |
| Escherichia coli | 64 | 128 | 8 | 2 | 4 | 2 |
| Proteus mirabilis | 32 | 128 | 4 | 0.5 | 2 | 1 |
| Indole + P. mirabilis | >500 | >500 | >128 | 64 | 4 | 1 |
| Hemophilus influenzaea | 0.4 | 6.3 | 25 | 0.25 | 0.012 | 0.03 |
| Salmonella sp. | 8 | 128 | 2 | 1 | 2 | 2 |
| Shigella sp. | 16 | 64 | 4 | |||
| Serratia marcescens | >128 | >128 | >128 | >500 | 16 | 88 |
| Klebsiella sp. | >128 | >128 | >128 | 64 | >64 | 16 |
| Enterobacter sp. | >128 | >128 | >128 | 128 | 16 | 8 |
| Citrobacter sp. | >128 | >128 | >128 | 32 | 2 | 2 |
| Acinetobacter sp. | >128 | >128 | >128 | 16 | 8 | 16 |
| Pseudomonas aeruginosa | >128 | >128 | >128 | >500 | 16 | 8 |
| Anaerobes | ||||||
| Peptostreptococcus | 0.1 | >32 | 0.5 | 0.5 | 0.5 | |
| Fusobacterium nucleatum | <0.1 | >64 | <0.1 | 0.5 | <0.1 | |
| Clostridium perfringens | 0.5 | >64 | <0.1 | 0.5 | 0.5 | |
| Bacteroides fragilis | 16 | >64 | 16 | 16 | 32 | |
a non-beta-lactamase producing strains
Table 3. Minimal Inhibitory Concentrations (MIC-90) of Specific Organisms
MIC-90 (µg/mL) |
|||||
|---|---|---|---|---|---|
| Organism | Penicillin G | Oxacillin | AmpicillinAmoxicillin | Ticarcillin | Mezlocillin Piperacillin |
| Gram-Positive aerobes | |||||
| Enterococcus sp. | 2.0 | >100 | 4 | 128 | 4 |
| Staphylococcus aureus | |||||
| (Non Penicillinase producing) | 0.03 | 3.1 | 0.125 | 1.0 | 0.06 |
| (Penicillinase-producing) | >32 | 6.3 | >32 | 32 | 32 |
| Staphlyococcus epidermidis | 1.0 | 1.0 | |||
| Streptococcus pneumoniae | 0.03 | 0.8 | 0.02 | 1 | 0.02 |
| Viridans streptococci | 8 | 0.25 | |||
| Streptococcus pyogenes | 0.015 | 0.4 | 0.03 | 0.25 | 0.125 |
| Listeria monocytogenes | 0.5 | >4 | 0.5 | 4 | 0.5 |
| Gram-negative aerobes | |||||
| Neisseria gonorrhoeaea | 0.5 | 12.5 | 0.06 | 0.03 | 0.015 |
| Neisseria meningitidis | 0.06 | 6.3 | 0.25 | 0.03 | 0.03 |
| Escherichia coli | 64 | >128 | >256 | >256 | 256 |
| Proteus mirabilis | 32 | >128 | >256 | 128 | 16 |
| Indole + P. mirabilus | >500 | >128 | >256 | >256 | 64 |
| Hemophilus influenzaea | 4 | 100 | 0.5 | 0.25 | 0.5 |
| Salmonella sp. | 16 | >128 | >256 | >256 | >256 |
| Shigella sp. | 32 | ||||
| Serratia marcescens | >128 | >128 | >128 | >256 | 128 |
| Klebsiella sp. | >128 | >128 | >128 | 500 | 16 |
| Enterobacter sp. | >128 | >128 | >128 | >256 | 128 |
| Citrobacter sp. | >128 | >128 | 128 | 256 | 8 |
| Acinetobacter sp. | >128 | >128 | 64 | 64 | 128 |
| Pseudomonas aeruginosa | >128 | >128 | >128 | 128 | 32 |
| Anaerobes | |||||
| Peptostreptococcus | 1.0 | >32 | 8.0 | 2.0 | 4.0 |
| Fusobacterium nucleatum | 1.0 | >64 | 16.0 | 0.5 | 0.5 |
| Clostridium perfringens | 0.5 | >64 | <0.1b | 0.5 | 0.5 |
| Bacteroides fragilis | >64 | >64 | >64 | 128 | 128 |
a non-beta-lactamase producing strains
b amoxicillin’s MIC90 is 0.5.
