Borrelia burgdorferi (Lyme Disease)
Authors: Eugene D. Shapiro, M.D., Paul Auwaerter, M.D. Previous author: David L. Coleman, M.D., 1997 Edition, 2002 Edition
MICROBIOLOGY
Lyme disease is caused by the spirochete, Borrelia burgdorferi sensu lato, a fastidious, microaerophilic bacterium that replicates slowly and requires special media to grow in the laboratory (161, 185, 192). Its cell membrane is covered by flagella and a loosely-associated outer membrane. The major outer-surface proteins include OspA, OspB and OspC (which are highly charged basic proteins of molecular weights of about 31, 34, and 23 kilodaltons, respectively), as well as the 41-kd flagellar protein, are important targets for the immune response of humans. Based on studies of its DNA, the organism has been subclassified into several genospecies: B. burgdorferi sensu stricto, B. garinii, and B. afzelii. In the United States only B. burgdorferi sensu stricto has been isolated from humans. In contrast, there is substantial variability among isolates of B. burgdorferi from humans in Europe, most of which are either B. garinii, or B. afzelii.
EPIDEMIOLOGY
B. burgdorferi is transmitted by Ixodid ticks--in the United States, primarily by Ixodes scapularis, the deer tick. In the United States most cases of Lyme disease occur in costal and riparian regions of southern New England, southeastern New York, New Jersey, eastern Pennsylvania, eastern Maryland, Delaware, and parts of Minnesota and Wisconsin. Some infections that occur in northern California and the upper Pacific Northwest are transmitted by Ixodes pacificus, the western black-legged tick. In 2006, a total of 19,931 Lyme disease cases were reported in the United States. The incidence in the ten states with the highest numbers of cases averaged 302 cases per 100,000 persons (147). In Europe, most cases occur in the Scandinavian countries and in central and eastern Europe (especially in Germany, Austria and Slovenia), although cases have been reported from throughout the continent.
The proportion of ticks infected with B. burgdorferi varies greatly both by geographic area and by the stage of the tick in its three-stage life cycle. In endemic areas persons who have either occupational or recreational exposure to tick-infested woodlands or fields are at increased risk of Lyme disease, although the risk is also substantial on the lawns of suburban homes that border wooded areas (140). Acute cases start to occur in the early spring with the incidence peaking typically in early July due to the frequent feeding characteristics of nymphal ticks. Onset of early disease can be seen through the fall until the first hard frost when ticks no longer are active. Due to outdoor predilections, children and middle-aged adults are most commonly afflicted, although ages can range from early childhood to advanced ages.
CLINICAL MANIFESTATIONS
The clinical manifestations of Lyme disease generally are divided into early localized disease, early disseminated disease and late disease (161, 185). Depending on the stage of infection, Borrelia burgdorferi tends to most commonly affect the skin, nervous system, heart and joints.
Early Localized Lyme Disease
Early localized disease is manifest by the characteristic single round or ovoid rash called erythema migrans (EM), that is observed in approximately 70-80% of infected individuals between 3-30 days [average 7-10 days] after an infected tick bite (182). In this study, only 31% of individuals directly recalled of a tick bite at the site of their erythema migrans rash. Another study, in children, suggested that the rash was observed in 90% of individuals (56).
The rash may begin as a macule or papule at the site of the bite and then often rapidly expands over days if untreated. Dimensions greater than five centimeters help differentiate an erythema migrans rash from other processes, including insect bites or tick hypersensitivity reactions than tend to remain quarter-sized or smaller. Although the classic erythema migrans description is that of a "bull's eye" because of central clearing that occurs as the rash expands (Figure 1), it is seen in only approximately 20% of U.S. patients as opposed to nearly 80% of European patients (203). In the North America, erythema migrans is more commonly identified at earlier stages without central clearing, so actually the most common erythema migrans rash is homogenous in appearance (Figure 2) (174). Favored locations include warmer and moist regions of the body such as in the axillae, the inguinal or popliteal regions, the torso and in women under the breasts though the rash may occur anywhere. The area of rash is usually light to more intensely red and typically flat but may be raised on occasion. Untreated, the rash may expand considerably with sizes up to 20cm or beyond in a circumferential pattern that distinguishes it from typical cellulitis or ringworm. The rash is usually painless, but may be warm and occasionally pruritic. A central punctum may be seen at the near center where the tick bite occurred (Figure 3). Occasionally, erythema migrans may have atypical features including unusual shapes, central duskiness or bluish discoloration, induration, or features of necrosis that may lead to confusion with spider bites (Figure 4) (150). Vesicle formation may be identified in up to 5% of erythema migrans rashes (57). Others occasionally show multiple red rings that may be confused with erythema multiforme.
Most patients have some form of accompanying symptoms including constitutional complaints such as fatigue or fever, or more localized problems such as headache, myalgia, arthralgia or enlarged lymph nodes. This may well represent spirochetal dissemination as studies have found evidence of B. burgdorferi blood stream infection in patients with localized erythema migrans (27, 126). For a minority, the patients feel well with only a rash as a presenting compliant. Many patients have no recollection of a prior tick bite (182). Even if untreated, the erythema migrans rash will resolve within 2-4 weeks. Occasionally, a patient may present with flu-like illness or headache representing early Lyme disease without evidence of a rash (194). Securing a definite Lyme diagnosis in these patients may be difficult because serological testing along cannot easily distinguish among the possibilities of a viral infection or infection with tick-borne agents such as Ehrlichia or Babesia species. Culture or PCR identification is mostly limited to research laboratories, so clinical determination of a rash as erythema migrans remains the usual method for identification of Lyme disease at this early, localized stage. If uncertainty exists, options include observing the rash as erythema migrans tends to expand over a period of days, or one can obtain acute and convalescent Lyme serology.
Early, Disseminated Lyme Disease
Cutaneous
The most common manifestation of early disseminated disease is multiple erythema migrans that almost always is accompanied by fever and flu-like symptoms reflecting hematogenous spread of the spirochete. Fatigue and lethargy are common. The lesions of multiple erythema migrans tend to be smaller and fainter with less indurated centers (182). These rashes occur beyond the site of a tick bite and may occur newly with others fading over the course of 3-4 weeks, anywhere on the body. Antibiotics yield improvement, but the rashes will resolve even without antibiotic therapy (185).
Neurologic
Neurological manifestations may afflict 10-15% of untreated patients, usually within four to eight weeks after erythema migrans, although they may also occur earlier and concomitantly with the rash (63). Aseptic lymphocytic meningitis, cranial neuropathy (especially facial nerve palsy) or radiculoneuritis are most commonly encountered in these early stages of neuroborreliosis, and may occur together (134). More rarely encountered early neurological involvement includes transverse myelitis, encephalitis and inflammatory myopathy.
For Lyme-related meningitis, expected symptoms may include severe headache, neck pain and stiffness but on occasional include signs consistent with mild encephalopathy. For cranial palsies, in an endemic region, up to 25% of acute facial paralysis may be ascribed to B. burgdorferi which has also been implicated as causing bilateral presentations distinguishing it from usual idiopathic Bell's palsy (25, 62). Though a facial palsy may be a presenting sign of Lyme disease, it usually occurs within four weeks of erythema migrans onset (25). Other cranial nerve palsies have been described, but are occur much less frequently (134, 95, 97). The radiculoneuropathy of neuroborreliosis can occur in a single nerve or nerve root/dermatomal pattern, or involve multiple regions of the torso or the extremities. The pain can be severe, disrupting sleep. Beyond pain, neurologic deficits may accrue including loss of sensation, motor weakness and depressed deep tendon reflexes (104). Lyme radiculoneuritis will need to be differentiated from other causes such as spinal disc or bone impingement of nerves, zoster (without rash, so-called zoster sine herpte), ischemia or virally-induced neuritis.
Cardiac
Lyme carditis may cause varying degrees of heart block, but only advanced cases resulting in reversible third degree-block causing severe fatigue, dizziness or syncope usually come to medical attention. Temporary pacemaker insertion may be required. The exact incidence of B. burgdorferi cardiac involvement is not known; however, asymptomatic primary and second degree heart block may be more common than recognized as electrocardiograms are not performed routinely in patients with Lyme disease (115). One series of 105 patients with Lyme carditis found the degree of heart block as 12% with first-degree, 16% with second-degree and 49% with third-degree (208). Pericarditis and myocarditis have been rarely described due to Lyme disease; however, there is no convincing evidence linking it as a common cause for cardiomyopathy (18).
Other Manifestations
Diffuse and migratory musculoskeletal pains may occur in the early, disseminated states of Lyme disease. Descriptions of intense arthralgia in both small and large joints are common along with myalgia, tendonitis and bursitis (182). Mild elevations of liver function tests may be seen in a minority of patients with acute infection (76). A sore throat or dry cough have also been identified in some patients with early Lyme disease (182). Conjunctivitis, keratitis, and uveitis have all been described as ocular manifestations (220). Although optic neuritis has been linked to B. burgdorferi infection by some, more careful study has failed to find an association (164).
Late Lyme Disease
Arthritis
The classic manifestation of late Lyme disease is arthritis that may begin months after acquiring the infection. Arthritis was stated in early study of Lyme disease to occur in up to 60% of Lyme disease patients, who would develop intermittent attacks of arthritis typically afflicting large, weight-bearing joints such as the knee, though smaller joints can be involved (184). This rate is likely far less now due to better recognition and earlier treatment of Lyme disease. Typical patients suffering from late Lyme arthritis will complain of joint swelling in one or two joints with significant pain that lasts for weeks or months but will resolve even without antibiotic therapy. Joint symptoms tend to remit completely before another flare. Over time, there may be fewer attacks in some patients but those that occur tend be of longer duration. Late Lyme arthritis existing for more than five years is rare (78).
Antibiotic treatment hastens arthritis resolution, but another important role is to stave off further attacks. In some patients, despite multiple course of appropriate oral or parenteral antibiotic therapy, arthritis continues. These patients are often labeled as having "persistent Lyme arthritis" or "antibiotic-resistant chronic Lyme arthritis." Approximately 10% of patients with late Lyme arthritis fall into this category. These patients have no evidence of remaining B. burgdorferi infection by either culture or PCR (23). Certain genetics have been associated including HLA-DRB1*0401 and HLA-DRB1*101 markers corresponding to specific host immune responses that interact with the B. burgdorferi OspA protein (193, 195, 197). These patients tend to behave much like other forms of chronic inflammatory arthritis.
Neurologic
Late manifestations of neuroborreliosis can affect either the central or the peripheral nervous systems months to years after acquisition of infection. Perhaps because of better recognition and treatment, encephalomyelitis due to B. burgdorferi is now considered extremely rare in the United States. Among the authors of the Infectious Disease Society of America (IDSA) Guideline, only one case of encephalomyelitis was diagnosed over the five period dating from 2000 (217). Encephalomyelitis is a more common entity in Europe that may account for combined presentations of motor and sensory dysfunction, objective cognitive abnormalities, cerebellar findings and cranial neuropathies (3, 65).
A chronic encephalopathy may result in problems with memory, mood disorders and sleep disturbance without the objective neurologic features of an encephalomyelitis (102). Borrelial encephalopathy may be difficult to discriminate from entities such as fibromyalgia or chronic fatigue syndrome. One study suggested that patients with Lyme encephalopathy displayed less physical complaints and less mood disturbance when compared with fibromyalgia or depression (82). An abnormal cerebrospinal fluid (CSF) profile or evidence of active B. burgdorferi infection by either specific polymerase chain reaction (PCR) or intrathecal antibody production correlated with objective measures of neurocognitive dysfunction may help distinguish between post-treatment Lyme disease syndrome (see below) or fibromyalgia/chronic fatigue syndrome (82). The distinction can be difficult to make clinically but it is important, since objective measures of neurocognitive dysfunction or CSF abnormalities may suggest an active, late B. burgdorferi infection that would necessitate parenteral antibiotic therapy as opposed to a post-infectious syndrome.
A chronic neuropathy can be another manifestation of late Lyme disease. This may be in the form of a radiculoneuropathy similar to that described for earlier stages of Lyme disease. Degrees of both sensory and motor polyneuropathies have been attributed to Lyme disease with objective evidence of nerve involvement, positive serology and clinical improvement with antibiotic therapy (64). It should be noted that peripheral neuropathy after arthropod bites not related to Lyme disease have also been described (28).
Cutaneous
Two forms of late cutaneous Lyme disease have been well described. Acrodermatitis atrophicans, causing red to violaceous skin lesions that can be sclerotic or atrophic, is mostly seen in elderly women (121). B. burgdorferi spirochetes may be cultured from these lesions many years after onset. Borrelial lymphocytoma is a problem mostly described in children, as a solitary, bluish nodule characteristically affecting the breast region or the ear lobe. It is an immunological reaction that resembles a pseudolymphoma in response toB. burgdorferi antigens (10). Both acrodermatitis atrophicans and borrelial lymphocytoma are rare manifestations of Lyme disease and almost uniformly seen only in Europe.
