Quinolones
Authors: Susan L. Davis, Pharm.D. , Melinda M. Neuhauser, Pharm.D., Peggy S. McKinnon, Pharm.D.
CLASS
Structure-Activity Relationship
The quinolones have contributed an increasingly important chapter to the evolution of antimicrobials, and their value and role in the treatment of bacterial infections continues to expand. The development of the quinolones began in 1962 with the discovery of nalidixic acid, the prototype 4-quinolone antibiotic (144). Nalidixic acid had adequate activity against Gram-negative aerobes, but its modest serum and tissue concentrations coupled with its relatively high minimal inhibitory concentrations (MICs) precluded its use to treat systemic infections. The advancement of quinolones proceeded slowly over the next fifteen years as researchers focused on structural modifications that would increase antibacterial activity and/or enhance pharmacokinetic properties.
The main quinolone nucleus is a nitrogen-containing, 8-membered heterocyclic aromatic quinoline ring. In studying the structure-activity relationships of the early 4-quinolones, the 3-carboxyl group and 4-oxo groups were linked to antimicrobial activity. Structure-activity relationships of the fluoroquinolones are shown in Figure 1. Subsequent derivatives were synthesized with the 3-carboxy and the 4-oxo units, which included cinoxacin, pipemidic acid, flumequine, miloxacin, and rosoxacin. Many of these agents were toxic, but cinoxacin and pipemidic acid were considered improvements over nalidixic acid. Pipemidic acid had improved tissue penetration and was used to treat otitis media and sinusitis. Pipemidic acid was also moderately active against Pseudomonas aeruginosa, which was credited to the presence of the piperazinyl group at position 7 of the ring. Ten years later, this structural characteristic was used to enhance the activity of the norfloxacin molecule and the other 6-fluoroquinolones. Examples of the structure of the fluoroquinolones are represented in (Figure 2).
The fluoroquinolones contain 6-fluoro substituents, which greatly broadens the spectrum of activity against both Gram-negative and Gram-positive pathogens, possibly by improving tissue penetration and binding to the DNA gyrase enzyme. Fluorine at this position provides greater activity than any other halogen or substituent (48, 96, 179, 221). Other structural modifications have been made primarily on ring positions 1, 2, 5, 7, and 8 (48, 96, 179, 221). Norfloxacin, having both the 6-fluoro group and the 7-piperazinyl group, was discovered in 1978. This fluoroquinolone compound demonstrated more potent antibacterial activity than the previously developed quinolones, and its antibacterial spectrum included some Gram-positive bacteria. In addition, norfloxacin penetrated well into tissues. However, this agent had poor oral absorption and only modest activity against Gram-positive bacteria and pseudomonads. As a result, the clinical utility of norfloxacin was limited to treatment of urinary tract infections. Further quinolone development focused on improved oral absorption and included agents such as enoxacin, pefloxacin, and ofloxacin. Ciprofloxacin, which was discovered in 1981 and marketed in the United States in 1987, possessed considerably more potent antibacterial activity than earlier fluoroquinolones. It featured a cyclopropyl group on position 1 of the quinolone ring structure. The cyclopropyl group became a component of compounds such as grepafloxacin, sparfloxacin, gatifloxacin, and others, as seen in Figure 2.
Recent structural modifications among the fluoroquinolones include additional fluorine atoms at position 8 and substituents other than piperazine at position 7. These new compounds exhibited enhanced Gram-positive activity at the expense of antipseudomonal activity. Fluorine or chlorine atoms at position 8, seem to enhance the phototoxicity of these compounds(48); and examples of agents with these modifications are sparfloxacin and clinafloxacin, respectfully. A methoxy group in place of fluorine or chlorine at position 8 , as seen with noxifloxacin and gatifloxacin, appears to minimize the risk of phototoxicity.
Analogues that feature an additional nitrogen at position 8 are called naphthyridines and examples derived from this core structure include enoxacin, trovafloxacin, and gemifloxacin. Hepatic toxicities associated with trovafloxacin are thought to be related the 1-difluoro side chain (17) which is not present in the chemical structure of gemifloxacin. In comparison, gemifloxacin has a 1-cyclopropyl group similar to ciprofloxacin and a pyrolidine derivative at the C-7 position, which may provide for its enhanced activity against Streptococcus pneumoniae (18).
ANTIMICROBIAL ACTIVITY
Spectrum
Fluoroquinolones have excellent in vitro activity against a wide range of Gram-negative and Gram-positive organisms. The in vitro activity of the fluoroquinolones against a selected group of pathogens is provided in Table 1. The table presents the minimum concentration (μg/mL) of antibiotic required to inhibit growth in 50% (MIC50) of the tested strains and 90% (MIC90) of the strains. The table provides a range of MIC50 and MIC90 values for each organism reported in the literature. Multiple references were utilized for the MIC ranges; therefore, the isolates represent a heterogeneous patient population and it should be noted that differences in study methodology exist.
Quinolones are considered bactericidal agents, as minimum bactericidal concentrations (MBCs) are typically no more than one to two serial dilution steps higher than MICs. Fluoroquinolones possess excellent activity against members of the family Enterobacteriaceae and other Gram-negative organisms, such as Haemophilus influenzae, Neisseria gonorrhoeae, Neisseria meningitides, and Moraxella catarrhalis. They also exhibit in vitro activity against methicillin-susceptible Staphylococcus aureusand Staphylococcus epidermidis. The older quinolones such as norfloxacin, ofloxacin, ciprofloxacin are less active against streptococcal species, including Streptococcus pyogenes, S. pneumoniae, and viridans streptococci and their activity againstEnterococcus is variable to poor. These older quinolones are also inactive against anaerobic bacteria. Levofloxacin has more potent activity against Gram-positive and atypical organisms than ciprofloxacin; this agent also maintains activity against Gram-negative pathogens including P. aeruginosa. The newer agents such as gatifloxacin, moxifloxacin, and gemifloxacin demonstrate even greater activity against Gram-positive and atypical organisms compared to levofloxacin. However, these newer agents have considerably less activity against P. aeruginosa than ciprofloxacin and levofloxacin.
Antimicrobial Activity In Vitro
Gram-Negative Organisms
Ciprofloxacin, levofloxacin, gatifloxacin, moxifloxacin, and gemifloxacin all have excellent in vitroactivity against Enterobacteriaceae, fastidious Gram-negative species such as H. influenzae and other Haemophilus species, N. gonorrhoeae, N. meningitides, and M. catarrhalis (Table 1). A recent surveillance study that collected 48,440 Enterobacteriaceaeisolates worldwide from 1997 – 2001 reported susceptibility rates of 90. 5% for ciprofloxacin, 91.7% for gatifloxacin, and 91.7% for levofloxacin (225). High susceptibility rates (99.8-100%) were also seen with several fluoroquinolones (ciprofloxacin, levofloxacin, gatifloxacin, moxifloxacin, gemifloxacin) against clinical isolates of H. influenzae and M. catarrhalis (120, 125, 289). Similarly, these agents demonstrate potent activity against N. meningitides with MIC90 ranges of 0.004 - 0.008 μg/mL (30).
Against P. aeruginosa, ciprofloxacin and levofloxacin retain more potent activity than newer agents such as gatifloxacin, moxifloxacin and gemifloxacin (116). Therefore, these newer agents are not used in the treatment of infections caused by P. aeruginosa. Over the past decade, surveillance studies have noted a significant reduction in fluoroquinolone susceptibility (approximately 20 – 25%) with P. aeruginosa, which has been associated with increased fluoroquinolone usage during this time period (178, 288). Susceptibility rates of ciprofloxacin may vary based upon geographical and hospital demographics. One study showed that overall susceptibility rates were 73% for ciprofloxacin (MIC50: 0.25 μg/mL) and 71% for levofloxacin (MIC50: 0.5 μg/mL) against 11, 968 P. aeruginosa isolates collected during 1997 – 2001 (130). Further decline in fluoroquinolone susceptibility will limit the usage of these agents for infections caused by P. aeruginosa.