Table 4. In Vitro Post-Antibiotic Effect of Selected Penicillins
| S. aureus | S. pneumoniae | E. faecalis | |
|---|---|---|---|
| penicillin G | 2-3.5 hrs | 2.5-3.5 hrs | 2.5-3.5 hrs |
| ampicillin | 2-2.5 hrs | 2-6 hrs | 0.5-2.5 hrs |
| nafcillin | 1.5-2 hrs | ND | ND |
| piperacillin | £0.5 hr | ND | £0.5 hr |
ND = no data
Table 5. Prevalence of Resistance of Organisms to Penicillins
| Organism | Prevalence |
|---|---|
| Gram-positive | |
| Streptococcus pneumoniaea,b | Intermediate pcn resistance 11-28% Highly PCN resistant 11-33% |
| Staphylococcus aureus | PCN resistant >95%, Methicillin resistant (nosocomial) 23-38% |
| Gram-negative | |
| Haemophilus influenzae | ampicillin 1-64% |
| Moraxella catarrhalis | up to 85% |
| Escherichia coli | ampicillin 30-50% |
| Neisseria gonorrhoeae | 1.2-38% |
| Neisseria meningitidis | up to 20% |
| Extended-spectrum beta-lactamase producers | |
| Klebsiella pneumoniae | up to 24% |
| Pseudomonas aeruginosa | piperacillin 5-30% |
aintermediate resistant strains have MICs of 0.12-1.0 µg/mL
highly resistant strains have MICs of ³2.0 µg/mL
bUnited States data
Table 6. Pharmacokinetic Properties of Penicillins
| Drug | Dose | Peak Serum Conc.1 | % Bioavailability | Half-Life (hours) | %PPB2 |
|---|---|---|---|---|---|
| Natural penicillins | |||||
| benzylpenicillin | 2g | 20 µg/mL | na | 0.5 | 50-60 |
| penicillin G oral3 | 400,000 u | 0.3 µg/mL | 15-30 | 0.5 | |
| penicillin VK | 250 mg | 3 µg/mL | 60 | 0.5 | 75-85 |
| procaine pen G IM | 300,000 u | 0.9 µg/mL | |||
| benzathine pen G IM | 1.2 mu | 0.09 µg/mL | |||
| Penicillinase-resistant penicillins | |||||
| nafcillin IV | 1g | 20 µg/mL | na | 0.5-1.0 | 90 |
| oxacillin IV | 500mg | 52-63µg/mL | na | 0.5-0.7 | 94 |
| oxacillin oral | 500mg | 5-7 µg/mL | 30-35% | 0.5-0.7 | |
| cloxacillin oral | 500mg | 7.5-14µg/mL | 50 | 0.5 | 95 |
| dicloxacillin oral | 500mg | 10-17µg/mL | 37 | 0.8 | 98 |
| Aminopenicillins | |||||
| ampicillin IV | 1g | 40 µg/mL | na | 1-1.3 | 20 |
| ampicillin oral | 1g | 3 µg/mL | 30-50 | 1-1.3 | |
| amoxicillin oral | 1g | 7.5 µg/mL | 80 | 1-1.3 | 20 |
| Extended-spectrum penicillins | |||||
| carbenicillin IV | 3g | 223 µg/mL | na | 1.1 | 50 |
| carbenicillin oral | 1g | 9 µg/mL | 30 | ||
| ticarcillin | 3.5g | 210 µg/mL | na | 1.2 | 45 |
| mezlocillin | 3g | 263 µg/mL | na | 0.8 | 16-42 |
| piperacillin | 4g | 240 µg/mL | na | 1.0 | 16 |
1 data complied from product package information and Donowitz 1988.