Post-Infectious Sequelae
Subjective symptoms such as fatigue and musculoskeletal aches may persist after antibiotic therapy for Lyme disease. One prospective study of culture-confirmed Lyme disease described 10% of patients with such complaints one year after diagnosis, but only 4% reliably had symptoms at every clinic visit during long-term follow-up (131). The exact incidence of such symptoms is not well understood, in part because careful studies of pre-existing disorders or subjective symptoms have not been performed. The term "post-Lyme disease syndrome" has been proposed as a name for a symptom complex that persists beyond six months after appropriate antibiotic therapy that otherwise cannot be explained by an alternative medical condition (217). These persistent symptoms which may encompass fatigue, arthralgia, myalgia, subjective memory difficulties, mood disturbance and sleep irregularities are poorly understood but do not appear respond to additional antibiotic therapy (12). Some practitioners espouse long-term oral, parenteral or combination antibiotic therapy to alleviate these symptoms, although there is little good human data to back up this practice (47, 198).
LABORATORY DIAGNOSIS
Misdiagnosis can be a common problem for treatment failure as a consequence of both under- and over-diagnosis (46, 143, 146, 188). Erythema migrans remains the best marker for Lyme disease in early infection (54). In the absence of the characteristic rash, diagnosis of Lyme disease may be difficult, since the other clinical manifestations of Lyme disease are not specific. Consideration of the diagnosis should depend on whether an individual has been in a region endemic for B. burgdorferi as well as whether they have likelihood for outdoor exposure. Because of the relative insensitivity of culture or PCR for B. burgdorferi and its limitation to research laboratories, this strategy is rarely employed for diagnosis (11). Consequently, the confirmation of Lyme disease without erythema migrans usually depends on the demonstration of antibodies to B. burgdorferi in the patient's serum.
Both the sensitivity and specificity of antibody test results for Lyme disease can vary substantially (14, 15,74). Use of Western immunoblots improves the specificity of serologic testing for Lyme disease (39), although the accuracy of some commercial test kits can be less than tests performed by reference laboratories. Official recommendations from the Second National Conference on Serologic Diagnosis of Lyme Disease are that clinicians use a two-step procedure when ordering antibody tests for Lyme disease-first, a sensitive screening test, either an enzyme-linked immunosorbent assay (ELISA) or an immunofluorescent assay (IFA) and, if that result is positive or equivocal, followed by a Western immunoblot to confirm the result (9). If the result of the ELISA or of the IFA is negative, an immunoblot is not necessary.
An antibody test directed against the B. burgdorferi C6 peptide has been developed and is available in both Europe and the U.S, with the potential advantage of not requiring Western blot analysis. This enzyme-linked immunosorbent assay (ELISA) compares relatively favorably to the standard two-tier approach (116). Some have reported that titer of C6 antibodies decline represents successful response to antibiotic therapy, although others have suggested this is not the case especially in patients with persisting subjective symptoms (50, 139).
Antibody tests generally are not useful for the diagnosis of early localized Lyme disease, since only a minority of patients with solitary erythema migrans will have a positive test result (54, 56). In confusing cases, serology can be ordered four weeks or more after the rash, although early antibiotic treatment may blunt development of characteristic serological responses (7, 130). Lyme serology tends to be robustly positive in late Lyme arthritis as well as a majority of cases with neurological involvement. In contrast to the serologic tests for antibody (which have been shown to be reasonably reproducible in a good laboratory), unvalidated or poorly sensitive or specific tests for Lyme disease including the alternative readings of Western immunoblots, B. burgdorferi PCR testing of blood or urine, and the Lyme urine antigen test. These tests may be inaccurate and display poor reproducibility (90). Certain "Lyme specialty" laboratories offer these and other tests, and warnings have been issued against their use (11).
The predictive value of results of antibody tests (even of very accurate tests) is highly dependent on the prevalence of the infection among patients who are tested. Because many lay persons as well as many physicians erroneously believe that nonspecific symptoms alone (e.g., headache, fatigue or arthralgia) may be manifestations of Lyme disease, patients with only nonspecific symptoms frequently are tested for Lyme disease (99). However, because the specificity of the test results is poor (the specificity of results of even excellent antibody tests for Lyme disease rarely exceeds 90%), some of the results in patients who do not have Lyme disease will be positive; the overwhelming majority (>95%) of these in patients with only nonspecific symptoms will be false-positive results (154, 204). An especially common mistake is to diagnosis active Lyme disease in a patient with chronic symptoms based solely on a positive IgM assay. A positive ELISA based only on a confirmatory IgM Western blot should not be used for any patient with symptoms persisting beyond one month due to poor specificity (145). Lyme disease will be the cause of the nonspecific symptoms in very few (if any) such patients. False positive tests have also been described as occurring due to other tick-borne infections, syphilis, infectious mononucleosis, systemic lupus erythematosis and other autoimmune condition (21).
Even though a symptomatic patient has a positive serologic test result for antibodies toB. burgdorferi, Lyme disease may not be the cause of that patient's symptoms, especially if they are of a subjective nature. In addition to the possibility that the positive result is falsely positive (a common occurrence), the patient may have been infected with B. burgdorferi previously, or the patient's symptoms may be unrelated to that previous infection. Once serum antibodies toB. burgdorferi develop, they (both IgG and IgM) may persist for many years despite adequate treatment and clinical cure of the disease (44, 75, 81). In addition, because some people who become infected with B. burgdorferi never develop symptoms (69,183), in endemic areas there will be a background rate of seropositivity among patients who have never had clinically apparent Lyme disease. When patients with previous Lyme disease (whether asymptomatic and untreated or clinically apparent and adequately treated) develop any kind of symptoms and are tested for antibodies against B. burgdorferi, their symptoms may erroneously be attributed to active Lyme disease. Clinicians should not routinely order antibody tests for Lyme disease either in patients who have not been in endemic areas or in patients with only nonspecific symptoms.
Some certain diagnostic situations may lend to consideration of tests beyond routine serology. Abnormal brain MRI findings that show possible demyelination may due to Lyme disease, although multiple sclerosis, vasculitis and sarcoidosis is often in the differential diagnosis (92). Brain SPECT scans showing reversible cortical and subcortical hypoperfusion have been described in Lyme disease, although the specificity of the finding is unclear (103). Evidence of intrathecal antibody production may lend support for active B. burgdorferi infection of the central nervous system (187). Because intrathecal IgG antibodies can persist for years after treatment, some advocate the use of a CSF index that examines the ratio of protein normalized CSF:serum (68). For ratios greater than 1.0 (some use >1.2-1.4) is taken as evidence of active neuroborreliosis, although the CSF:serum Lyme index is more commonly employed in Europe (80, 205). PCR analysis of the CSF is relatively insensitive for neuroborreliosis; however, it may be helpful in cases of late Lyme arthritis to assess whether antibiotic therapy has eradicated the bacteria (11, 128). In some areas, patients may be co-infected with tick-borne pathogens that may be transmitted by the same tick vector. Human granulocytic anaplasmosis or babesiosis was described in 2% of patients with Lyme disease in areas of Connecticut and Rhode Island in one prospective study (196).
PATHOGENESIS
The pathogenesis of Lyme disease is incompletely understood. In contrast to infections caused by bacteria, which elaborate or contain classic toxins, B. burgdorferi does not produce a toxin (16). In addition, the number of organisms found in infected tissues is relatively small. These observations suggest a complex interaction between the organism and the host in the pathogenesis of Lyme disease.
B. burgdorferi can be found in the skin at the site of the bite after inoculation by the tick. The organism spreads centrifugally through the extracellular matrix and induces a mild inflammatory reaction manifest clinically as erythema migrans (88). The organism disseminates hematogenously to the heart, the central nervous system and other sites shortly after inoculation. B. burgdorferi can adhere to a variety of mesenchymal cells and penetrates cell monolayers through intercellular junctions or, occasionally, through the cytoplasm of cells (202). In vitro, the organism grows within macrophages, endothelial cells, and fibroblasts (53,111,117). The ability of the organism to survive within these cell types in vivo is unknown.
After infection, IgM antibodies develop over a three to six week period and IgG is detectable within 4-12 weeks after infection. Antibody is required for killing of the spirochete via the classical complement pathway (91).
Direct invasion of the synovium by the organism is likely to be responsible for the symptomatic arthritis in Lyme disease. Without treatment, the organism may persist in the joint for months and may produce chronic arthritis (175). The role of autoimmunity in Lyme arthritis in humans has not been proved. However, it does appear that autoimmunity may be important in some patients, since B. burgdorferi was not detected in the synovial fluid of patients with recurrent Lyme arthritis who had received a standard course of antimicrobial treatment (110). In addition, there appears to be an association between chronic arthritis and certain HLA types (186).
The experimental data on the potential pathogenic role of outer surface protein A (OspA) have been conflicting. In an animal model of antigen-induced arthritis, an extract of B. burgdorferi that contained OspA induced arthritis in the knee (58). In contrast, mice made transgenic for both OspA and Osp B develop arthritis after exposure to B. burgdorferi which is similar to the arthritis that develops in nontransgenic control mice (49). The pathogenesis of the neuropathy associated with Lyme disease also is unclear. B. burgdorferi does not appear to be present in affected nerves. Antiaxonal antibodies have been identified in patients with Lyme disease (168). Antiaxonal antibodies similar to those found in patients with Lyme disease have been shown to alter axonal transport (166). In addition to these findings in peripheral nerves, cerebrospinal fluid of patients with Lyme disease contains antibodies reactive with myelin basic protein (52, 170). The role of these antibodies in the pathogenesis of the neurologic manifestations of Lyme disease is unknown.
SUSCEPTIBILITY IN VITRO AND IN VIVO
Results of in vitro and in vivo antimicrobial susceptibility tests for B. burgdorferi have been conflicting. Agents which appear to be quite active in vitro have been ineffective in vivo. The discrepancy between in vitro and in vivo activity has been most notable for the macrolide antibiotics (142). Moreover, antibiotics with good activity in vitro as well as in animal models have not necessarily been effective in treating humans with Lyme disease. For example, roxithromycin has good activity in vitro (MBC: 0.06-0.25 ug/ml) and has been effective for treating gerbils infected with B. burgdorferi. However, treatment with roxithromycin failed in 5 of 19 patients with early Lyme disease (70). Therefore, the results of in vitro and in vivosusceptibility tests should not be used to guide therapy in the same manner in which they are used for many other bacterial infections.
The in vitro susceptibility tests utilize macro- or microdilution methods in standard Barbour-Stoenner-Kelly medium. The spirochetes are inoculated at a concentration of 105 organisms/ml and are incubated for 3-8 days at 32-34 degrees Centigrade. Absence of spirochetes by darkfield microscopy after the initial seven day incubation period is used to determine the minimal inhibitory concentration (MIC). An aliquot of medium is subcultured for an additional 7-42 days and then examined microscopically to determine the minimal bactericidal concentration (MBC). The results of in vitro susceptibility tests of B. burgdorferi may be influenced by the following factors (35, 79, 98, 122):
1) Strain of the organism
2) Prior passage of the organism in vitro
3) Duration of incubation before determination of the MIC or the MBC
In other studies, investigators have evaluated the susceptibility of multiple different strains of the organism, have standardized the preparation of laboratory strains, and have incubated the organisms for 7 days prior to determination of the MIC and for 7-21 days prior to determination of the MBC (35,98). The most active agents in vitro are the tetracyclines, clarithromycin, amoxicillin, cefotaxime, and ceftriaxone (35, 79, 98). In vivo susceptibility testing has been conducted primarily in gerbils, hamsters (61), rats (17), rabbits (118) and mice (119). The animals are inoculated intradermally or intraperitoneally with 104 to 108 organisms and then treated with standard antibiotic regimens. Hamsters may be treated with cyclophosphamide prior to inoculation to induce immunosuppression (8). The animals are sacrificed and tissues are subcultured and examined histologically for the presence of spirochetes. In the hamster model of hind paw inoculation (8), therapeutic efficacy is determined by measuring the reduction in swelling of the paws.
The results of antimicrobial susceptibility testing using animal models of Borrelial infection may be influenced by the following factors:
1) Species of animal used
2) Strain of B. burgdorferi
3) Method used to detect surviving organisms
The most active agents in vivo are ceftriaxone, clarithromycin, and the tetracyclines (8,119). The basis for the occasional discrepancy between in vitro and in vivo results of antimicrobial susceptibility tests for B. burgdorferi is unknown. The organism may persist intracellularly where antibiotic agents may vary widely in their effectiveness (53, 111, 117). For example, B. burgdorferi has been shown to persist within fibroblasts where they may avoid the antimicrobial effects of ceftriaxone (53). The organism may also interact with host factors to alter its pathogenicity. For example, addition of cytokines to the culture medium enhances the pathogenicity of B. burgdorferi (60, 61).