Fluoroquinolones demonstrate in vitro activity against other non-fermentative Gram-negative bacilli such asStenotrophomonas maltophilia. The newer fluoroquinolones such as levofloxacin, gatifloxacin and moxifloxacin have improved activity against S. maltophilia compared to ciprofloxacin (130, 132 ,269). Jones et al. reported susceptibility rates of 86% for both gatifloxacin and levofloxacin compared to 32% for ciprofloxacin (based upon the National Committee for Clinical Laboratory Standards (NCCLS) breakpoints for P. aeruginosa) (130). Similarly, another study demonstrated more potent activity with moxifloxacin (MIC50/MIC90: 0.5/4 μg/mL), levofloxacin (MIC50/MIC90: 1/8 μg/mL), and gatifloxacin (MIC50/MIC90: 1/8 μg/mL) against S. maltophilia compared to ciprofloxacin (MIC50/MIC90: 4/16 μg/mL) (132). Against Acinetobacter spp., fluoroquinolones have overall susceptibility rates of ~ 50%; however, increasing resistance rates continue to be problematic with this multi-drug resistant organism (130, 132). Fluoroquinolones demonstrate minimal activity against Burkholderia cepacia with MIC90 ranges of 16-32 μg/mL, which correlates with susceptibility rates of approximately 10 to 25% (108).
Gram-Positive Organisms
The older generations of fluoroquinolones such as ciprofloxacin have moderate activity against Gram-positive organisms; however, the clinical utilization of these agents as monotherapy is typically avoided for treatment of Gram-positive infections due to the potential for rapid development of resistance. In comparison, the newer fluoroquinolones have demonstrated improved activity against Gram-positive organisms and are often used to treat for a wide-range of infections caused by these microorganisms.
The susceptibility rates to the newer agents are approximately 90 – 95% for methicillin-susceptible S. aureus (MSSA) (97, 109). The rank order of fluoroquinolone potency against MSSA has been reported as gemifloxacin > moxifloxacin > levofloxacin > ciprofloxacin (30,270). The newer agents may display elevated MICs with ciprofloxacin-resistant strains of S. aureus(77, 136) and are also inactive against most isolates of methicillin-resistant S. aureus (MRSA) and methicillin- resistant coagulase negative staphylococci (97,109). Although a few of the methicillin-resistant staphylococcal isolates may display in vitrosusceptibility to the fluoroquinolones, the clinical use of these agents is generally avoided because of concerns about rapid development of resistance and limited efficacy data.
The newer fluoroquinolones exhibit favorable activity against most clinical isolates of streptococci (Table 1). These agents remain active against S. pneumoniae independent of penicillin and/or macrolide susceptibility (30, 106, 128). Recent surveillance studies have shown overall susceptibility of S. pneumoniae to fluoroquinolones to be approximately 98 – 99% (120,127). Similarly, fluoroquinolones possess excellent in vitro activity against all five serogroups of beta-hemolytic streptococci (MIC50/MIC90: 0.12-0.25/0.25 μg/mL for gatifloxacin; 0.25-0.5/0.5-1 μg/mL for levofloxacin) (27).
The activity of fluoroquinolones against enterococcal species is variable to poor. In a recent surveillance study, quinolone susceptibility was 66% among Enterococcus faecalis isolates (MIC50/MIC90: 0.06/2 μg/mL for gemifloxacin; 1/>16 μg/mL for levofloxacin) and 9-12% for Enterococcus faecium (MIC50/MIC90: 16/32 μg/mL for gemifloxacin; >16/>16 μg/mL for levofloxacin) (108). Despite the fact that some strains of E. faecalis are inhibited by clinically achievable plasma concentrations, the fluoroquinolones are not typically used clinically for the treatment of enterococcal infections with the possible exception of urinary tract infections.
The newer fluoroquinolones are more active against Listeria monocytogenes than ciprofloxacin (112,164,165). An in vitrosusceptibility study showed that gemifloxacin (MIC50/MIC90: 0.12/0.25 μg/mL) had the most potent activity followed by moxifloxacin (MIC50/MIC90: 0.25/0.5 μg/mL), levofloxacin (MIC50/MIC90: 1/1 μg/mL), and ciprofloxacin (MIC50/MIC90: 1/1 μg/mL) (164). Another in vitro study that tested gatifloxacin demonstrated more potent activity against 26 isolates of L. monocytogenescompared to ciprofloxacin (MIC range: 0.25 – 0.5 μg/mL for gatifloxacin vs. 1 – 2 μg/mL for ciprofloxacin) (78).
Anaerobic Organisms
Anaerobic activity with the fluoroquinolones has evolved with advancement of these agents. Fluoroquinolones such as ciprofloxacin and levofloxacin have minimal activity against anaerobes. Both trovafloxacin and clinafloxacin exhibit improved anti-anaerobic activity compared to the older compounds (115, 136). In fact, trovafloxacin received FDA indications for various anaerobic infections including complicated intra-abdominal infections, gynecologic and pelvic infections, and complicated skin and skin structure infections, including diabetic foot infections. However, toxicities associated with these agents have halted the clinical usage. Gatifloxacin, moxifloxacin, and gemifloxacin exhibit variable activity against anaerobes particularly against Gram-negative anaerobes (92, 115, 136). Against 145 Gram-Negative anaerobes, gatifloxacin displayed MIC (μg/mL) ranges of ≤0.06 – 16 (MIC50/MIC90: 0.5/4) compared to ≤0.125-64 (MIC50/MIC90: 1/8) for levofloxacin and ≤0.06-256 (MIC50/MIC90: 4/32) for ciprofloxacin (55). Similarly, moxifloxacin displayed markedly improved activity compared to ciprofloxacin against Gram-negative anaerobes (MIC50/MIC90: 0.25/1 μg/mL for moxifloxacin vs. 4/32 μg/mL for ciprofloxacin) (24).
In general, the newer fluoroquinolones have greater activity against Gram-positive versus Gram-negative anaerobes. Gatifloxacin inhibited Gram-positive anaerobes (n=59) in the range of ≤0.12 – 8 μg/mL (MIC50/MIC90: 0.25/1) compared to ≤0.12 - >64 μg/mL (MIC50/MIC90: 0.5/4) for levofloxacin (55). Another study found the vitro activity of moxifloxacin against Gram-positive anaerobes was 2-3 times more potent than ciprofloxacin (MIC50/MIC90: 0.25/1 for moxifloxacin vs.1 /8 for ciprofloxacin) (24).