2 plasma protein bound
3 no longer commercially available in the US
Table 7. Guidelines for Adult and Pediatric Dosing of Penicillins
| Drug | Normal adult dosea,b | Normal pediatric dosec | Dosage adjustment in renal impairmentd,e |
|---|---|---|---|
| Natural penicillins | |||
| benzylpenicillin (penicillin G) | enterococcal endocarditis: 4-6 mub IV q4h | ≤ 1week and > 2kg: 20,000-50,000 u/kg IV q8h | CrCL 10-50 mL/min: Increase dosing interval to q6-8h |
| ≤ 1 week and ≤ 2kg: 20,000-50,000 u/kg IV q12h | |||
| > 1 week and ≤ 2kg: 25,000-65,000 u/kg IV q8h | |||
| Streptococcal meningitis: 2-3 mu IV q4h | > 1 week and > 2kg: 25,000-65,000 u/kg IV q6h | ||
| Streptococcal infection: 2mu IV q4-6h | > 1month and < 12 years, severe infection: 40,000-60,000 u/kg IV q4-6h > 12 years: usual adult dose | CrCL < 10 mL/min: Increase dosing interval to q12h | |
| anthrax (setting of bioterrorism): 4 mg IV q4h | <12 years: 50,000 u/kg IV q6h | ||
| >12 years: usual adult dose | |||
| neurosyphilis: 3-4 mu IV q4h | |||
| penicillin VK | Streptococcal pharyngitis: 500 mg po bid-tid for 10 days | > 1 month: 15-62.5 mg/kg/day po in 3-6 divided doses | Little data available. Adjust dose if CrCL < 10 mL/min. |
| Lyme disease: 250-500mg po qid for 10-30 days | Lyme disease and < 9 years: 25-50 mg/kgday po in 3 divided doses for 10-30 days | ||
| procaine penicillin G (PPG) | Streptococcal infection: 600,000-1.2 mu IM qd for 10 days | neonates: 50,000 u/kg/day IM | Not necessary |
| >1 month and < 12 years: 25,000-50,000 u/kgday IM | |||
| ≥12 years: usual adult dose | |||
| benzathine penicillin G | early syphilis: 2.4 mu IM | <27kg: 300,000-600,000 u IM | Not necessary |
| group A strep infection and prophylaxis of recurrent rheumatic fever: 1.2 mu IM | ≥27 kg: 1.2 mu IM | ||
| Penicillinase-resistant penicillins | |||
| nafcillin | 500mg-1g po q4-6h | > 1 month: 50-100 mg/kg/day po in 3-4 divided doses | CrCL < 10 mL/min: Extend dosage interval to q8-12 hrs |
| 1-2g IV q4-6h | > 1 month: IV data limited, 100-200mg/kg/day in 4-6 divided doses | ||
| oxacillin | 500mg-1g po q4-6h | > 1 month and < 40kg: 50-100 mg/kg/day po or IV in 4 divided doses | CrCL < 10 mL/min: use lower range of usual dose |
| 1-2g IV q4-6h | ≥40 kg: usual adult dose | ||
| cloxacillin | 250-500mg po q6h | > 1 month and < 20 kg: 50-100 mg/kg/day po in 4 divided doses | |
| ≥20 kg: usual adult dose | |||
| dicloxacillin | 125-250 mg po q6h | > 1 month and < 40 kg: 12.5 - 25 mg/kg/day po in 4 divided doses | not necessary |
| ≥40 kg: usual adult dose | |||
| Staphylococcal osteomyelitis: 50-100 mg/kg/day in 4 divided doses | |||
| Aminopenicillins | |||
| ampicillin | 250-500mg po q6h 1-2g IV q4h | ³ 1 month and < 40 kg: 50-200 mg/kg day IV in 4-6 divided doses | CrCL 10-50 mL/min: Extend dosing interval to q 6-12h |
| < 1 week: 25 mg/kg IV/IM q 8-12h | |||
| ³ 1 week and < 1 month: 25 mg/kg IV/IM q6-8h | |||
| > 40 kg: usual adult dose | CrCL < 10 mL/min: Extend dosing interval to q8-16h | ||
| amoxicillin | 500mg po q12h or 250-500mg po q8h or 875mg po q12h | > 1 month and < 20 kg: 20-40 mg/kg/day in 3 divided doses | CrCL 10-50 mL/min: Consider extending dosing interval to q12h. |
| > 20 kg: usual adult dose | CrCL < 10 mL/min: Extend dosing interval to q12-24h. | ||
| Extended-spectrum penicillins | |||
| carbenicillin | 382-764 mg po qid | No data | No data |
| ticarcillin | 3g IV q4h | > 1 month and < 40 kg: 100-300 mg/kg/day in 4-6 divided doses | CrCL 30-60 mL/min: 2g q4h |
| CrCL 10-30 mL/min: 2g q8h | |||
| CrCL < 10 mL/min: 2g q12h | |||
| ≥40 kg: usual adult dose | Supplement after HD: 3g | ||
| mezlocillin | 3-4g IV q4-6h | < 1 week: 75 mg/kg q12h | CrCL 10-50 mL/min: 1.5-3g q6-8h |
| ≥1 week and < 1 month: 75 mg/kg q6-8 hrs | |||
| ≥1 month and < 12 yrs: 50-75 mg/kg q4h | CrCL < 10 mL/min: 1.5-2g q8h | ||
| ≥12 years: usual adult dose | Supplement after HD: 3-4g | ||
| piperacillin | 3-4g IV q4-6h | 1 month -12 years: 50 mg/kg q4h | CrCL 20-40 mL/min: 3-4g q8h |
| CrCL < 20 mL/min: 3-4g q12h | |||
| Supplement after HD: 1g | |||
aintramuscular
bmillion units
cThese dosages are ranges of acceptable doses. The lower range of usual dose is generally used for mild infection, upper range for severe infection (e.g. meningitis, endocarditis). Higher dosages than recommended may be used in certain circumstances. Clinical judgment should be used when dosing and prescribing information for the specific drugs should be consulted for more information.