The selection of antimicrobials appropriate to treat B. burgdorferi should be based on results of clinical trials in addition to studies conducted in vitro or in animal models. Reliance solely on either in vitro susceptibility results or data from animal models may lead to the use of antimicrobial agents that are ineffective.
ANTIMICROBIAL THERAPY
Choice of treatment for Lyme disease depends on the manifestations of the illness. Practice guidelines for the treatment of persons with Lyme disease have been issued by the Infectious Diseases Society of America and by the American Academy of Neurology (66, 217). Although there is substantial evidence that treatment of Lyme disease, whatever the stage, usually is highly effective, there are few controlled clinical trials in which different doses of medication and/or different durations of treatment are compared (34, 125). In addition, the "success rates" of treatment that have been reported in various studies are highly dependent on the definition of cure. Many studies classified patients as treatment failures if they had any symptoms, even nonspecific symptoms such as fatigue or intermittent arthralgia, after antimicrobial treatment was discontinued. It is clear that some patients have such nonspecific symptoms (without objective signs of active infection) that persist for some time after treatment (219, 36, 165). There is no evidence that such nonspecific symptoms indicate that the previous treatment was inadequate or that additional antimicrobial treatment is necessary (217, 218). Indeed, in the vast majority of patients these symptoms resolve in a matter of weeks without additional antimicrobial treatment. A large number of studies now indicate that the long-term outcomes of the vast majority of persons treated for Lyme disease are excellent (84,155, 156, 157, 211). Recommended treatment for Lyme disease is shown in the table (2, 144,167, 218).
Children <9 years of age and pregnant women generally should not be treated with eitherdoxycycline or tetracycline because they may cause permanent discoloration of the teeth of children or of a fetus, although there are some recent data to indicate this risk is relatively low in children treated with a short course of doxycycline (101). Patients who are treated with doxycycline should be alerted to risk of drug-related, sun-induced dermatitis. Some patients with Lyme disease who receive antimicrobial treatment develop a Jarisch-Herxheimer reaction,marked by increased fever, myalgia and chills, within 48 hours after treatment is begun (120). The symptoms, presumably a reaction to bacteriolysis and release of bacterial products, usually resolve within one to three days. Antimicrobial treatment should not be stopped. Non-steroidal anti-inflammatory agents may be useful in treating the symptoms of the Jarisch-Herxheimer reaction as well as the myalgia, arthralgia, headache and other symptoms of Lyme disease.
Early Localized Disease
The goals of treatment of early localized disease are to alleviate symptoms and to prevent development of either early disseminated or late Lyme disease. Early studies found penicillin and tetracycline to be effective in the treatment of early Lyme disease (178, 179). Subsequently it was shown that treatment of early Lyme disease with doxycycline, amoxicillinor cefuroxime was as or more effective than treatment with penicillin or tetracycline and could be administered in fewer daily doses (125, 130). Overall success rates ranged from 90-100% with these drugs. Although in most studies treatment was administered for 3 weeks, some patients were cured with 10 days of treatment (114). Optimal duration of treatment is unknown (218). In most instances, persons who developed objective neurologic manifestations of Lyme disease despite treatment had symptoms that suggested that early neurologic involvement already was present at the time that treatment was initiated. Treatment sterilizes skin lesions rapidly (124). Limited data on treatment of patients with Lyme disease with macrolide antibiotics, including erythromycin, azithromycin and clarithromycin, suggests that these drugs may not be as effective as the above-cited agents, and should be reserved for patients with contraindications to the preferred drugs (22, 109, 110, 114, 151).
Early Disseminated Disease
Patients with early disseminated Lyme disease manifest as either multiple erythema migrans or cranial neuritis (including seventh nerve palsy) can be treated for 14-21 days with the same agents used to treat early localized disease (34, 38, 71, 144, 218). In the only comparative clinical trial of treatment of patients with disseminated Lyme disease (without meningitis), there were no significant differences in the outcomes of patients treated withdoxycycline (100 mg bid for 21 days) vs. ceftriaxone (2 grams qd for 14 days) (34). Both regimens were extremely efficacious. Because of lower cost and fewer side effects, treatment with doxycycline (or another oral agent, such as amoxicillin) is recommended.
There has been controversy about treatment of patients with facial palsy (160). Some believe that a patient with facial palsy due to Lyme disease should undergo lumbar puncture and, if the results of the analyses of the cerebrospinal fluid are abnormal, the patient should be treated parenterally with antibiotics. Although there is evidence that B. burgdorferi may affect the central nervous system in patients with early Lyme disease (107), even among patients with no apparent neurologic involvement (94), there is little evidence that parenterally administered treatment necessarily is indicated. On the contrary, there is substantial evidence that orally administered therapy is highly effective (66,25,34,56,75,84,85,167,218). Nevertheless, this remains an area of controversy. There is no evidence that use of corticosteroids improves the outcomes of patients with facial nerve palsy due to Lyme disease (25).
Patients with Bannwarth’s syndrome (radiculoneuritis) have been treated successfully with either ceftriaxone or cefotaxime as well as with doxycycline (31, 33, 84, 124, 128). There is some evidence that corticosteroids are effective for treatment of patients with painful radiculitis (136). Many experts recommend that patients with meningitis be treated parenterally with ceftriaxone, cefotaxime or penicillin, although there are many reports of successful treatment with orally administered tetracycline or doxycycline (4, 31, 33, 137, 138, 178). Indeed, the practice parameter issued by the American Academy of Neurology in 2007 concludes that doxycycline administered orally is an acceptable alternative to parenteral treatment of Lyme meninigitis for most patients (66).
There are no clinical trials of treatment of carditis with antimicrobials and no evidence that antimicrobial treatment hastens the resolution of carditis. There are reports of resolution of carditis after both orally and parenterally administered antimicrobial treatment as well as after no antimicrobial treatment (71, 106, 133, 148, 177, 178, 207). Temporary cardiac pacing may be necessary in patients with high-grade heart block (106). Nevertheless, experts generally recommend 2-3 weeks of parenteral treatment for patients with symptomatic carditis with high-grade heart block, although patients with only mild manifestations or in whom low-grade heart block is an incidental finding may be treated orally (144, 210). Corticosteroids may be useful if there is any delay in the resolution of the heart block (115).
Late Disease
Lyme arthritis can be treated effectively with either orally administered or parenterally administered treatment (25, 30, 34, 56, 72, 159, 167, 181, 189, 206). Treatment with an oral antimicrobial is more cost-effective than parenteral treatment (40). In most instances the arthritis will resolve within 2 months. In some patients the arthritis persists or recurs. Such patients should be treated with another course of antibiotics--some would repeat a course of an orally administered antimicrobial, while others would initiate parenteral treatment with a drug such as ceftriaxone. Rarely, patients with Lyme disease may develop chronic or recurrent arthritis; there appears to be an association with certain HLA types and this apparent autoimmune disease that is triggered by infection with B. burgdorferi (59, 186). There is little evidence of persistent infection in patients with chronic or recurrent arthritis who have received adequate antimicrobial treatment (128). Patients with chronic arthritis unresponsive to antimicrobial treatment have been treated with remitting agents, such as hydroxychloroquine, or with synovectomy (32, 138, 152, 176,189).
There is controversy about the entity of late neurologic Lyme disease (sometimes called either chronic or tertiary neuroborreliosis). Although there are some well-documented cases of patients with late neurologic Lyme disease (102), there are also many poorly substantiated reports (214). Although there are no clinical trials of treatment, there are reports of success using either penicillin or ceftriaxone (105,180, 218). Most experts recommend a course (2-4 weeks) of a parenterally administered antimicrobial such as ceftriaxone (105, 167, 218).
There has been considerable publicity about so-called “chronic Lyme disease,” usually applied to patients with chronic non-specific symptoms (such as pain or fatigue) with no objective physical findings. There is no evidence that such a condition exists (47) nor that prolonged antimicrobial treatment is of any benefit (67, 89).
Special Infections
Ocular Disease
A wide range of ocular manifestations of Lyme disease have been reported (86, 96). While the most commonly reported ocular manifestations are relatively minor (e.g., conjunctivitis) and respond to standard, orally administered regimens, occasionally more serious ocular disease, including retinitis, has been reported. Since most such reports base the association with Lyme disease on a positive serology (which could be from past infection or could be a false-positive result), the true spectrum of ocular disease is unclear. There are no clinical trials of treatment. Successful treatment of intraocular Lyme borreliosis withceftriaxone has been reported (201).
Borrelial Lymphocytoma and Acrodermatitis Atrophicans
These skin manifestations of Lyme disease occur primarily in Europe (10, 77). They are rare in the United States, presumably because of regional, strain-specific differences in the organism. Lymphocytoma, a dense inflammatory infiltrate of lymphocytes that occurs at the site of inoculation of the organism, is usually treated with orally administered drugs. Either doxycycline, penicillin or amoxicillin, administered for 2 weeks, has been effective (10, 77, 190, 199). Acrodermatitis atrophicans, a late manifestation of Lyme disease, is a chronic, sclerotic skin lesion. In a clinical trial, 3-4 weeks of orally administered penicillin or doxycycline was more effective than a 2-week course of ceftriaxone (1). Hence, patients with acrodermatitis atrophicans should be treated for 4 weeks with either doxycycline or amoxicillin (1, 87).
Pregnancy
Because clinical syndromes caused by congenital infection have been recognized with other spirochetal infections such as syphilis, there has been substantial concern about the possible transmission of B. burgdorferi from an infected pregnant woman to her unborn fetus. Although there are case reports in which B. burgdorferi has been identified from several abortuses and from a few live-born children with congenital anomalies, the placentas, abortuses and tissues in which the spirochete was identified did not show histologic evidence of inflammation (112, 151, 213). In addition, no consistent pattern of congenital malformations (as would be expected in a "syndrome" due to congenital infection) has been identified. In longitudinal studies of pregnant women who developed Lyme disease that were conducted by the Centers for Disease Control, the adverse outcomes that occurred could not be attributed to infection with B. burgdorferi (113). Furthermore, in two serosurveys and in a prospective study that were conducted in endemic areas, there were no significant differences in the prevalence of congenital malformations among the offspring of women with serum antibodies against B. burgdorferi and the offspring of those without such antibodies (151, 200, 215). Moreover, in a large-scale survey no children with neurologic abnormalities attributable to Lyme disease could be identified by child neurologists in endemic areas (55).
There is no definite evidence that congenital disease caused by B. burgdorferi occurs, although the existence of such a syndrome also has not been ruled out (173). If it does occur, congenital Lyme disease must be extremely rare. Consequently, in theory, pregnant women who develop Lyme disease should not be treated differently than others with Lyme disease. There is no evidence that either longer courses of treatment or parenteral treatment is indicated for pregnant women with early Lyme disease. However, because of the potential adverse effects oftetracyclines on the fetus, these antimicrobials (including doxycycline) should be avoided. Consequently, amoxicillin is the drug of choice to treat pregnant women with uncomplicated Lyme disease. Cefuroxime and Erythromycin are alternative agents for women who are allergic to penicillin.
Tick Bites
Physicians in endemic areas often are asked whether a patient who is bitten by a deer tick should receive antimicrobial prophylaxis. There is substantial evidence that the risk of Lyme disease after a recognized deer tick bite, even in hyperendemic areas, does not exceed 3% (126, 159). Presumably this is because ticks must be infected with B. burgdorferi (only a minority of ticks are infected) and, even then, the infected tick must feed for 48 hours or longer before the risk of transmission of B. burgdorferi becomes substantial (127, 141). Nearly 75% of persons who remove ticks do so before the tick has fed for 48 hours (42).
Investigators who conducted different randomized, double-blind clinical trials in which >600 subjects bitten by deer ticks were treated with either a placebo or an antibiotic (penicillin,tetracycline, or amoxicillin) concluded that routine antimicrobial prophylaxis is not warranted (6, 26, 159). However, a double-blind randomized trial of antimicrobial prophylaxis for ticks bites conducted in Westchester County, NY found that a single, 200 mg dose of doxycycline was 87% effective in preventing Lyme disease, although the 95% confidence interval around this estimate of efficacy was wide (the lower bound was 25% or less, depending on the method used) (127). In that study, the only persons who developed Lyme disease had been bitten by nymphal stage ticks that were at least partially engorged; among recipients of placebo, the risk of Lyme disease among persons bitten by an engorged nymphal-stage tick was 9.9%, while it was 0% for persons bitten by all larval and adult deer ticks. Unfortunately, the expertise to identify the species, stage and degree of engorgement of a tick, and thereby to assess the degree of risk, is rarely available to persons who are bitten. Consequently, routine use of antimicrobial agents to prevent Lyme disease in persons who are bitten by a deer tick, even in highly endemic areas, is still not generally recommended because the overall risk of Lyme disease is low (1-3%), treatment for Lyme disease, if it does develop, is very effective, and a substantial proportion of patients who took doxycycline developed nausea and/or vomiting (127, 162). In the unusual instance in which doxycycline prophylaxis is used (e.g., in a patient who removes a fully engorged nymphal-stage deer tick in an endemic area), only a single dose of doxycycline (200 mg) should be used, and it should taken with food to minimize nausea.