Mycobacteria
The fluoroquinolones have variable activity against mycobacteria. In general, they are more active againstMycobacterium tuberculosis than against other mycobacteria. Against M. tuberculosis, the C-8 methoxy-fluoroquinolones, moxifloxacin and gatifloxacin, demonstrate more potent in vitro activity than ciprofloxacin and levofloxacin (Table 1) (11,90,216). In contrast, the naphthyridines, gemifloxacin and trovafloxacin, are less active against isolates of M. tuberculosis (223). Fluoroquinolone activity varies against species of non-tuberculosis mycobacteria; the trend of increased activity of the C-8 methoxy-fluoroquinolones continues against these isolates (90,91,259,260). Against rapid growing mycobacteria, moxifloxacin and gatifloxacin demonstrate more potent activity against M. fortuitum (MIC90: 0.5 μg/mL) than levofloxacin (MIC90: 1 μg/mL) compared to M. chelonae (MIC90: 16 μg/mL for both moxifloxacin and gatifloxacin; MIC90: 32 μg/mL for levofloxacin) (90,215). Against slow growing mycobacteria, moxifloxacin exhibited greater activity against M. kansasii > M. intracellulare > M. avium than gatifloxacin and levofloxacin (90,215). The MIC50/MIC90 (μg/mL) values for moxifloxacin, gatifloxacin, and levofloxacin, respectively, were: M. kansasii 0.125/2, 1/4, and 2/16; M. intracellulare 1/2, 4/4, 8/8; and M. avium 2/4, 8/16 and 16/16. Other investigators reported the range of moxifloxacin MICs between 0.125– 2 μg/mL against M. avium including 5-macrolide resistant strains (25,90).
Atypical Pathogens
Fluoroquinolones display in vitro activity against Legionella pneumophila, Chlamydia pneumoniae andMycoplasma pneumoniae (Table 1). Similar potency among the newer and older fluoroquinolones has been observed for isolates of L. pneumophila (128). These agents can be differentiated on their activity against C. pneumoniae and M. pneumoniae. Gatifloxacin, moxifloxacin, and gemifloxacin have lower MICs ranges than levofloxacin and ciprofloxacin against these organisms (102, 128).
Pharmacodynamic Effects
Bactericidal Effects
Quinolone pharmacodynamics can be expressed in terms of their in vivo concentration versus bacterial-killing capabilities as well as concentration (or dose) relationships to bacterial resistance (also a pharmacodynamic effect). The preferred way to express these relationships is to define the interaction of a pharmacokinetic parameter such as area under the curve (AUC) or peak concentration and an index of organism susceptibility such as MIC (232).
Fluoroquinolones kill bacteria in a concentration-dependent fashion. In general, the MBCs are similar to MICs for most bacteria, and there are minimal differences in MICs as the inoculum size is increased, with the exception of activity against pseudomonads. In vitro pharmacodynamic models, which mimic changes in serum concentrations over time, have shown that fluoroquinolones kill bacteria rapidly at peak concentrations and continue to kill bacteria as long as concentrations exceed the MIC (29, 57, 158). It is relatively easy to show more rapid bacterial killing effects in patients with the fluoroquinolones. An example of these comparative studies is shown in Figure 3, which compares data from ciprofloxacin and a third-generation cephalosporin, cefmenoxime (98). For both agents, in vivo bacterial killing rates were monitored using daily cultures. These data show that in patients with nosocomial pneumonia, the killing rate of ciprofloxacin at the same area under the inhibitory curve (AUIC) ratio is considerably faster, even though both agents killed the same number of organisms by the end of therapy.
The other relevant in vitro observation in the pharmacodynamic models is the emergence of an initially small population (< 103) of resistant organisms, which becomes the dominant flora after 24 h of antibiotic exposure. High peak concentrations prolong the time before this emergence occurs, presumably by killing even greater numbers (those with the highest MICs) of the small minority population of highly resistant microbes. This argument has been used to support dosing regimens that provide high peak concentrations. There is a strong relationship between the in vitro and in vivo activity and emergence of resistance in patients with lower respiratory tract infections (LRTI). In vitro data demonstrated that fluoroquinolone resistance emerges in P. aeruginosa at AUC:MIC ratios below 100 (158). Unfortunately, such low drug exposure conditions may be encountered in the nosocomial pneumonia patient (258). When the same conditions are reproduced in animal models, resistance also results, presumably because of weak bactericidal activity and the resulting selective pressure (169).
Postantibiotic Effects
Fluoroquinolones demonstrate a 3- to 6-h in vitro post antibiotic effect (PAE) on staphylococci,Enterobacteriaceae, and P. aeruginosa. Organisms that are killed very rapidly, such as Haemophilus spp., E. coli and Klebsiellaspp., have shown almost no in vitro PAE, and the PAE for E. faecalis is considerably shorter than that of other organisms such as pseudomonas and staphylococci. A modest in vivo PAE, 2 to 6 hours depending on the bacterial species, has also been demonstrated in animal models. The significance of PAE in designing a dosing regimen in humans is largely unknown.
At least a portion of the argument for PAE, in fact, may be confusion caused by the effects of sub-MIC concentrations in vivo. For example, the long half-life of most quinolones, even in animal models, ensures that serum and tissue concentrations remain measurable for 3 to 6 h after they fall below the MIC of the organism. It could easily be the residual effects of these concentrations that are perceived as an in vivo PAE. Further, it is assumed that sub-MIC concentrations have a role in selection of resistant subpopulations, an intrinsic mechanism of resistance in the patient care setting.
Important Effects on Host Immunity
Quinolones have been shown to prevent release of endotoxin in vitro, even though they kiIl bacteria quite rapidly. This may result in a lesser host response to bacterial challenge (181). The clinical implications of this are unknown.
Pharmacodynamic Correlates with Outcome
Clinical efficacy of the fluoroquinolones has been associated with both peak: MIC and AUC:MIC values (12,56,72,181,198,233). Area under the inhibitory curve, or AUIC, is the nomenclature used to describe the integrated AUC above MIC versus time (232), a value similar to the AUC: MIC. The genesis of the relationship between these parameters was reported in a study conducted in 1985 by Barriere et al. (22). An overall integration of serum concentrations with bacterial MICs is shown for ciprofloxacin in Figure 4. The AUC of 64 for the regimen shown, in relationship to an MIC of 0.25 μg/mL, yields an AUC:MIC of 256. This corresponds to a peak:MIC ratio of 10:1. A peak:MIC ratio of 5:1 is approximately equal to an AUC:MIC ratio of 125 (117,159). Thus there is a strong concordance among the pharmacokinetic and pharmacodynamic parameters that describe this class of antibiotics (51,232,233).
Clinical cure of infection is also a relevant pharmacodynamic end point, as clinical cure is a composite parameter. The components of a clinical cure include bacterial killing versus antibiotic concentration, and the rates of host repair and resolution of inflammation. The relative importance of these two factors differs with the type of infection. For this reason, it is more enlightening to model antibiotic concentration versus bacterial killing than versus clinical cure. These parameters also may correlate, but to a different degree in different types of infections.
In Vitro MICs as Pharmacodynamic Predictors of Outcome
Although in vitro pharmacodynamic models yield mechanistic information on the killing rates of these antibiotics versus one or two organisms, most of the available information regarding fluoroquinolone pharmacodynamics must be gleaned from in vitro studies of organism MICs. While low MICs do indicate greaterin vitro potency, MIC data must be interpreted from the perspective of achievable quinolone concentrations in vivo. The peak serum concentrations for most fluoroquinolones exceed the MICs for Haemophilus, Neisseria, Moraxella, andEnterobacteriaceae for 4 to 6 h. Newer agents with long half-lives (e.g., trovafloxacin, moxifloxacin) achieve serum levels above the MICs of Gram-positive organisms for 24 h. The peak plasma concentrations of many of the newer quinolones exceed the MICs of streptococci including S. pneumoniae.