dclinical judgment should be utilized when making decisions regarding dosage adjustment in renally impaired patients, taking into account severity of renal impairment, site of infection, expected length of therapy, organism isolated, etc.
edata complied using McEvoy 2003 AHFS Drug Information and product package inserts
HD = hemodialysis
Table 8. Approximate Cost of Typical Therapeutic Regimens of Selected Penicillins1
| Drug | Dosing regimen | Cost1 |
|---|---|---|
| Oral agents | ||
| ampicillin | 500mg po q6h x 10d | $5.00 |
| penicillin VK | 500mg po q8h x 10d | $4.00 |
| amoxicillin | 500mg po q8h x 10d | $5.00 |
| Long-acting agents | ||
| procaine penicillin G | 1.2 mu IM x1 | $5.00 |
| benzathine penicillin G | 2.4mu IM x1 | $15.00 |
| Intravenous agents | ||
| ampicillin | 2g IV q4h x 10d | $57.00 |
| penicillin G potassium | 2 mu IV q4h x 10d | $21.00 |
| penicillin G sodium | 2 mu IV q4h x 10d | $158.00 |
| oxacillin | 1g IV q6h x 10d | $109.00 |
| ticarcillin | 3g IV q4h x 10d | $375.00 |
| piperacillin | 4g IV q6h x 10d | $390.00 |
1 = drug acquisition costs for Albany Medical Center Hospital, Albany, New York.
Table 9. Dosing During Continuous Renal Replacement Therapy (Oxacillin and Nafcillin)
| CVVH (Continuous venovenous hemofiltration) | 2g IV q4-6h |
| CVVHD (Continuous venovenous hemodialysis) | 2g IV q4-6h |
| CVVHDF (Continuous venovenous hemodiafiltration) | 2g IV q4-6h |
GUIDED MEDLINE SEARCH FOR:
Pharmacokinetics/Pharmacodynamics
What's New
Bard JD, et al. Rationale for Eliminating Staphylococcus Breakpoints for β-lactam Agents Other Than Penicillin, Oxacillin or Cefoxitin, and Ceftaroline. Clin Infect Dis 2014;58:1287-1296.
Turner S, et al. NDM-1-The Newest Recruit to the Army of β-lactamases. 2013.
GUIDED MEDLINE SEARCH FOR RECENT REVIEWS
History
Geddes A. 80th Anniversary of the discovery of penicillin. An appreciation of Sir Alexander Fleming. Int J Antimicrob Agents 2008;32:373.
Guided Medline Search For Historical Aspects:
Table of Contents
- Introduction
- Class
- Antimicrobial Activity
- Mechanisms of Action
- Mechanisms of Resistance
- Pharmacokinetics
- Dosage
- Penicillin Hypersensitivity
- Adverse Effects
- Drug Interactions
- Clindical Indications
- Bone and Joint Infections
- Central Nervous System Infections
- Endocarditis
- Intra-abdominal Infection
- Obstetrics and Gynecological Infections
- Respiratory Tract Infections
- Sexually Transmitted Diseases
- Skin and Soft Tissue Infections
- Urinary Tract Infections
- Anthrax Resulting from Bioterrorism
- Miscellaneous
- Summary