Serologic testing for Lyme disease after a recognized tick bite also is not recommended. Antibodies to B. burgdorferi that are present at the time that the tick is removed likely would be due either to a false-positive test result or to an earlier infection with B. burgdorferi rather than to a new infection from the recent bite. Likewise, in this setting the predictive value of a positive result is very low.
Ascertainment of whether the tick is infected, using tests such as the polymerase chain reaction (PCR), is not useful. While testing ticks with PCR may provide important epidemiologic information, the predictive values for infection of humans of either a positive or a negative PCR test result is unknown. Routine serologic testing of persons bitten by a deer tick also is not indicated. Among more than 1000 subjects who have been followed in prospective studies after recognized tick bites, none has been reported to have developed either late disease or latent infection (i.e., asymptomatic seroconversion, the clinical significance of which is unknown). Moreover, serologic tests for Lyme disease do not have a high enough specificity to be useful for screening (22, 154, 204).
Co-Infections
The same tick that transmits B. burgdorferi also can transmit Anaplasma and Babesia. Co-infection with either or both of these organisms has been well documented (126, 212). However, except in the very rare instances of persons who are either asplenic or otherwise significantly immunocompromised and who develop Babesiosis, the outcomes of persons with such co-infections are generally excellent (212). Since it is not necessary routinely to treat for co-infections (and Anaplasma are susceptible to doxycycline), evidence of co-infection should be sought only if there are specific clinical findings that raise concern about the role of co-infection (127).
Seropositive Persons Who Are Asymptomatic
Although antibody tests for Lyme disease are not sufficiently accurate to be used for screening and should not be ordered for patients who are asymptomatic or who have only nonspecific symptoms (22, 154, 204), occasionally physicians are confronted with asymptomatic patients with positive results of antibody tests for B. burgdorferi. In many instances these will be false-positive results, but in some instances the test result may be accurate (see section above on Diagnosis). There are not adequate data to know whether asymptomatic persons with antibodies to B. burgdorferi should be treated. Presumably, one would be concerned about the possible development of late neurologic disease in such patients. However, if the purpose of treatment is to eradicate B. burgdorferi from the central nervous system, orally administered antimicrobials may not be effective. On the other hand, it seems unreasonable to commit a person to an expensive, inconvenient course of treatment with a parenterally administered drug such as ceftriaxone without evidence that such patients are at risk of poor outcomes without treatment.
Underlying Diseases
There is little information about Lyme disease in persons who are immunosuppresed or otherwise compromised
Alternative Therapy
Alternative drugs for persons who cannot tolerate the usual medications are listed in Table 1.
ADJUNCTIVE THERAPY
Nonsteroidal anti-inflammatory agents often are useful for treating associated symptoms such as arthralgia or myalgia. In addition, they may be effective in hastening the resolution of synovitis when it is present.
ENDPOINTS FOR MONITORING THERAPY
The most appropriate endpoint for monitoring the effects of treatment is careful clinical evaluation of the patient. Laboratory tests generally are not helpful. Antibodies against B. burgdorferi may develop, persist or even rise in concentration despite adequate treatment and clinical cure of the patient. Likewise, IgM antibodies may persist despite cure of the infection (75). Symptoms such as fatigue, arthralgia and myalgia sometimes persist for some time after completion of a course of treatment for Lyme disease. These nonspecific symptoms (which may accompany or follow more specific symptoms and signs of Lyme disease but almost never are the sole manifestations of Lyme disease) usually resolve over a period of weeks. There is little evidence that such symptoms are related to persistence of B. burgdorferi, and there is no evidence that either repeated or prolonged courses of antimicrobials speed the resolution of such symptoms. There are a number of possible reasons for persistence of symptoms after a patient is treated for Lyme disease (169). It is critical to differentiate patients with nonspecific subjective complaints from those with objective evidence of an abnormality (e.g., synovitis). In patients in whom subjective symptoms persist or recur despite appropriate antimicrobial treatment, it is unlikely that additional or prolonged antimicrobial treatment will be of benefit (171). Indeed, there are clear recommendations against such practice by expert panels sanctioned by both the Infectious Diseases Society of America and the American College of Rheumatology (108,218). Moreover, such treatment is not cost-effective (100). In addition, there are reports of serious morbidity and even of death from such inappropriate prolonged treatment (41, 135).
VACCINES
A vaccine for Lyme disease that uses recombinant outer surface protein A (rOspA) as the antigen is approved for persons 15-70 years of age (5, 191). LYMErix™ (SmithKline Beecham Pharmaceuticals) contains 30 ug of purified rOspA lipidated protein combined with 0.5 mg of aluminum adjuvant. Because the vaccine was not profitable, the manufacturer announced in February 2002 that they would stop marketing this product. The efficacy of Lyme vaccine in preventing clinically apparent Lyme disease was 49% (95% confidence interval: 15-69%) in the first year, after two doses (191). After the third dose, the vaccine’s efficacy in preventing symptomatic Lyme disease was 76% (95% confidence interval: 58-86%). Three doses of the vaccine are required for optimal protection in adults (the vaccine is more immunogenic in children) (45, 172); the second dose is given one month after the first dose and the third dose is given 12 months after the first dose. Preliminary data suggest that other immunization schedules (e.g., 0,1, 6 months or 0, 1, 2 months) are safe and induce antibody responses similar to the 0,1, 12 month schedule (153). However, at this time, only the 0, 1, 12 month schedule is approved by the FDA.
This rOspA vaccine has a unique mode of action. OspA is expressed by B. burgdorferithat reside in the midguts of ticks; expression of OspA is later down-regulated in response to a blood meal. Since ticks must become engorged with blood before they transmit the organism, the human host who becomes infected with B. burgdorferi has little exposure to the OspA protein (at least in the early stages of infection). When an immunized host is bitten by a tick infected with B. burgdorferi, the host’s vaccine-induced antibodies against OspA are ingested by the tick. Antibody-dependent killing of B. burgdorferi occurs within the tick, thereby preventing transmission to the host. However, unlike with many other vaccines, since the host is not directly exposed to the OspA antigen, boosting of the immune response to OspA through natural exposure does not occur. Consequently, it is likely that additional booster doses of the vaccine will be needed to maintain immunity. Studies suggest that Lyme disease is cost-effective only if the patient’s risk of Lyme disease is extremely high (>1%/year) (158).
Indications
Lyme vaccine currently is approved only for persons 15-70 years of age. The decision to administer Lyme vaccine should be based on determination of the person’s risk of developing Lyme disease, which depends on the likelihood of being bitten by ticks infected withB. burgdorferi (for most people who live in endemic areas, the overall risk is relatively low). The potential benefits of the vaccine (compared with other protective measures, including early diagnosis and treatment of Lyme disease) should be considered, as should the costs and adverse side effects of the vaccine. Lyme vaccine does not protect all recipients from infection with B. burgdorferi and provides no protection against other tick-borne diseases. Therefore, vaccinated persons should continue to practice personal protective measures against tick bites. Because the risk of Lyme disease, even in endemic areas, is relatively low, and treatment of Lyme disease usually is very effective, the Advisory Committee on Immunization Practices recommends that Lyme vaccine be considered for persons in endemic areas who are at increased risk because of either occupational or recreational activities that frequently put them in tick-infested woods or fields (5). Although persons who have had Lyme disease may be given the vaccine (indeed, those with early localized disease that was treated with antibiotics may be good candidates, since they have shown that they are at risk and they may become infected again), the vaccine should not be given to patients with treatment-resistant Lyme arthritis. Dose Lyme vaccine is administered as a 0.5 ml dose intramuscularly (into the deltoid muscle). A second dose should be administered one month later and a booster dose should be give 12 months later. New, accelerated schedules and additional booster doses may be recommended in the future. Adverse Effects The most frequently reported adverse side effects of the vaccine are pain, rubor, and swelling at the site of the injection. These side effects are usually mild and self-limited. In the prelicensure studies, there was no evidence that the vaccine exacerbated prior Lyme arthritis, caused neurologic disease, or caused arthritis in subjects (including those with a history of Lyme disease).
PREVENTION
Reducing the risk of tick bites is one strategy to prevent Lyme disease (73, 218). In endemic areas, clearing brush and trees, removing leaf litter and woodpiles, and keeping grass mowed may reduce exposure to ticks (163). Application of pesticides to residential properties is effective in suppressing populations of ticks, but may be harmful both to other wildlife and to people (29). Erecting fences to exclude deer from residential yards and maintaining tick-free pets also may reduce exposure to ticks. Tick and insect repellents that contain n,n-diethylmetatoluamide (DEET) applied to the skin provide additional protection, but require reapplication every 1 to 2 hours for maximum effectiveness (20, 51). Serious neurologic complications from either frequent or excessive application of DEET-containing repellents have been reported, but they are rare and the risk is low when these products are used according to instructions on their labels (24). Use of products with concentrations of DEET greater than 30% is not necessary and increases the risk of adverse effects. DEET should be applied sparingly only to exposed skin. Permethrin (a synthetic pyrethroid) is available in a spray for application to clothing only and is particularly effective because it kills ticks on contact. Persons should be taught to inspect themselves and their clothing daily after possible exposure to Ixodid ticks. An attached tick should be grasped with medium-tipped tweezers as close to the skin as possible and removed by gently pulling the tick straight out. If some of the mouth parts remain embedded in the skin, they should be left alone, since they usually are extruded eventually; additional attempts to remove them often result in unnecessary damage to tissue and may increase the risk of local bacterial infection.
CONTROVERSIES
There have been efforts to characterize Lyme disease as a difficult-to-treat infection that often leads to chronic symptoms that do not respond to anything but very prolonged antimicrobial treatment. At the current time the evidence indicates that the vast majority of patients with Lyme disease respond well to conventional courses of antimicrobial treatment. Moreover, there is evidence that prolonged courses of treatment do more harm than good (41,100, 108, 135). The committee of the Infectious Diseases Society of America that developed Practice Guidelines for patients with Lyme disease concluded that there was no such diagnostic entity as "chronic Lyme disease" that represents active infection requiring long-term antibiotic therapy (217). There is additional evidence that patients with "chronic Lyme disease" are not suffering from chronic infection with the organism. Patients with "chronic Lyme disease" were enrolled in a double-blind randomized clinical trial of long-term antibiotic treatment (89). Patients were stratified by whether they were seropositive or seronegative, and within each stratum were randomized to receive treatment with either one month of ceftriaxone followed by two months of doxycycline or identical placebos. The clinical trials were stopped (by the data monitoring and safety oversight committee) before the projected enrollment was completed, because there were virtually no differences in outcomes between the antibiotic and control groups. Three other prospective studies investigating whether longer term antibiotic therapy can yield significant, durable improvement have failed to find any benefit compared to placebo (93,132, 43). Other diagnoses should be considered for persons who fail to respond to conventional treatment. If objective abnormalities persist for several months despite adequate courses of antimicrobial treatment, the patient should be referred to an appropriate specialist experienced in the diagnosis and treatment of Lyme disease.
REFERENCES
1. Aberer E, Breier F, Stanek G, Schmidt B. Success and failure in the treatment of acrodermatitis chronica atrophicans. Infection 1996;24:85-7. [PubMed]
2. Abramowicz M. Treatment of Lyme disease. Med Letter 2000;42:37-9.
3. Ackermann, R., et al., Chronic neurologic manifestations of erythema migrans borreliosis. Ann N Y Acad Sci, 1988; 539:16-23.