Integration of MIC and Antibiotic Concentration
Pharmacokinetics and pharmacodynamics can be simultaneously considered by integrating these endpoints to form activity indices during comparative evaluation of the fluoroquinolones. Fluoroquinolones can be dosed to optimize both peak:MIC and AUC:MIC values, both of which have been linked to efficacy (56, 72, 198, 233). Blaser et al. noted that peak concentrations of enoxacin greater than 3 times the MIC were associated with >99% reduction in the initial inoculum at 4 hours (29); however, bacterial regrowth occurred at 24 hours unless the peak:MIC ratio exceeded 8:1. Using in vitromethods, it has been demonstrated that the estimated AUC:MIC ratio for maximal killing with ciprofloxacin in vitro is 350 to 450 (118). Dudley et al. examined the effect of ciprofloxacin against E. coli and P. aeruginosa in an in vitro dynamic model (56). Complete killing of the E. coli strain was noted after the first dose. Although an initial bactericidal effect was observed against theP. aeruginosa strain, regrowth of resistant organisms occurred. A peak:MIC ratio of 150:1 was noted for the E. coli strain while the peak:MIC ratio for the P. aeruginosa was <5:1. These results are consistent with clinical outcomes achieved in humans with similar antibiotic exposure. In a study evaluating levofloxacin for the treatment of respiratory, skin or urinary tract infections due toS. aureus, S. pneumoniae, or P. aeruginosa, clinical and microbiological outcomes were found to be optimal when peak:MIC was at least 12.2 (198).
Forrest et al. (73) assessed the relationship between antimicrobial exposure and bacterial susceptibility for ciprofloxacin. A threshold AUC:MIC of 125 (SIT-1•24 h) was determined to be necessary for onset of effective antibacterial action (232). A ciprofloxacin AUC:MIC of 125 to 250 predicted slow bacterial killing, with bacterial eradication requiring about 7 days. When AUC:MIC values exceeded 250, bacterial killing was extremely fast with eradication averaging 1.9 days, regardless of the species of bacteria (73). The results were similar in an in vitro study evaluating the rate of kill for ciprofloxacin against strains of S. pneumoniae, S. aureus, and P. aeruginosa, each strain having the same MIC values of 0.5 μg/mL. At in vitro concentrations that achieved AUC:MIC values >250, optimal and similar kill rates were attained for all 3 organisms (118). As the earlier clinical study had demonstrated, MIC was predictive of antibacterial activity across bacterial species, when the same AUC:MIC value of 250 was achieved. Thus, quinolones are acting similarly in the in vitro models and in vivo with respect to the relationships between their concentrations and the rate at which they kill bacteria. When dosages are normalized to those that produce an AUC:MIC of 125 to 250, all of these quinolones should be useful for treating a variety of bacterial infections, because their peak:MIC ratios exceed 5:1. However, the peak concentration: MIC ratio is below 5 when typical doses of any of these agents (except ciprofloxacin) are used to treat patients infected with P. aeruginosa. At its recommended dose of 1500 mg/day, only ciprofloxacin fulfills this 5:1 ratio criterion for Pseudomonas sp.
In more recent evaluations, the optimal pharmacodynamic endpoint for the fluoroquinolones has been found to vary by pathogen. Ambrose et al. (12) identified an AUC/MIC breakpoint of 33.7 against S. pneumoniae using free drug concentrations for levofloxacin and gatifloxacin. This lower AUC/MIC requirement of 30-40 for newer fluoroquinolones against S. pneumoniae is supported by numerous in vitro pharmacokinetic and animal models (152,153). Monte Carlo analysis suggests that moxifloxacin is more potent against S. pneumoniae, with 4-8-fold greater activity and higher attainable AUC:MIC values than levofloxacin or gatifloxacin (231).
Human Pharmacodynamics
A large body of human pharmacokinetic data has been accumulated during studies of the various quinolones. A pharmacodynamic perspective on these kinetic data comes from attempts to correlate either microbiologic or clinical endpoints to these kinetic indices. However, because there are numerous difficulties in conducting adequately powered clinical pharmacodynamic analyses, there remains a high degree of uncertainty over the proper dosage of these drugs in patients. The AUC:MIC of ciprofloxacin in one study ranged from 6 to over 5500 (73). A range of this magnitude may occur in any patient population due to variability in pharmacokinetics and organism susceptibility. This partially explains why clinical studies that do not directly measure AUC:MIC or another measure of the interaction between pharmacokinetics and pharmacodynamics cannot possibly differentiate between two doses of the same antibiotic or between two different antibiotics. The studies cited previously by Forrest, Preston and Ambrose remain the landmark investigations of fluoroquinolone pharmacodynamic parameters which relate to patient outcomes. These investigations demonstrate the contributions of both the peak:MIC and the AUC/MIC to clinical patient outcomes. Efficacy against Gram-negative organisms was optimal when peak:MIC was at least 12.2 (198) or AUC:MIC exceeded 125. For Gram-positive pathogens, a free AUC:MIC exceeding 33.7 was most predictive of positive clinical outcomes when evaluating gatifloxacin or levofloxacin against S. pneumoniae (12).
MECHANISMS OF ACTION
The fluoroquinolones selectively inhibit bacterial DNA synthesis in the presence of competent RNA and protein synthesis. More specifically, these agents target the action of topoisomerase II (also called DNA gyrase) and topoisomerase IV, which belong to a group of related enzymes known as DNA topoisomerases found in all organisms (50). DNA gyrase, which is a tetramer that consists of two subunits (GyrA and GyrB), has the unique ability to insert negative supercoils into DNA. Since DNA gyrase maintains the chromosome in a supercoiled state and repairs small single-strand breaks in DNA that occur during replication, its inhibition provides a possible explanation for the bactericidal activity of these agents. The A subunit (encoded bygyrA gene) is involved in breakage and reunion of DNA, while the B subunit (encoded by gyrB gene) is the site of ATP hydrolysis and conformational changes in the complete enzyme to allow DNA strand passage as new molecules are produced (50,221). The role of topoisomerase IV in bacteria is to separate the daughter chromosomes following the replication process. Similar to DNA gyrase, topoisomerase IV is comprised of two subunits, ParC and ParE, which are encoded by parC and parE genes, respectively. Ultimately, the quinolones exert their antibacterial effect by binding to complexes of DNA and topoisomerase II or IV, which leads to interference in the DNA replication process.
MECHANISMS OF RESISTANCE
Resistance to fluoroquinolones can occur through the following mechanisms: alteration of the target sites (DNA gyrase and topoisomerase IV), alteration in the cell wall permeability due to a loss of outer membrane proteins, and mutations that regulate active antibiotic efflux out of the cell. Bacterial resistance to the fluoroquinolones is primarily chromosomally mediated. Fluoroquinolones have two primary targets with multiple subunits: DNA gyrase (gyr A or gyr B) and topoisomerase IV (parC, parE of S. pneumoniae and grl A and grl B of S. aureus). Both are critical to the organism for active replication. Different quinolones exhibit different primary targets within the Quinolone-Resistance-Determining-Region (QRDR) of bacteria. Ciprofloxacin and levofloxacin preferentially bind to the C subunit of topoisomerase IV, while gatifloxacin and moxifloxacin show a greater affinity for the A subunit of DNA gyrase.
Resistance which develops in Gram-positive organisms occurs in this step-wise manner. In general, a mutation in topoisomerase IV will occur first, producing only a small-to-moderate increase in MIC. The second-step mutation in gyr A or gyr B will result in a larger increase in MIC and usually lead to the production of a resistant organism. Ideally, use of a fluoroquinolone which prevents the first-step mutation by targeting both gyr A or gyr B plus topoisomerase IV will offer the greatest advantage in prevention of resistance (50, 253). This enhanced activity leads to a lower likelihood of development of S. pneumoniae resistance (in vitro), which may be seen with use of moxifloxacin, gatifloxacin or gemifloxacin compared to older fluoroquinolones.
The mechanisms of resistance for Gram-negative organisms may include alterations in the primary target, DNA gyr A subunit, secondary targets such as gyrB or ParC, and active efflux. A change in a single amino acid in the DNA gyrase due to mutation in the gyr A gene has been shown to be associated with resistance. Mutants isolated from patients were found to have alterations in the serine residue. In general, changes in the gyr A gene produce an 8- to 16-fold increase in the concentration of a fluoroquinolone required to inhibit or kill bacteria.
Resistance to quinolones can also result from changes in permeability. Yoshida et al. (50, 286) described decreased expression of OmpF because of changes in the nixB and cixB genes that prevented fluoroquinolone accumulation in cells, as normally occurs in an energy-dependent fashion. Permeation and DNA gyrase mutations have been described in P. aeruginosaand Citrobacter freundii. Several active efflux systems have been characterized in E. coli, Klebsiella sp. and P. aeruginosa(50, 138). While some efflux systems preferentially act on select agents, several confer resistance to all fluoroquinolones and some provide cross resistance with other classes of antimicrobials. The magnitude of increase in MIC resulting from hyperexpression of genes that express efflux pump proteins is dependent on the quinolones and the particular efflux system.
Video: Mechanism of Resistance -- Mutation, Efflux
PHARMACOKINETICS
The fluoroquinolones are characterized by rapid oral absorption, blood and urine concentrations that markedly exceed the MICs for many common bacterial pathogens, wide distribution into body tissues, with serum and tissue concentrations above the MIC for most Gram-negative and many Gram-positive aerobic organisms, and half-lives sufficiently long to permit dosing every 12 to 24 h. The pharmacokinetic parameters of the newer fluoroquinolones have many similarities, although there are differences in half-life, degree of absorption, metabolism, and elimination. In general, quinolones exhibit linear pharmacokinetics, with increases in serum concentrations directly proportional to dose size, and pharmacokinetic properties (serum half-life, total body clearance, etc.) independent of dose.
Renal clearance mechanisms are the most important for removal of ofloxacin, levofloxacin, and gatifloxacin. Renal excretion of these compounds occurs via both tubular secretion and glomerular filtration, with glomerular filtration as the major component. Hepatic mechanisms of elimination are more important for removal of trovafloxacin, and multiple mechanisms of elimination contribute to norfloxacin, ciprofloxacin, moxifloxacin and gemifloxacin elimination. Fluoroquinolones are excreted across the bowel wall into the intestinal lumen, which also explains their efficacy in diarrheal diseases.
Absorption
The excellent bioavailability of the quinolones allows oral dosing in place of the more traditional parenteral administration. With most of the new fluoroquinolones, oral absorption is sufficient to achieve adequate serum bactericidal activity for systemic infections (50, 171, 172, 184, 256, 267, 277). Fluoroquinolones are absorbed primarily in the duodenum and the proximal jejunum. Absorption does not require acidity or an alkaline environment and fluoroquinolones are absorbed to a similar extent in a fasting state or with a meal (141, 184, 287). The absolute bioavailability of several of these compounds has not been characterized because intravenous dosage forms are not available for human studies. Bioavailability data for the quinolones is summarized in Table 2.
The fluoroquinolones are rapidly absorbed after oral dosing, reaching peak serum concentrations in 1 to 2 h. Peak plasma levels differ for each drug, as shown in Table 2. For comparison purposes, all values were normalized to a 100-mg oral dose, making the assumption that peak serum concentrations and AUC are proportional to dose. Levofloxacin, trovafloxacin and moxifloxacin reach the highest peak values; norfloxacin, gemifloxacin, and ciprofloxacin are lower (96,184). These latter compounds have larger steady-state volumes of distribution (Table 2). Elderly and critically ill individuals absorb the drugs normally, but peak concentrations in these individuals are generally delayed and are usually higher, since such patients frequently have a concomitant decrease in renal function.
The 24-h areas under the serum concentration-time curve (AUC) for typical doses of each new quinolone are listed in Table 2 (96, 184, 239). For comparison purposes, all values were normalized to a 100-mg oral dose, assuming that peak serum concentrations and AUC are proportional to dose. In some studies, peak concentration or AUC changed out of proportion to dose; however, the deviations from linearity were not substantial. The potential clinical significance of the large differences between compounds in peak serum concentrations and AUC will be addressed as these AUC values are integrated with relative antimicrobial potency.
Distribution
Fluoroquinolones have a large volume of distribution, ranging from 1 to more than 4 L/kg. Values are provided for each of these agents in Table 2 (96, 179, 184, 239, 277). Clearly, the apparent volume of distribution of all fluoroquinolones exceeds the 0.6 L/kg that corresponds to total body water. However, the derived values in the literature vary considerably, even for the same quinolone, presumably because few studies used intravenous forms of these drugs to determine precise volumes of distribution. The accuracy of the derived value depends upon knowing bioavailability accurately enough to factor it out. For example, after intravenous administration of ciprofloxacin, the apparent volume of distribution was 2.2 to 2.7 L/kg. If an oral dose were used to assess this parameter, it would appear to be higher (in the range of 3.2 L/kg). Adjustment with a bioavailability of 70 to 85% corrects the derived volume parameter. This effect deserves attention because most of the fluoroquinolones that appear to have incomplete distribution volumes actually have incomplete bioavailability.
All of the newer fluoroquinolones are widely distributed throughout the body. Table 3 summarizes some of the extracellular site concentrations in relation to the simultaneous serum concentrations. Interstitial fluid concentrations range from 50 to 100% of peak plasma levels after 2 h, and between 4 and 24 h they generally exceed serum concentrations. Concentrations significantly above those in serum are attained in the kidney, liver, and lung; levels in saliva, bronchial secretions, and prostatic fluid are lower than those in serum (96, 184, 239).
Urine drug concentrations are high and remain above the MICs of common urinary pathogens. In most instances, they exceed inhibitory levels for urinary pathogens for a full 24 h. Urinary concentrations above 10 mg/L often can be detected up to 48 h after ingestion of a single dose. The lowest concentrations of fluoroquinolones in urine are seen with trovafloxacin (41, 256, 257), and moxifloxacin (249). The highest urinary concentrations are noted with gatifloxacin (96), and levofloxacin (36, 43, 70), because these compounds are well absorbed and are excreted by the kidney completely unchanged. Most fluoroquinolones continue to achieve adequate therapeutic concentrations in the urine, even when renal function is greatly reduced. Consistent with transintestinal elimination (212, 219, 245), the fecal levels of most quinolones are sufficient to inhibit most gastrointestinal bacterial pathogens.
The cerebrospinal fluid levels of ciprofloxacin and ofloxacin in patients with inflamed meninges are 40 to 90% of serum concentrations. The levels of ciprofloxacin and ofloxacin in human aqueous humor range from 3.8 to 25% and 44 to 88% of serum levels, respectively. The total areas under the blister fluid–concentration-time curves exceed serum levels by 120% for ciprofloxacin, norfloxacin, and ofloxacin (277). Ciprofloxacin, ofloxacin, and other quinolones appear to penetrate into prostate tissue and seminal fluid reaching concentrations exceeding those achieved in serum. Ciprofloxacin penetrates well into pancreatic tissue. The penetration ratio in one study was 1.0 for pancreatic tissue and 0.83 for pancreatic juice (119). Biliary concentrations also exceed those in serum (62).