4. Ackley A Jr., Lupovici M. Lyme-disease meningitis treated with tetracycline. Ann Intern Med 1986;105:630-1. [PubMed]
5. Advisory Committee on Immunization Practices. Centers for Disease Control and Prevention. Recommendations for the use of Lyme disease vaccine. MMWR 1999;48(RR-7):1-25. [PubMed]
6. Agre F, Schwartz R. The value of early treatment of deer tick bite for the prevention of Lyme disease. Am J Dis Child 1993;147:945-7. [PubMed]
7. Aguero-Rosenfeld ME, et al., Evolution of the serologic response to Borrelia burgdorferi in treated patients with culture-confirmed erythema migrans. J Clin Microbiol, 1996;34(1):1-9.[PubMed]
8. Alder J, Mitten M, Jarvis K, Gupta P, Clement J. Efficacy of clarithromycin for treatment of experimental Lyme Disease in vivo. Antimicrob Agents Chemother 1993;37:1329-35. [PubMed]
9. Anonymous. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme disease. MMWR 1995;44:590. [PubMed]
10. Asbrink E, Hovmark A. Early and late cutaneous manifestations in Ixodes-borne borreliosis. Ann NY Acad Sci 1988;539:4-15. [PubMed]
11. Auwaerter PG, Aucott J, Dumler JS. Lyme borreliosis (Lyme disease): molecular and cellular pathobiology and prospects for prevention, diagnosis and treatment. Expert Rev Mol Med, 2004:1-22. [PubMed]
12. Auwaerter PG. Point: antibiotic therapy is not the answer for patients with persisting symptoms attributable to lyme disease. Clin Infect Dis, 2007;45(2):143-8. [PubMed]
13. Bacterial Zoonoses Branch, Centers for Disease Control: Evaluation of serologic tests for Lyme disease: Report of a national evaluation. Lyme Disease Surveillance Summary 1991;2:1-3.
14. Bakken LL, Case KL, Callister SM, Bourdeau NJ, Schell RF. Performance of 45 laboratories participating in a proficiency testing program for Lyme disease serology. JAMA 1992;268:891-5. [PubMed]
15. Bakken LL, Callister SM, Wand PJ, Schell RF. Interlaboratory comparison of test results for detection of Lyme disease by 516 participants in the Wisconsin State Laboratory of Hygiene/College of American Pathologists proficiency testing program. J Clin Microbiol 1997;35:537-43. [PubMed]
16. Barbour A, Hayes SF. Biology of Borrelia species. Microbiol Rev 1986;50:381-400.[PubMed]
17. Barthold SE, Moody KD, Terwilliger GA, Duray PH, Jacoby RO, Steere AC. Experimental Lyme arthritis in rats infected with Borrelia burgdorferi Experimental Lyme arthritis in rats infected with Borrelia burgdorferi. J Infect. Dis 1988;157:842-6. [PubMed]
18. Bateman H, Sigal L. Update on Lyme Carditis. Curr Infect Dis Rep, 2000;2(4):299-301.[PubMed]
19. Bedell SE, Pastor BM, Cohen SI. Symptomatic high grade heart block in Lyme disease. Chest 1981;79:236-7. [PubMed]
20. Brown M, Hebert AA. Insect repellents: An overview. J Am Acad Dermatol. 1997;36:243-9. [PubMed]
21. Bunikis J, Barbour AG: Laboratory testing for suspected Lyme disease. Med Clin North Am, 2002;86(2):311-40. [PubMed]
22. Burlington DB. FDA Public Health Advisory: Assays for antibodies to Borrelia burgdorferi: Limitations, use, and interpretation for supporting a clinical diagnosis of Lyme disease. US Government Printing Office 1997;520-050 July 7, 1997.
23. Carlson D, et al., Lack of Borrelia burgdorferi DNA in synovial samples from patients with antibiotic treatment-resistant Lyme arthritis. Arthritis Rheum, 1999;42(12): 2705-9. [PubMed]
24. Centers for Disease Control. Seizures temporally associated with use of DEET insect repellent - New York and Connecticut. Morbid Mortal Wkly Rep. 1989;38:678-80. [PubMed]
25. Clark JR, Carlson RD, Sasaki CT, Pachner AR, Steere AC. Facial paralysis in Lyme disease. Laryngoscope 1985; 95:1341-5. [PubMed]
26. Costello CM, Steere AC, Pinkerton RE, Feder HM Jr. A prospective study of tick bites in an endemic area for Lyme disease. J Infect Dis 1989;159:136-9. [PubMed]
27. Coulter P, et al., Two-year evaluation of Borrelia burgdorferi culture and supplemental tests for definitive diagnosis of Lyme disease. J Clin Microbiol, 2005;43(10):5080-4. [PubMed]
28. Creange A, et al., Peripheral neuropathies after arthropod stings not due to Lyme disease: a report of five cases and review of the literature. Neurology, 1993;43(8): 483-8. [PubMed]
29. Curran KL, Fish D, Piesman J. Reduction of nymphal Ixodes dammini (Acari: Ixodidae) in a residential suburban landscape by area application of insecticides. J Med Entomol 1993;30:107-13. [PubMed]
30. Dattwyler RJ, Halperin JJ, Pass H, Luft BJ. Ceftriaxone as effective therapy in refractory Lyme disease. J Infect Dis 1987;155:1322-5. [PubMed]
31. Dattwyler RJ, Halperin JJ, Volkman DJ, Luft BJ. Treatment of late Lyme borreliosis -- Randomised comparison of ceftriaxone and penicillin. Lancet 1988; 1:1191-4. [PubMed]
32. Dattwyler RJ, Volkman DJ, Conaty SM. Amoxycillin plus probenecid versus doxycycline for treatment of erythema migrans borreliosis. Lancet 1990; 336:1404-6. [PubMed]
33. Dattwyler RJ, Grunwaldt E, Luft BJ. Clarithromycin in treatment of early Lyme disease: A pilot study. Antimicrob Agents Chemother 1996; 40:468-9. [PubMed]
34. Dattwyler RJ, Luft BJ, Kunkel MJ, Finkel MF, Wormser GP, Rush TJ, Grunwaldt E, Agger WA, Franklin M, Oswald D, Cockey L, Maladorno D. Ceftriaxone compared with doxycycline for the treatment of acute disseminated Lyme disease. N Engl J Med 1997;337:289-94. [PubMed]
35. Dever LL, Jorgensen JH, Barbour AG. Comparative in vitro activities of clarithromycin, azithromycin, and erythromycin against Borrelia burgdorferi. Antimicrob Agents Chemother 1993; 37:1704-6. [PubMed]
36. Dinerman H, Steere AC. Lyme disease associated with fibromyalgia. Ann Intern Med 1992; 117:281-5. [PubMed] [PubMed]
37. Dotevall L, Alestig K, Hanner P, Norkrans G, Hagberg L. The use of doxycycline in nervous system Borrelia burgdorferi infection. Scandinavian Journal of Infect Dis 1988;53(Suppl):74-79. [PubMed]
38. Dotevall L, Hagberg L. Penetration of doxycycline into cerebrospinal fluid in patients treated for suspected Lyme neuroborreliosis. Antimicrob Agents Chemother 1989; 33:1078-80. [PubMed]
39. Dressler F, Whalen JA, Reinhardt BN, Steere AC. Western blotting in the serodiagnosis of Lyme disease. J Infect Dis 1993; 167:392-400. [PubMed]
40. Eckman MH, Steere AC, Kalish RA, Pauker SG. Cost effectiveness of oral as compared with intravenous antibiotic therapy for patients with early Lyme disease or Lyme arthritis. N Engl J Med 1997;337:357-63. [PubMed]
41. Ettestad PJ, Campbell GL, Welbel SF, Genese CA, Spitalny KC, Marchetti CM, Dennis DT. Biliary complications in the treatment of unsubstantiated Lyme disease. J Infect Dis 1995; 171:356-61. [PubMed]
42. Falco RC, Fish D, Piesman J: Duration of Tick Bites in a Lyme Disease-endemic area. Am J Epidemiol 1996;143:187-92. [PubMed]
43. Fallon BA, et al., A randomized, placebo-controlled trial of repeated IV antibiotic therapy for Lyme encephalopathy. Neurology, 2008;70(13):992-1003. [PubMed]
44. Feder HM Jr, Gerber MA, Luger SW, Ryan RW. Persistence of serum antibodies to Borrelia burgdorferi in patients treated for Lyme disease. Clin Infect Dis 1992;15:788-93. [PubMed]
45. Feder HM Jr, Beran J, Van Hoecke C, Abraham B, De Clercq N, Buscarino C, Parenti DL. Immunogenicity of a recombinant Borrelia burgdorferi outer surface protein A vaccine against Lyme disease in children. J Pediatr 1999:135:575-9. [PubMed]
46. Feder HM Jr, Hunt MS. Pitfalls in the diagnosis and treatment of Lyme disease in children. JAMA 1995;274:66-8. [PubMed]
47. Feder HM Jr, Johnson BJB, O’Connell S, Shapiro ED, Steere AC, Wormser GP, the Ad Hoc International Lyme Disease Group. A critical appraisal of “Chronic Lyme Disease.” N Engl J Med 2007;357:1422-30. [PubMed]
48. Feder HM Jr., et al., A critical appraisal of "chronic Lyme disease". N Engl J Med, 2007. 357(14): p. 1422-30. [PubMed]
49. Fikrig E, Tao H, Chen M, Barthold SW, Flavell RA. Lyme borreliosis in transgenic mice tolerant to Borrelia burgdorferi OspA or B. J Clin Invest 1995; 96:1706-14. [PubMed]
50. Fleming RV, et al., Pre-treatment and post-treatment assessment of the C(6) test in patients with persistent symptoms and a history of Lyme borreliosis. Eur J Clin Microbiol Infect Dis, 2004. 23(8): p. 615-8. [PubMed]
51. Fradin MS. Mosquitoes and mosquito repellents: A clinician's guide. Ann Intern Med. 1998;128:931-40. [PubMed]
52. Garcia-Monco JC, Coleman JL, Benach JL. Antibodies to myelin basic protein in Lyme disease. J Infect Dis 1988; 158:667-8. [PubMed]
53. Georgilis K, Peacocke M, Klempner MS. Fibroblasts protect the Lyme disease spirochete, Borrelia burgdorferi, from ceftriaxone in vitro. J Infect Dis 1992; 166:440-4. [PubMed]
54. Gerber MA, Shapiro ED. Diagnosis of Lyme disease in children. J Pediatr 1992; 121:157-62. [PubMed]
55. Gerber MA, Zalneraitis EL. Childhood neurologic disorders and lyme disease during pregnancy. Pediatr Neuro 1994;11:41-3. [PubMed]
56. Gerber MA, Shapiro ED, Burke GS, Parcells VJ, Bell GL. Lyme disease in children in southeastern Connecticut. N Engl J Med 1996;335:1270-4. [PubMed]
57. Goldberg NS, et al., Vesicular erythema migrans. Arch Dermatol, 1992;128(11):1495-8.[PubMed]
58. Gondolf KB, Mihatsch M, Curschellas E, Dunn JJ, Batsford SR. Induction of experimental allergic arthritis with outer surface proteins of Borrelia burgdorferi. Arthritis Rheum 1994; 37:1070-7.[PubMed]
59. Gross DM, Forsthuber T, Tary-Lehmann M, et al. Identification of LFA-1 as a candidate autoantigen in treatment-resistant Lyme arthritis. Science 1998; 281:703-6. [PubMed]
60. Guner ES. Retention of B. burgdorferi pathogenicity and infectivity after multiple passages in a co-culture system. Experientia 1994; 50:54-9. [PubMed]
61. Guner ES. Complement evasion by the Lyme disease spirochete Borrelia burgdorferi grown in host-derived tissue co cultures: Role of fibronectin in complement resistance. Experientia 1996; 52:364-72. [PubMed]
62. Halperin JJ, Golightly M. Lyme borreliosis in Bell's palsy. Long Island Neuroborreliosis Collaborative Study Group. Neurology, 1992;42(7):1268-70. [PubMed]
63. Halperin JJ. Nervous system Lyme disease. J Neurol Sci, 1998;153(2):182-91. [PubMed]
64. Halperin JJ. Lyme disease and the peripheral nervous system. Muscle Nerve, 2003;28(2): 133-43. [PubMed]
65. Halperin JJ. Central nervous system lyme disease. Curr Neurol Neurosci Rep, 2005;5(6):446-52. [PubMed]
66. Halperin JJ, Shapiro ED, Logigian E, Belman AL, Dotevall L, Wormser GP, Krupp L, Gronseth G, Bever CT Jr. Practice parameter: Treatment of nervous system Lyme disease (An evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2007;69;91-102. [PubMed]
67. Halperin JJ. Prolonged Lyme disease treatment: Enough is enough. Neurology In Press (published on- line ahead of print: http://www.neurology.org/cgi/rapidpdf/01.WNL.0000291407.40667.69v1.pdf) [PubMed]
68. Hammers-Berggren S, et al., Borrelia burgdorferi-specific intrathecal antibody production in neuroborreliosis: a follow-up study. Neurology, 1993;43(1):169-75. [PubMed]
69. Hanrahan JP, Benach JL, Coleman JL, Bosler EM, Morse DL, Cameron DJ, Edelman R, Kaslow RA. Incidence and cumulative frequency of endemic Lyme disease in a community. J Infect Dis 1984; 150:489-96. [PubMed]
70. Hansen K, Hovmark A, Lebech AM, Lebech K, Olsson I, Halkier Sorensen L, Olsson E, Asbrink E. Roxithromycin in Lyme borreliosis: Discrepant results of an in vitro and in vivo animal susceptibility study and a clinical trial in patients with erythema migrans. Acta Derm Venereol (Stockh) 1992; 72:297-300. [PubMed]
71. Hansen K, Madsen JK. Myocarditis associated with tickborne Borrelia burgdorferi infection. Lancet 1986;2:1323-4 [PubMed]