After a single 200-mg intravenous dose, concentrations of ciprofloxacin in cortical bone and cancellous bone were 6.9 and 9.7 ug/g. Other quinolones also appear to penetrate bone. However, these values should be interpreted cautiously because tissue:serum ratios change in relation to time after administration, and study designs differ among investigations. Bone marrow tissue concentrations are excellent and in almost every case exceed MICs for infecting bacteria (184).
Quinolones also reach high concentrations inside many cells. Fluoroquinolones enter polymorphonuclear cells, alveolar macrophages, peritoneal macrophages, and phagocytic cells within the liver, producing concentrations ranging from 3 to 10 ug/mL (235,278,281). An anionic transport mechanism removes the compounds from white blood cells (96,184,239). Tissue concentrations are generally higher in infected tissues than in uninfected tissues, because of WBC accumulation. There are more white blood cells in infected tissue, and these compounds are probably present intracellularly in concentrations higher than those in extracellular fluids, though the degree of antimicrobial activity of these drugs at intracellular sites has not been well studied.
Protein binding has been measured for most of these compounds and values are reported in Table 2 (96, 184, 239). Most of the fluoroquinolones have relatively low protein binding of 14 to 45% (187). Ciprofloxacin, levofloxacin, gatifloxacin, and ofloxacin are 10 to 25% protein bound (184, 187). Thus any compromise of antimicrobial activity by the presence of serum protein should be minimal. Some of the newer fluoroquinolones have higher protein binding: trovafloxacin approaches 65 to 70% (256, 257), moxifloxacin 40-50% (249), and gemifloxacin 60% (9).
Routes of Elimination
Metabolism
Hepatic metabolism is essential for clearance of several fluoroquinolones. In the case of fluoroquinolones such as norfloxacin and ciprofloxacin, most metabolism occurs at the piperazine substituent on ring position 7 (Figure 1). In contrast to the pathway for the earlier quinolone compounds, metabolic alteration of the newer fluoroquinolones does not typically occur on the position 7 ring. And whereas the metabolic products of earlier quinolones had antimicrobial activity, glucuronides formed at position 3 are clearly inactive because this part of the molecule is essential for antimicrobial activity.
As with most oxoquinolone metabolites, the oxo- metabolite of ciprofloxacin is active, although less so than ciprofloxacin. In urine, 36% of a 500-mg oral dose is recovered as unchanged ciprofloxacin, 9.6% as the oxo- metabolite, 2 to 4% as the dioxo- metabolite, and less than 2% as other metabolites (256). In animal studies using 14C-Iabeled ciprofloxacin, all of the radiolabeled drug can be accounted for in the combined collections of urine and stool. In rats, 90% of a 10-mg/kg oral dose and 48% of an equal intravenous dose was recovered in stool. The remainder was recovered in urine as unchanged drug and metabolites (111, 140, 219).
Extensive data on the metabolism of many of the newest fluoroquinolones are not yet available. However, gatifloxacin and gemifloxacin appear to undergo minimal metabolic conversion. Trovafloxacin is approximately 25% hepatically metabolized. (166). Moxifloxacin is metabolized to an N-sulfate conjugate (38%) and an acyl glucuronide (14%) which are then excreted in the feces and urine, respectively. To some extent, all fluoroquinolones are excreted in bile, unchanged and as metabolites.
For reasons detailed above, hepatic disease in the presence of normal renal function does not produce major changes in the serum half-life of the renally excreted agents ciprofloxacin, ofloxacin, levofloxacin, and gatifloxacin (272). Little is known about the disposition of the hepatically cleared fluoroquinolones such as trovafloxacin and moxifloxacin in patients with hepatic disease. Dramatic changes in half-life, similar to those occurring for renally excreted fluoroquinolones in renal failure, would be anticipated for these agents. Quantitative studies in patients with severe renal and hepatic disease are rare. Further studies should be conducted to characterize pharmacokinetic alterations in these patients.
Renal Excretion
Quinolones are eliminated by renal mechanisms, including glomerular filtration and tubular secretion, as well as by nonrenal routes, such as hepatic metabolism and transintestinal transport (219). The terminal half-lives of the fluoroquinolones range from 3.5 to 15 h (Table 2). Since the elimination of the compounds is different, terminal half-lives increase to varying degrees, depending on the degree of elimination via renal and hepatic function. Fluoroquinolones excreted primarily by hepatic metabolism (e.g., trovafloxacin, moxifloxacin) have longer half-lives in many cases (41, 96, 256, 257) than quinolones excreted primarily by renal mechanisms.
Renal clearance usually exceeds the glomerular filtration rate, suggesting that tubular secretion plays a major role in the elimination of these drugs. The fact that most of these antibiotics interact with probenecid (184, 240, 251), is further evidence that these compounds undergo renal tubular secretion. Renal clearance of the fluoroquinolone ranges from 140 to 425 mL/min in patients with normal renal function. Administration of probenecid reduces the renal clearance of ciprofloxacin by 50% and total urine recovery by 24%. Probenecid also reduces the renal excretion rate of norfloxacin (184, 251), gemifloxacin, levofloxacin, and gatifloxacin.
Fluoroquinolone compounds are moderately affected by renal disease, with levofloxacin, ofloxacin, and gatifloxacin showing the greatest sensitivity (69). In anuric patients (creatinine clearance < 10 mL/min) serum half-lives increase to 8 to 10 h for ciprofloxacin and norfloxacin and 25 to 45 h for ofloxacin, levofloxacin, and gatifloxacin. (Table 2) (69,173,184,240). The AUC of gemifloxacin increases approximately 70% in severe renal failure. With the exception of moxifloxacin, the available fluoroquinolones require dosage reduction for patients with renal insufficiency (Table 6). In patient reciving hemodialysis, the half-life of fluoroquinolones is decreased approximately 50% by hemodialysis while the procedure is underway; however, minimal amounts of the drugs are removed. Accordingly, patients undergoing hemodialysis should receive the same reduced doses that would normally be given to patients with end stage renal function, and supplemental doses are not needed between dialysis sessions in anuric patients (184).
Frequently, alternate pathways account for excretion of drugs in patients with renal failure (69,184). This is evidenced in the finding that for some compounds only modest dose reductions are required even for patients with severe renal dysfunction. Renal impairment does not markedly affect trovafloxacin and moxifloxacin, because these compounds are primarily eliminated by non-renal pathways. Conversely, half-lives of ofloxacin, levofloxacin, and gatifloxacin can increase up to fivefold in severe renal dysfunction (69, 184). No data are presently available regarding the impact of renal dysfunction on the half-life of gemifloxacin.
Hepatic Metabolism
Compared to the other fluoroquinolones, drug metabolism occurs to the greatest extent for trovafloxacin (41, 256, 257) and moxifloxacin (250). Metabolites constitute between 15 and 30% of norfloxacin and ciprofloxacin recoverable from urine. As shown in Table 2, the total clearance of hepatically eliminated compounds (e.g., trovafloxacin, moxifloxacin) is only modestly altered by renal dysfunction. This comparison verifies a shift in the excretion pattern of these drugs in patients with renal failure, for more of the compound is metabolized or excreted in bile. As with all drugs subject to excretion by combinations of renal and metabolic pathways, patients with multiple organ failure and resulting impairment of both pathways would show extreme prolongations of serum half-life. In this case, neither elimination pathway can compensate for failure of the other, and marked accumulation would occur. Severe hepatic disease also would be expected to prolong the serum half-lives of trovafloxacin, moxifloxacin and norfloxacin. In fact, ciprofloxacin and norfloxacin may accumulate in patients with hepatic failure (80), particularly with concomitant renal impairment (75, 272).