72. Hassler D, Zöller L, Haude M, Hufnagel HD, Heinrich F, Sonntag HG. Cefotaxime versus penicillin in the late stage of Lyme disease -- prospective, randomized therapeutic study. Infection 1990;18:24-8. [PubMed]
73. Hayes EB, Maupin GO, Mount GA, Piesman J. Assessing the effectiveness of local Lyme disease control. J Public Health Manag Pract. 1999;5:84-92. [PubMed]
74. Hedberg CW, Osterholm MT. Serologic tests for antibody to Borrelia burgdorferi: Another Pandora's box for medicine? Arch Intern Med 150:732-3, 1990 (editorial). [PubMed]
75. Hilton E, Tramontano A, DeVoti J, Sood SK. Temporal study of immunoglobulin M seroreactivity to Borrelia burgdorferi in patients treated for Lyme borreliosis. J Clin Microbiol 1997;35:774-6. [PubMed]
76. Horowitz HW, et al., Liver function in early Lyme disease. Hepatology, 1996;23(6): 1412-7.[PubMed]
77. Hovmark A. Role of Borrelia burgdorferi in lymphocytomas and sclerotic skin lesions. Clin Dermatol 1993;11:363-7. [PubMed]
78. Hu L. Lyme arthritis. Infect Dis Clin North Am, 2005;19(4): 947-61. [PubMed]
79. Johnson RC, Kodner C, Russell M. In vitro and in vivo susceptibility of the Lyme disease spirochete Borrelia burgdorferi, to four antimicrobial agents. Antimicrob Agents Chemother 1987; 31:164-7. [PubMed]
80. Kaiser R, Lucking CH. Intrathecal synthesis of specific antibodies in neuroborreliosis. Comparison of different ELISA techniques and calculation methods. J Neurol Sci 1993;118(1):64-72. [PubMed]
81. Kalish RA, McHugh G, Granquist J, Shea B, Ruthazer R, Steere AC. Persistence of Immunoglobin M or Immunoglobin G antibody responses to Borrelia burgdorferi 10-20 years after active Lyme disease. Clin Infect Dis 2001;33:780-5. [[PubMed]
82. Kaplan RF, et al., Memory impairment and depression in patients with Lyme encephalopathy: comparison with fibromyalgia and nonpsychotically depressed patients. Neurology. 1992;42(7):1263-7. [PubMed]
83. Kaplan RF, et al., Neuropsychological deficits in Lyme disease patients with and without other evidence of central nervous system pathology. Appl Neuropsychol, 1999;6(1):3-11.[PubMed]
84. Karkkonen K, Stiernstedt SH, Karlsson M. Follow-up of patients treated with oral doxycycline for Lyme neuroborreliosis. Scand J Infect Dis 2001;33:259-62. [PubMed]
85. Karlsson M, Hammers S, Nilsson-Ehle I, Malmborg AS, Wretlind B. Concentrations of doxycycline and penicillin G in sera and cerebrospinal fluid of patients treated for neuroborreliosis. Antimicrob Agents Chemother 1996; 40:1104-7. [PubMed]
86. Kauffmann DJ, Wormser G. Ocular Lyme disease: case report and review of the literature. Brit J Ophthalmol 1990;74:325-7. [PubMed]
87. Kaufman LD, Gruber BL, Phillips ME, Benach JL. Late cutaneous Lyme disease: Acrodermatitis chronic atrophicans. Am J Med 1989;86:828-30. [PubMed]
88. Kimsey RB, Spielman A. Motility of Lyme disease spirochetes in fluid as viscous as the extracellular matrix. J Infect Dis 1990; 162:1205-8. [PubMed]
89. Klempner MS, Hu LT, Evans J, Schmid CH, Johnson GM, Trevino RP, Norton D, Levy L, Wall D, McCall J, Kosinski M, Weinstein A. Two controlled trials of antibiotic treatment in patients with persistent symptoms and a history of Lyme disease. N Engl J Med 2001;345:85-92. [PubMed]
90. Klempner MS, Schmid CH, Hu L, Steere AC, Johnson G, McCloud B, Noring R, Weinstein A. Intralaboratory reliability of serologic and urine testing for Lyme disease. Am J Med 2001;110: 217-9.[PubMed]
91. Kochi SK, Johnson RC. Role of immunoglobulin G in killing of Borrelia burgdorferi in the classical complement pathway. Infect Immun 1988;56:314-21. [PubMed]
92. Kruger H, et al., Acute and chronic neuroborreliosis with and without CNS involvement: a clinical, MRI, and HLA study of 27 cases. J Neurol, 1991;238(5): 271-80. [PubMed]
93. Krupp LB, et al., Study and treatment of post Lyme disease (STOP-LD): a randomized double masked clinical trial. Neurology, 2003;60(12):1923-30. [PubMed]
94. Kuiper H, de Jongh BM, van Dam AP, Dodge DE, Ramselaar AC, Spanjaard L, Dankert J. Evaluation of central nervous system involvement in Lyme borreliosis patients with a solitary erythema migrans lesion. Eur J Clin Microbiol Infect Dis 1994;13:379-87. [PubMed]
95. Larrosa F, Aguilar F, Benitez P. [Otoneurological manifestations of Lyme's disease]. Acta Otorrinolaringol Esp, 1999;50(8):644-8. [PubMed]
96. Lesser RL, Kornmehl EW, Pachner AR, Kattah J, Hedges TR 3rd, Newman NM, Ecker PA, Glassman MI. Neuro-ophthalmologic manifestations of Lyme disease. Ophthalmology 1990;97:699-706. [PubMed]
97. Lesser RL. Ocular manifestations of Lyme disease. Am J Med, 1995;98(4A): 60S-62S.[PubMed]
98. Levin JM, Nelson JA, Segreti J, Harrison B, Benson CA, Strle F. In vitro susceptibility of Borrelia burgdorferi to 11 antimicrobial agents. Antimicrob Agents Chemother 1993;37:1444-6.[PubMed]
99. Ley C, Le C, Olshen EM, Reingold AL. The use of serologic tests for Lyme disease in a prepaid health plan in California. JAMA. 1994;271:460-3. [PubMed]
100. Lightfoot RW, Luft BJ, Rahn DW, Steere AC, Sigel LH, Zoschke DC, Gardner P, Britton MC, Kaufman RL. Empiric parenteral antibiotic treatment of patients with fibromyalgia and fatigue and a positive serologic result for Lyme disease: A cost-effectiveness analysis. Ann Intern Med 1993;119:503-9. [PubMed]
101. Lochary ME, Lockhart PB, Williams WT. Doxycycline and staining of permanent teeth. Pediatr Infect Dis J 1998; 17:429–31. [PubMed]
102. Logigian EL, Kaplan RF, Steere AC. Chronic neurologic manifestations of Lyme disease. N Engl J Med 1990; 323:1438-44. [PubMed]
103. Logigian EL, et al., Reversible cerebral hypoperfusion in Lyme encephalopathy. Neurology, 1997;49(6):1661-70. [PubMed]
104. Logigian EL. Neurological Manifestations of Lyme Disease, in Lyme Disease, D.W. Rahn and J. Evans, Editors. 1998, American College of Physicians: Philadelphia, PA. p. 89-106.
105. Logigian EL, Kaplan RF, Steere AC. Successful treatment of Lyme encephalopathy with intravenous ceftriaxone. J Infect Dis 1999;180:377-83. [PubMed]
106. Lorincz I, Lakos A, Kovacs P, Varvolgyi C, Polgar P,Worum F. Temporary pacing in complete heart block due to Lyme disease: a case report. Pacing Clin Electrophysiol 1989;12:1433-6. [PubMed]
107. Luft BJ, Steinman CR, Neimark HC, Muralidhar B, Rush T, Finkel MF, Kunkel M, Dattwyler RJ. Invasion of the central nervous system by Borrelia burgdorferi in acute disseminated infection. JAMA 1992;267:1364-7. [PubMed]
108. Luft BJ, Gardner P, Lightfoot RW Jr, Joint Committee of the American College of Rheumatology and the Council of the Infectious Diseases Society of America. Appropriateness of parenteral antibiotic treatment for patients with presumed Lyme disease. Clin Infect Dis 1994;18:112. [PubMed]
109. Luft BJ, Dattwyler RJ, Johnson RC, Luger SW, Bosler EM, Rahn DW, Masters EJ, Grunwaldt E, Gadgil SD. Azithromycin compared with amoxicillin in the treatment of erythema migrans. A double blind, randomized, controlled trial. Ann Intern Med 1996;124:785 91.[PubMed]
110. Luger SW, Paparone P, Wormser GP, Nadelman RB, Grunwaldt E, Gomez G, Wisniewski M, Collins JJ. Comparison of cefuroxime axetil and doxycycline in treatment of patients with early Lyme disease associated with erythema migrans. Antimicrob Agents Chemother 1995;39:661-7. [PubMed]
111. Ma Y, Sturrock A, Weis J. Intracellular localization of Borrelia burgdorferi within human endothelial cells. Infect Immun 1991;59:671-8. [PubMed]
112. MacDonald AB, Benach JL, Burgdorfer W. Stillbirth following maternal Lyme disease. NY State J Med 1987;87:615-6. [PubMed]
113. Markowitz LE, Steere AC, Benach JL, Slade JD, Broome CV. Lyme disease during pregnancy. JAMA 1986;255:3394-6. [PubMed]
114. Massarotti EM, Luger SW, Rahn DW, Messner RP, Wong JB, Johnson RC, Steere AC. Treatment of early Lyme disease. Am J Med 1992;92:396-403. [PubMed]
115. McAlister HF, Klementowicz PT, Andrews C, Fisher JD, Feld M, Furman S. Lyme carditis: an important cause of reversible heart block. Ann Intern Med 1989;110:339-45. [PubMed]
116. Mogilyansky E, et al. Comparison of Western immunoblotting and the C6 Lyme antibody test for laboratory detection of Lyme disease. Clin Diagn Lab Immunol, 2004;11(5): p. 924-9.[PubMed]
117. Montgomery RR, Nathanson MH, Malawista SE. The fate of Borrelia burgdorferi, the agent for Lyme Disease, in mouse macrophages. Destruction, survival, recovery J Immunol 1993;150:909-15. [PubMed]
118. Moody KD, Barthold SW, Terwilliger GA. Lyme borreliosis in laboratory animals: effect of host species and in vitro passage level of Borrelia burgdorferi. Am J Trop Med Hyg 1990;43:87-92. [PubMed]
119. Moody KD, Adams RL, Barthold SW. Effectiveness of antimicrobial treatment against Borrelia burgdorferi infection in mice. Antimicrob Agents Chemother 1994;38:1567-72.[PubMed]
120. Moore JA. Jarisch-Herxheimer reaction in Lyme disease. Cutis 1987; 39:397-8.[PubMed]
121. Mullegger RR. Dermatological manifestations of Lyme borreliosis. Eur J Dermatol, 2004;14:296-309. [PubMed]
122. Mursic VP, Wilske B, Schierz G, Holmburger M, Suss E. In vitro and in vivo susceptibility of Borrelia burgdorferi. Eur J Clin Microbiol 1987;6:424-6. [PubMed]
123. Nadal D, Hunziker UA, Bucher HU, Hitzig WH, Duc G. Infants born to mothers with antibodies against Borrelia burgdorferi at delivery. Eur J Pediatr 1989;148:426-7. [PubMed]
124. Nadelman RB, Nowakowski J, Forseter G, Bittker S, Cooper D, Goldberg N, McKenna D, Wormser GP. Failure to isolate Borrelia burgdorferi after antimicrobial therapy in culture-documented Lyme borreliosis associated with erythema migrans: Report of a prospective study. Am J Med 1993;94:583-8. [PubMed]
125. Nadelman RB, Luger SW, Frank E, Wisniewski M, Collins JJ, Wormser GP. Comparison of cefuroxime axetil and doxycycline in the treatment of early Lyme disease. Ann Intern Med 1992; 117:273-80. [PubMed]
126. Nadelman RB, Nowakowski J, Fish D, Falco RC, Freeman K, McKenna D, Welch P, Marcus R, Aguero-Rosenfeld ME, Dennis DT, Wormser GP, Tick Bite Study Group. Prophylaxis with single-dose doxycycline for the prevention of Lyme disease after an Ixodes scapularis tick bite. N Engl J Med 2001;345:79-84. [PubMed]
127. Nadelman RB, Horowitz HW, Hsieh TC, Wu JM, Aguero-Rosenfeld ME, Schwartz I, Nowakowski J, Varde S, Wormser GP. Simultaneous human ehrlichiosis and Lyme borreliosis. N Engl J Med 1997; 337:27-30. [PubMed]
128. Nocton JJ, Dressler F, Rutledge BJ, Rys PN, Persing DH, Steere AC. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med 1994; 330:229-34. [PubMed]
129. Notice to Readers: Caution Regarding Testing for Lyme Disease MMWR Morb Mortal Wkly Rep, 2005;54(05): 125.
130. Nowakowski J, Nadelman RB, Forseter G, McKenna D, Wormser GP. Doxycycline versus tetracycline therapy for Lyme disease associated with erythema migrans. J Am Acad Dermatol 1995; 32:223-7. [PubMed]
131. Nowakowski, J, et al., Long-term follow-up of patients with culture-confirmed Lyme disease. Am J Med, 2003; 115:91-6. [PubMed]
132. Oksi, J, et al., Duration of antibiotic treatment in disseminated Lyme borreliosis: a double-blind, randomized, placebo-controlled, multicenter clinical study. Eur J Clin Microbiol Infect Dis, 2007;26:571-81. [PubMed]
133. Olson LJ, Okafor EC, Clements IP. Cardiac involvement in Lyme disease: Manifestations and management. Mayo Clin Proceed 1986; 61:745-9. [PubMed]
134. Pachner AR, Steere AC. The triad of neurologic manifestations of Lyme disease: meningitis, cranial neuritis, and radiculoneuritis. Neurology, 1985;35:47-53. [PubMed]
135. Patel R, Grogg KL, Edwards WD, Wright AJ, Schwenk NM. Death from inappropriate therapy for Lyme disease. Clin Infect Dis 2000;31:1107-9.[PubMed]
136. Pfister HW, Einhaupl KM, Franz P, Garner C. Corticosteroids for radicular pain in Bannwarth’s syndrome. Ann NY Acad Sci 1988;539:485-7.