Pharmacokinetic Parameters
The pharmacokinetic parameters for the fluoroquinolones are shown in Table 2.
DOSAGE
Adults and Children
The usual adult dosage ranges for oral and parenteral fluoroquinolones in the treatment of various infections are provided in Table 4. Potential risk of bone and cartilage adverse effects has limited the use of fluoroquinolones for pediatric infections. For limited indications, ciprofloxacin doses in pediatric patients range from 6 -10 mg/kg every 8 to 12 hours for intravenous administration and 10 – 20 mg/kg every 12 hours for oral administration (199).
Renal Failure
Patients with impaired renal function may require decreased doses and/or frequency of administration of ciprofloxacin, gatifloxacin, gemifloxacin, levofloxacin, and norfloxacin. Dosage adjustment should be based on the degree of renal impairment, severity of the infection, susceptibility of the pathogenic organism, and expected serum concentrations of the agent. Dose adjustments recommended for patients with renal insufficiency, receiving dialysis, or continuous renal replacement are summarized in Table 6.
Hepatic Failure
Pharmacokinetic studies in hepatically impaired patients are limited. Higher peak plasma levels of certain quinolones may be observed; however, dose adjustments may not be necessary unless there is concomitant renal impairment.
ADVERSE EFFECTS
The most frequent adverse effects experienced by quinolone recipients include nausea, upper gastrointestinal tract (GI) discomfort and central nervous system (CNS) effects such as headache, insomnia, and dizziness (21, 185, 221). The adverse events associated with the quinolones are typically mild, self-limited, and only rarely require discontinuation of treatment. Some adverse effects of quinolones (e.g. GI symptoms and arthropathy) do not appear to be related to specific structural modifications of the drug; whereas phototoxicity and CNS effects are linked to specific structure-activity relationships (48). Each quinolone tends to produce a characteristic profile of adverse effects. The frequency of these various effects has not been evaluated in head-to-head comparative studies, because the clinical trials conducted for registration purposes usually study comparators from other antibiotic classes. The relative frequency of the more common events compiled from a large body of literature is presented in relative rank order in Table 5.
Rare but severe adverse reactions resulted in market withdrawal of several fluoroquinolones in the 1990s. Clinical development of temafloxacin was halted in 1992 after 92 cases of hemolytic-uremic syndrome were reported after the product’s release in Europe. Since then, lomefloxacin has been associated with CNS toxicity and phototoxicity, and tosufloxacin has been linked to severe thrombocytopenia and nephritis. Sparfloxacin was voluntarily removed from the US market in 1994 due to concerns of phototoxicity and QT interval prolongation. In 1999, clinafloxacin development was stopped in light of safety concerns regarding liver toxicity, arrhythmias, and phototoxicity. Also in 1999, grepafloxacin was voluntarily withdrawn from the worldwide market after reports of a small number of cardiovascular events related to QT prolongation. The production of trovafloxacin was limited shortly after its launch, then was subsequently withdrawn, due to reports of rare but serious liver injury. In May of 2006, three months after receiving a new contraindication against use in patients with diabetes mellitus, the manufacture of gatifloxacin was stopped, following data demonstrating its relationship with altered glucose metabolism (3, 156, 284). Fortunately, for compounds which remain on the market, adverse effect profiles are generally mild and more predictable.
Corrado et al. (39), summarized tolerability data from clinical trials involving 1540 norfloxacin-treated patients. From this patient population the investigators reported that 4% of patients experienced adverse drug events probably or possibly related to norfloxacin therapy. Adverse drug events required discontinuation of therapy in approximately 1% of patients and were considered serious in less than 1% of patients. The most frequently reported symptoms included nausea (1.9%), headache (1.8%), dizziness (1.2%), abdominal pain, dyspepsia, constipation, and flatulence (0.3% each). Arcieri et al. (14) analyzed safety data for 2829 ciprofloxacin recipients. The attending physician determined that an adverse drug event was either probably or possibly related to ciprofloxacin therapy in approximately 16% of the patients; 8% complained of signs or symptoms associated with the GI tract (primarily nausea, vomiting, or diarrhea), and 3.3% experienced CNS abnormalities (most notably dizziness, headache, tremors, and restlessness). For the most commonly prescribed quinolones, levofloxacin, ciprofloxacin, and moxifloxacin, the overall profile and rate of adverse events is similar. However, quinolone therapy can be associated with potentially serious adverse reactions which must be considered when prescribing any agent of this class.
Connective Tissue Damage
Several quinolones (pefloxacin, ciprofloxacin, norfloxacin, levofloxacin) have been linked to tendon injury in a number of case reports and retrospective surveillance studies. The mechanism by which fluoroquinolones are thought to damage connective tissue has not been established, but it is hypothesized to be related to oxidative damage and vascular ischemia. In animal studies, effects were more pronounced in subjects fed a magnesium-deficient diet, suggesting that chelation of magnesium could lead to radical formation and subsequent tissue damage (247). A review of published case reports (135) revealed that the most common site of tendopathy is the Achilles tendon, and injury can present as tendonitis or tendon rupture. The median duration of therapy prior to the onset of symptoms was six days, and renal dysfunction and corticosteroid use were implicated as possible risk factors. Patients who engage in sports or frequent exercise should use caution when receiving fluoroquinolones.
Another manifestation of connective tissue toxicity of the quinolones is inhibition of the epiphyseal growth plate and cartilage in the developmental stage. Arthropathy has primarily been observed in studies of young experimental animals, especially beagle dogs. Clinical experience with the quinolones in the human pediatric population is limited because routine use in this age group is discouraged. The lack of a reliable early marker for articular damage in pediatric patients and the uncertain causal relationship between the use of the newer fluoroquinolones (as opposed to nalidixic acid) may further complicate the question of risk-benefit. In carefully selected pediatric patients, the use of quinolones with close monitoring and limited ambulation may be justified for indications such as pulmonary infection in cystic fibrosis, salmonellosis, complicated urinary tract infection or after exposure to anthrax (8).
Renal Injury
Animal studies suggest the possibility of interstitial inflammatory reactions associated with precipitation of quinolone complexes within the kidney's tubular walls. Animals with distinct crystalluria have subsequently developed obstructive uropathy. The dose producing nephropathy exceeds the dose that produced crystalluria, and doses for both of these events produce serum concentrations well above the human therapeutic range. Acute renal failure, interstitial nephritis, and nonspecific nephritis were each reported once in the 2829 ciprofloxacin recipients reviewed by Arcieri et al. (14) even though causality can be very difficult to establish in these complicated clinical settings. Additional case reports have subsequently linked both ciprofloxacin (148) and levofloxacin (66,208) with the development of interstitial nephritis.
Central Nervous System
A number of the fluoroquinolones demonstrate a propensity for mild but detectable CNS effects. In clinical trials of newer fluoroquinolones, mild reactions such as headache and dizziness occurred in 2-6% of patients. Severe neurotoxic side effects are rare; however, hallucinations, depression, and psychotic reactions have been reported during therapy with many quinolones. The mechanism of neurotoxic effects is not clear but appears to be related to one of two properties: direction action on CNS receptors or interaction with co-administered medications. In vitro and in vivo experiments have revealed that some fluoroquinolones may inhibit transmission of gamma-aminobutryic acid (GABA). It appears that a large C-7 side chain may minimize the degree of GABA inhibition, and overall lipophilicity of the molecule is important for penetration across the blood-brain barrier (Figure 1). CNS effects may also be potentiated by interactions with other medications such as theophylline (47,151). Many patients reporting CNS symptoms in the early studies of fluoroquinolones had received concomitant theophylline or caffeine. Enoxacin and also ciprofloxacin, pefloxacin, and grepafloxacin significantly increase peak and trough serum theophylline concentrations. In 30% of patients experiencing increased theophylline concentrations, theophylline concentrations were clearly in the toxic range. In a study that was uncontrolled for theophylline administration, enoxacin (400-600 mg twice daily) was associated with a 42.3% incidence of nausea and dizziness and a 15.4% incidence of CNS symptomatology, compared with 6.3 and 2.5% incidences, respectively, for ciprofloxacin (500-1000 mg twice daily) and 4 and 0% for pefloxacin (400 mg twice daily), and no reactions for 30 ofloxacin patients given 400 mg once or twice daily (151,274). These results follow the rank order of potency of these fluoroquinolones in inhibiting the theophylline-metabolizing isozyme.