137. Pfister HW, Preac-Mursic V, Wilske B, Einhäupl KM. Cefotaxime vs. penicillin G for acute neurologic manifestations in Lyme borreliosis: A prospective randomized study. Arch Neurol 1989; 46:1190-4. [PubMed] [PubMed]
138. Pfister HW, Preac-Mursic V, Wilske B, Schielke E, Sorgel F, Einhäupl KM. Randomised comparison of ceftriaxone and cefotaxime in Lyme neuroborreliosis. J Infect Dis 1991;163:311-8. [PubMed]
139. Philipp MT, et al., A decline in C6 antibody titer occurs in successfully treated patients with culture-confirmed early localized or early disseminated Lyme Borreliosis. Clin Diagn Lab Immunol, 2005;12(9):1069-74. [PubMed]
140. Piacentino JD, Schwartz BS. Occupational risk of Lyme disease: an epidemiological review. Occup Environ Med, 2002;59(2):75-84. [PubMed]
141. Piesman J. Dynamics of Borrelia burgdorferi transmission by nymphal Ixodes dammini ticks. J Infect Dis 1993; 167:1082-5. [PubMed]
142. Preac-Mursic V, Wilske B, Schierz G, Suss E, Gross B. Comparative antimicrobial activity of the new macrolides against Borrelia burgdorferi. Eur J Clin Microbiol 1989; 8:651-3.[PubMed]
143. Qureshi MZ, et al., Overdiagnosis and overtreatment of Lyme disease in children. Pediatr Infect Dis J, 2002;21:12-4. [PubMed]
144. Rahn DW, Malawista SE. Lyme disease: Recommendations for diagnosis and treatment. Ann Intern Med 1991;114:472-81. [PubMed]
145. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep, 1995;44:590-1.
146. Reid MC, Schoen RT, Evans J, Rosenberg JC, Horwitz RI. The consequences of overdiagnosis and overtreatment of Lyme disease: an observational study. Ann Intern Med 1998;128:354-62. [PubMed]
147. Reported Cases of Lyme Disease by Year, United States, 1991-2006. 2007 September 27, 2007 [cited 2008 March 26]; Available from: http://www.cdc.gov/ncidod/dvbid/lyme/ld_UpClimbLymeDis.htm.
148. Reznick JW, Braunstein DB, Walsh RL, Smith CR,Wolfson PM, Gierke LW, Gorelkin L, Chandler FW. Lyme carditis: electrophysiologic and histopathologic study. Am J Med 1986; 81: 923-7. [PubMed]
149. Rohacova H, Hancil J, Hulinska D, Mailer H, Havlik J. Ceftriaxone in the treatment of Lyme neuroborreliosis. Infection 1996;24:88-90. [PubMed]
150. Rosenstein ED, Kramer N. Lyme disease misdiagnosed as a brown recluse spider bite. Ann Intern Med. 1987; 107:782. [PubMed]
151. Schlesinger PA, Duray PH, Burke BA, Steere AC, Stillman MT. Maternal-fetal transmission of the Lyme disease spirochete, Borrelia burgdorferi. Ann Intern Med 1985;103:67-8. [PubMed]
152. Schoen RT, Aversa JM, Rahn DW, Steere AC. Treatment of refractory chronic Lyme arthritis with arthroscopic synovectomy. Arthritis Rheum 1991;34:1056 60. [PubMed]
153. Schoen RT, Sikand VK, Caldwell MC, Van Hoecke C, Gillet M, Buscarino C, Parenti DL. Safety and immunogenicity profile of a recombinant outer surface protein A Lyme disease vaccine: clinical trial of a 3-dose schedule at 0,1, and 2 months. Clin Therapeutics 2000;22:315-25. [PubMed]
154. Seltzer EG, Shapiro ED. Misdiagnosis of Lyme disease: When not to order serologic tests. Pediatr Infect Dis J 1996;15:762-3. [PubMed]
155. Seltzer EG, Gerber MA, Cartter ML, Freudigman K, ShapiroED. Long-term outcomes of persons with Lyme disease. JAMA 2000;283:609-16. [PubMed]
156. Shadick NA, Phillips CA, Logigian EL, Steere AC, Kaplan RF, Berardi VP, Duray PH, Larson MG, Wright EA, Ginsburg KS. The long-term clinical outcomes of Lyme disease: A population-based retrospective cohort study. Ann Intern Med 1994;121:560-7.[PubMed]
157. Shadick NA, Phillips CB, Sangha O, Logigian EL, Kaplan RF, Wright EA, Fossel AH, Fossel K, Berardi V, Lew RA, Liang MH. Musculoskeletal and neurologic outcomes in patients with previously treated Lyme disease. Ann Intern Med 1999;131:919-26. [PubMed]
158. Shadick NA, Liang MH, Phillips CB, Fossel K, Kuntz KM. The cost-effectiveness of vaccination against Lyme disease. Arch Intern Med 2001;161:554-61. [PubMed]
159. Shapiro ED, Gerber MA, Holabird N, Berg AT, Feder HM, Bell GL, Rys PN, Persing DH: A controlled trial of antimicrobial prophylaxis for Lyme disease after deer-tick bites. N Engl J Med 1992;327:1769-73. [PubMed] [PubMed]
160. Shapiro ED, Gerber MA. Lyme disease and facial nerve palsy: More questions than answers. Arch Pediatr Adolesc Med 1997;151:1183-4 (editorial). [PubMed]
161. Shapiro ED, Gerber MA. State-of-the-Art Clinical Article: Lyme disease. Clin Infect Dis. 2000;31:533-42. [PubMed]
162. Shapiro ED. Doxycycline for tick bites-Not for everyone. N Engl J Med 2001; 345:133 4. (editorial) [PubMed] [[PubMed]
163. Schulze TL, Jordan RA, Hung RW. Suppression of subadult Ixodes scapularis (Acari: Ixodidae) following removal of leaf litter. J Med Entomol. 1995;32:730-3. [PubMed]
164. Sibony P, et al. Reactive Lyme serology in optic neuritis. J Neuroophthalmol, 2005;25: 71-82. [PubMed]
165. Sigal LH. Experience with the first one hundred patients referral to a Lyme Disease referral center. Am J Med 1987;88:577-81.
166. Sigal LH, Tatum A. Lyme disease patients' serum contains IgM antibodies to Borrelia burgdorferi that cross react with neuronal antigens. Neurology 1988; 38:1439-42. [PubMed]
167. Sigal LH. Current recommendations for the treatment of Lyme disease. Drugs 1992;43:683-99. [PubMed] [PubMed]
168. Sigal LH. The flagellin of Borrelia burgdorferi, the causative agent of Lyme disease, cross reacts with a human axonal 64,000 molecular weight protein. J Infect Dis 1993; 167:1372-8. [PubMed]
169. Sigal LH. Persisting complaints attributed to chronic Lyme disease: Possible mechanisms and implications for management. Am J Med 1994;96:365-74. [PubMed]
170. Sigal LH. Management of Lyme disease refractory to antibiotic therapy. Rheum Dis Clin N Amer 1995;21:217-30. [PubMed]
171. Sigal LH. The Lyme disease controversy: Social and financial costs of misdiagnosis and mismanagement. Arch Intern Med 1996;156: 1493-1500. [PubMed]
172. Sikand VK, Halsey N, Krause PJ, Sood SK, Geller R, Van Hoecke C, Buscarino C, Parenti D, Pediatric Lyme Vaccine Study Group. Safety and immunogenicity of a recombinant Borrelia burgdorferi outer surface protein A vaccine against Lyme disease in healthy children and adolescents: a randomized controlled trial. Pediatrics 2001;108:123-8. [PubMed]
173. Silver HM. Lyme disease during pregnancy. Infect Dis Clin N Amer 1997;11:93-7. [PubMed]
174. Smith RP, et al., Clinical characteristics and treatment outcome of early Lyme disease in patients with microbiologically confirmed erythema migrans. Ann Intern Med, 2002;136:421-8.[PubMed]
175. Snydman DR, Schenkein DP, Berardi VP, Lastavica CC, Pariser KM. Borrelia burgdorferi in joint fluid in chronic Lyme arthritis. Ann Intern Med. 1986; 104:798-800. [PubMed]
176. Steere AC, Gibofsky A, Patarroyo ME, Winchester RJ, Hardin JA, Malawista SE. Chronic Lyme arthritis: Clinical and immunogenetic differentiation from rheumatoid arthritis. Ann Intern Med 1979;90:896-901. [PubMed]
177. Steere AC, Batsford WP, Weinberg M, Alexander J, Berger HJ,Wolfson S, Malawista SE. Lyme carditis: cardiac abnormalities of Lyme disease. Ann Intern Med 1980; 93:8-16. [PubMed]
178. Steere AC, Malawista SE, Newman JH, Spieler PN, Bartenhagen NH. Antibiotic therapy in Lyme disease. Ann Intern Med 1980; 93:1-8. [PubMed]
179. Steere AC, Hutchinson GJ, Rahn DW, Sigal LH, Craft JE, DeSanna ET, Malawista SE. Treatment of the early manifestations of Lyme disease. Ann Intern Med 1983; 99:22-6. [PubMed]
180. Steere AC, Pachner AR, Malawista SE. Neurologic abnormalities of Lyme disease: successful treatment with high dose intravenous penicillin. Ann Intern Med 1983;99:767-72.[PubMed]
181. Steere AC, Green J, Schoen RT, Taylor E, Hutchinson GJ, Rahn DW, Malawista SE. Successful parenteral penicillin therapy of established Lyme arthritis. N Engl J Med 1985; 312:869-74. [PubMed]
182. Steere AC, et al., The early clinical manifestations of Lyme disease. Ann Intern Med, 1983;99:76-82. [PubMed]
183. Steere AC, Taylor E, Wilson ML, Levine JF, Spielman A. Longitudinal assessment of the clinical and epidemiological features of Lyme disease in a defined population. J Infect Dis 1986; 154:294-300. [PubMed]
184. Steere AC, Schoen RT, Taylor E. The clinical evolution of Lyme arthritis. Ann Intern Med, 1987:107:725-31. [PubMed]
185. Steere AC. Lyme disease. N Engl J Med 1989;321:586-96. [PubMed]
186. Steere AC, Dwyer E, Winchester R. Association of chronic Lyme arthritis with HLA-DR4 and HLA-DR2 Alleles. N Engl J Med 1990; 323:219-23. [PubMed]
187. Steere AC, et al., Evaluation of the intrathecal antibody response to Borrelia burgdorferi as a diagnostic test for Lyme neuroborreliosis. J Infect Dis, 1990. 161(6): p. 1203-9. [PubMed]
188. Steere AC, Taylor E, McHugh GL, Logigian EL. The overdiagnosis of Lyme disease. JAMA 1993;269:1812-26. [PubMed]
189. Steere AC, Levin RE, Molloy PJ, Kalish RA, Abraham JH III, Liu NY, Schmid CH. Treatment of Lyme arthritis. Arth Rheum 1994; 37:878-88. [PubMed]
190. Steere AC. Diagnosis and treatment of Lyme arthritis. Med Clin N Amer 1997; 81:179-94.[PubMed]
191. Steere AC, Sikand VK, Meurice F, Parenti DL, Fikrig E, Schoen RT, Nowakowski J, Schmid CH, Laukamp S, Buscarino C, Krause DS. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface lipoprotein A with adjuvant. N Engl J Med. 1998;339:209-16. [PubMed]
192. Steere AC. Lyme disease. N Engl J Med 2001;345:115 25. [PubMed]
193. Steere AC, et al., Autoimmune mechanisms in antibiotic treatment-resistant lyme arthritis. J Autoimmun. 2001;16:263-8. [PubMed]
194. Steere AC, et al., Systemic symptoms without erythema migrans as the presenting picture of early Lyme disease. Am J Med, 2003;114:58-62. [PubMed]
195. Steere AC, et al., Binding of outer surface protein A and human lymphocyte function-associated antigen 1 peptides to HLA-DR molecules associated with antibiotic treatment-resistant Lyme arthritis. Arthritis Rheum, 2003;48:534-40. [PubMed]
196. Steere AC, et al. Prospective study of coinfection in patients with erythema migrans. Clin Infect Dis, 2003;36: 1078-81. [PubMed]
197. Steere AC, et al. Antibiotic-refractory Lyme arthritis is associated with HLA-DR molecules that bind a Borrelia burgdorferi peptide. J Exp Med, 2006;203:961-71. [PubMed]
198. Stricker RB. Counterpoint: long-term antibiotic therapy improves persistent symptoms associated with lyme disease. Clin Infect Dis, 2007;45:149-57. [PubMed]
199. Strle F, MaraspinV, Pleterski-Rigler D, Lotric Furlan S, Ruzic Sabljic E, Jurca T, Cimperman J. Treatment of borrelial lymphocytoma. Infection 1996; 24:80-4. [PubMed]
200. Strobino BA, Williams CL, Abid S, Chalson R, Spierling P. Lyme disease and pregnancy outcome: Prospective study of two thousand prenatal patients. Am J Obstet Gynecol 1993;169:367-75. [PubMed]
201. Suttorp-Schulten MS, Kuiper H, Kijlstra A, van Dam AP, Rothova A. Long-term effects of ceftriaxone treatment on intraocular Lyme borreliosis. Am J Ophthalmol 1993; 116:571-5.[PubMed]
202. Szczepanski A, Furie MB, Benach JL, Lane BP, Fleit HB. Interaction between Borrelia burgdorferi and endothelium in vitro. J Clin Invest. 1990; 85:1637-47. [PubMed]
203. Tibbles CD, Edlow JA. Does this patient have erythema migrans? JAMA, 2007;297:2617-27. [PubMed]
204. Tugwell P, Dennis DT, Weinstein A, Wells G, Shea B, Nichol G, Hayward R, Lightfoot R, Baker P, Steere AC. Laboratory evaluation in the diagnosis of Lyme disease. Ann Intern Med 1997;127:1109 23. [PubMed]
205. Tumani H, Nolker G, Reiber H. Relevance of cerebrospinal fluid variables for early diagnosis of neuroborreliosis. Neurology, 1995;45:1663-70. [PubMed]
206. Valešová H, Mailer J, Havlik J, Hulinska D, Hercogova J. Long-term results in patients with Lyme arthritis following treatment with ceftriaxone. Infection 1996;24:100-4. [PubMed]
207. van der Linde MR, Crijns HJGM,de Koning J, Hoogkamp Korstanje JA, de Graaf JJ, Piers DA, van der Galien A, Lie KI. Range of atrioventricular conduction disturbances in Lyme borreliosis: a report of four cases and review of other published reports. Br Heart J 1990; 63:162-8. [PubMed]
208. van der Linde MR. Lyme carditis: clinical characteristics of 105 cases. Scand J Infect Dis Suppl, 1991;77: 81-4. [PubMed]
209. Vázquez M, Sparrow SS, Shapiro ED. Long-term neuropsychologic and health outcomes of children with facial nerve palsy due to Lyme disease. Pediatrics 2003;112:e93-7. [PubMed]
210. Vlay SC. Complete heart block due to Lyme disease. N Engl J Med 1986; 315:1418. (Letter) [PubMed] [PubMed]
211. Wang TJ, Sangha O, Phillips CB, et al. Outcomes of children treated for Lyme disease. J Rheumatol 1998;25:2249-53. [PubMed]
212. Wang TJ, Liang MH, Sangha O, et al. Coexposure to Borrelia burgdorferi and Babesia microti does not worsen the long-term outcome of Lyme disease. Clin Infect Dis 2000;31:1149-54. [PubMed]
213. Weber K, Bratzke H, Neubert U, Wilske B, Duray PH. Borrelia burgdorferi in a newborn despite oral penicillin for Lyme borreliosis during pregnancy. Pediatr Infect Dis J 1988;7:286-9.[PubMed]
214. Weder B, Wiedersheim P, Matter L, Steck A. Chronic progressive neurologic involvement in Borrelia burgdorferi infection. J Neurol 1987; 234:40-3. [PubMed]
215. Williams CL, Benach JL, Curran AS, Spierling P, Medici F. Lyme disease during pregnancy: A cord blood serosurvey. Ann NY Acad Sci 1988;539:504-6.
216. Wormser GP, et al., Comparison of the yields of blood cultures using serum or plasma from patients with early Lyme disease. J Clin Microbiol, 2000;38:1648-50. [PubMed]
217. Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, M Klempner MS, Krause PJ, Bakken JS, Strle F, Stanek G, Bockenstedt L, Fish D, Dumler JS, Nadelman RB. The clinical assessment, treatment and prevention of Lyme disease, human granulocytic Anaplasmosis, and Babesiosis: Clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006;43:1089-1134. [PubMed]
218. Wormser GP, Nadelman RB, Dattwyler RJ, Dennis DT, Shapiro ED, Steere AC, Rush TJ, Rahn DW, Coyle PK, Persing DH, Fish D, Luft BJ. Infectious Diseases Society of America Practice Guidelines for the Treatment of Lyme Disease. Clin Infect Dis 2000;31 (Suppl 1): S1-S14. (Also available at www.idsociety.org). [PubMed]
219. Wormser GP, Ramanathan R, Nowakowski J, McKenna D, Holmgren D, Visintainer P, Dornbush R, Singh B, Nadelman RB. Duration of antibiotic therapy for early Lyme disease: a randomized, double-blind, placebo-controlled trial. Ann Intern Med 2003;138:697–704. [PubMed]
220. Zaidman GW. The ocular manifestations of Lyme disease. Int Ophthalmol Clin, 1997;37:13-28. [PubMed]
Tables
Table 1: Antimicrobial Treatment of Lyme Disease
Early Disease
Erythema migrans and disseminated early disease (including facial nerve palsy)
Doxycycline, 100 mg bid for 14-21 days (for children, use 2/mg/kg/dose up to 100 mg/dose; do not use in children <8 years of age or in pregnant women), or amoxicillin, 500 mg tid for 14-21 days (for children, use 20/mg/kg/dose up to 500 mg/dose). All doses are PO.
The preferred alternative agents for those who cannot take either amoxicillin or doxycycline is cefuroxime axetil, 500 mg bid for 14-21 days (for children, use 15/mg/kg/dose bid up to 500 mg/dose). Other alternatives are azithromycin (500 mg qd for 7-10 days; for children, use 10 mg/kg/dose up to 500 mg/dose), clarithromycin (500 mg bid for 14-21 days, except during pregnancy; for children use 7.5 mg/kg/dose up to 500 mg/dose) or erythromycin, 250-500 mg qid for 14-21 days (for children, use 12.5 mg/kg/dose up to 500 mg/dose). All doses are PO.
Meningitis
Ceftriaxone, 2 grams/day in a single dose IV or IM for 14-28 days (for children, use 50-80 mg/kg/day in a single dose), or penicillin G, 18-24 million units/day administered IV divided q 4h for 14-28 days (for children, use 200,000-400,000 units/kg/day divided q 4h administered IV). For non-pregnant persons >8 years of age an alternative is doxycycline, 100-200 mg/dose bid taken PO (for children, use 2-4/mg/kg/dose).
An alternative agent is cefotaxime, 2 grams/day q 8h administered IV for 14-28 days (for children, use 150-200 mg/kg/day divided q 8h). For non-pregnant persons >8 years of age who cannot take either penicillins or cephalosporins, an alternative is doxycycline, 100-200 mg/dose bid (2-4 mg/kg/dose up to 200 mg/dose for children) taken PO.
Late Disease
Arthritis
Initial treatment is the same as for early disease except treat for 28 days. If symptoms fail to resolve after 2 months or there is a recurrence, then either repeat a course of an orally administered antimicrobial or treat as for meningitis for from 14-28 days.
Neurological disease*
Same as for meningitis.
* For facial nerve palsy, see early diseases.
Figure 1: Erythema multiforme with central clearing originating in the submammary fold.
Often there is a central area of erythema remaining at the site of the tick bite (punctum). Although this is the “classical" presentation of erythema migrans, it is not the most common (see Figures 2 and 3).

Figure 2: Erythema migrans with homogenous erythema (and lack of central clearing).
Note that this is the most common presentation of erythema migrans in the U.S.
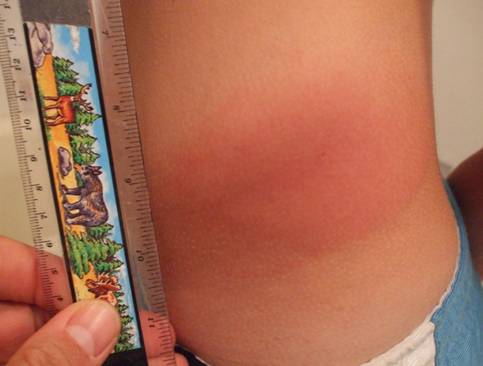
Figure 3: Erythema migrans rash without central clearing. Note the easily observed central punctum (arrow) at the site of tick bite.
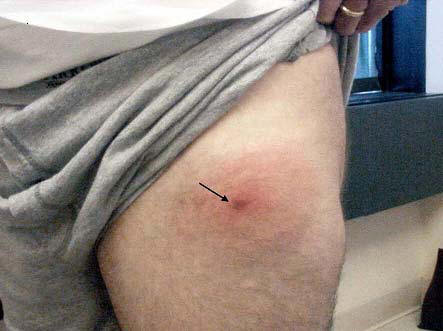
Figure 4: Vesicular rash surround by erythema occurring over the knee. This lesion was thought to represent a spider bite reaction and the patient was treated with cephalexin without response. Borrelia burgdorferi was isolated by culture of a skin biopsy of the leading margin of erythema.
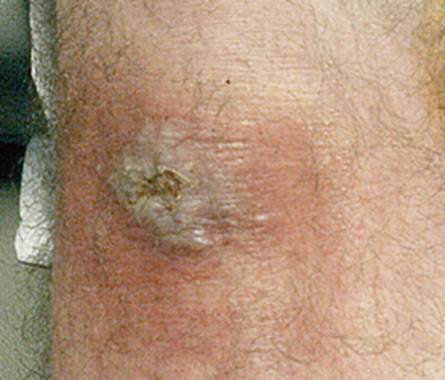
What's New
Blin-Rochemaure N, et al. Should a lumbar puncture be performed in any child with acute peripheral facial palsy and clinical suspicion of Lyme borreliosis? Arch Pediatr 2012;19:1354-1361.
Guided Medline Seach For:
Reviews
Auwaerter PG. Point: Antibiotic Therapy Is Not the Answer for Patients with Persisting Symptoms Attributable to Lyme Disease. Clin Infect Dis 2007;45:143-148.
Video: Borrelia burgdorferi. Provided by CytoViva.
Hingwe, A. Jarisch-Herxheimer reaction.
Lin JY. Tick-Borne Diseases. 2013.
Stricker RB. Counterpoint: Long-Term Antibiotic Therapy Improved Persistent Symptoms Associated with Lyme Disease. Clin Infect Dis 2007;45:149-157.
Guided Medline Search For Recent Reviews
History
Berger S. Emergence of Infectious Diseases into the 21st Century, 2008
Dixon B. Lyme Disease -- The Public Dimension. Microbe 2007;2:114-115.
Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, Krause PJ, Bakken JS, Strle F, Stanek G, Bockenstedt L, Fish D, Dumler JS, Nadelman RB. The Clinical Assessment, Treatment, and Prevention of Lyme Disease, Human Granulocytic Anaplasmosis, and Babesiosis. Clinical Practice Guidelines by the Infectious Disease Society of America. Clin Infect Dis 2006;43:1089-134.