Photosensitivity
Varying frequencies of photosensitization have been reported with the quinolones. Of the older quinolones, nalidixic acid is well known to cause occasional phototoxic skin reactions, and on the basis of previous reactions to this agent, the potential for phototoxicity with the newer fluoroquinolones was predicted. This side effect is dose related and can be avoided or prevented by avoiding exposure to sunlight or ultraviolet radiation. The potential for phototoxicity associated with ciprofloxacin, ofloxacin, trovafloxacin, levofloxacin, gatifloxacin, moxifloxacin, and gemifloxacin appears similar and are lower than that of clinafloxacin, lomefloxacin, and nalidixic acid, compounds that are substituted with an additional chlorine or fluorine moiety at the C-8 position. (Figure 1) Fluoroquinolones with a naphthyridone base, such as enoxacin, have also been linked to phototoxicity (48,151).(Figure 2) In clinical trials of gemifloxacin, which contains a naphthyridone base, photosensitivity reactions were rare (0.039%), but gemifloxacin was associated with a higher (~3%) risk of skin rash unrelated to sun exposure. These phototoxic quinolones induce free radical formation causing tissue damage (151,265). In an analysis by the Food and Drug Administration (FDA) of spontaneous reports, lomefloxacin phototoxicity occurred in 70 per 100,000 prescriptions, compared with less than 0.1 per 100,000 for ciprofloxacin and 0.4 per 100,000 for ofloxacin (61,151). Patients given these more definitively phototoxic fluoroquinolones should be cautioned to avoid exposure to sunlight.
Glucose Metabolism
Some quinolones have been shown in animal studies to increase insulin release from pancreatic islet cells (151,160). Reports of altered glucose metabolism with gatifloxacin led to its removal from the market; however, other quinolones have been infrequently associated with this complication (79). In many reports, patients experiencing hypoglycemia had diabetes mellitus and were also being treated with a hypoglycemic medication. Elderly patients with or without diabetes may also be at a higher risk for quinolone-associated alterations in glucose metabolism (16,28). In a retrospective study of over 900 patients in a Veterans Administration health care system, Lodise et al. reported gatifloxacin was associated with higher rates of both hyperglycemia and hypoglycemia than levofloxacin, regardless of the doses used (156).
Cardiovascular Toxicity
A great deal of attention has been directed to cardiovascular toxicity induced by some quinolones. Several quinolones have been linked to QT prolongation and arrhythmias including torsades de pointes. The potential of quinolones to prolong the QT interval appears to be related to blockade of a cardiac potassium channel (13). Extensive ECG testing was conducted during the development of moxifloxacin. In 2.8% of 787 patients assessed, significant QT prolongation was noted. Published rates of torsades de pointes vary with differences in reporting methodology (76, 189). Although individual quinolones alter cardiac action potential to varying degrees, cardiovascular toxicity appears to be a class effect. The relative potency of agents tested was sparfloxacin > grepafloxacin = moxifloxacin > levofloxacin = ciprofloxacin (65a, 193, 261a). Case reports and clinical studies show that moxifloxacin carries the greatest risk of QT prolongation from all available quinolones in clinical practice while ciprofloxacin appears to be associated with the lowest risk. Nevertheless, the actual effect for patient harm is exceedingly small for all fluoroquinolones. Caution should be exercised in patients with risk factors for torsades de pointes (bradycardia) or concurrently receiving other QT-prolonging agents, including class Ia or class III antiarrhythmic agents (quinidine, procainamide, amiodarone, sotalol) (204, 205). In such patients, continuous cardiac monitoring might be prudent if quinolones are indicated for therapy.
DRUG INTERACTIONS
The important drug interactions and adverse drug reactions of the quinolones are summarized in Table 5. As with the adverse events, there is no head-to-head comparative study, and the rank ordering is by inference on the basis of noncomparative reports and studies against the same or similar non-quinolone comparator antibiotics (122). Some significant and potentially significant interactions are summarized below.
Anticoagulants
Studies on the interactions between quinolones and warfarin demonstrate that norfloxacin prolongs the elimination half-life of (R)-warfarin, while not affecting (S)-warfarin. Because the (R)-enantiomer is five to eight times less active than the (S)- isomer, the overall norfloxacin-warfarin interaction should be of little clinical significance. In clinical trials of newer agents, no significant interaction with warfarin was described. However, several anecdotal cases have implied interactions between warfarin and commonly-prescribed quinolones (15, 64, 126, 186). Any patient receiving a quinolone along with warfarin anticoagulation should have prothrombin time closely monitored.
Divalent Cations
Fluoroquinolones form chelates with divalent cations, particularly aluminum and magnesium and, to a lesser degree, iron, zinc, and calcium. Thus, co-administration of fluoroquinolones with antacids or agents such as sucralfate reduces their bioavailability by as much as 85%, which can result in therapeutic failures. Iron preparations behave similarly to antacids, and adequate time should be allowed between doses. Multivitamin preparations that contain minerals should be avoided as well. Allowing a 4- to 6-h interval between the administration of antacids or sucralfate and fluoroquinolones will likely avoid the interaction, but this is not always a suitable alternative for patients on long-term antacid treatment. Histamine-2 antagonists do not affect the oral absorption of fluoroquinolones and can be used for acid control when the quinolones must be used in the presence of acid-reducing medications.
The expanded use of oral fluoroquinolones in hospitalized patients has lead to concern over the possibility of similar interactions with enteral nutrition formulations. Healy et al. (103) demonstrated that the bioavailability of orally administered ciprofloxacin may be reduced by 27-67% when enteral nutrition formulations are given orally or via jejunonstomy, suggesting that an interruption in enteral feeding may be necessary when co-administering ciprofloxacin. No significant reduction was seen in peak ciprofloxacin concentrations when nutrition was administered via gastrostomy.
Theophylline, Caffeine, and the Xanthines
Clearance of theophylline and caffeine is inhibited by some of the quinolones, in approximately the rank order shown in Table 5. Given the different affinity for the cytochrome P-450 isozyme 1A-2, the fluoroquinolones vary in their relative degree of interaction with theophylline. The effect is strongest with enoxacin, which, in combination with theophylline, results in an approximate doubling of theophylline levels. Norfloxacin and ciprofloxacin interact with theophylline to a lesser extent than enoxacin and raise the serum concentration of theophylline by 2 to 5 µg/mL. No clinically significant interaction was demonstrated upon coadministration of theophylline with moxifloxacin, gatifloxacin, gemifloxacin, or trovafloxacin (45, 180, 248, 264). Caffeine, a chemical analogue of theophylline, interacts similarly when coadministered with quinolones. Patients receiving certain fluoroquinolones should be advised against excessive caffeine intake, and if CNS effects develop, they should be instructed to cease caffeine intake.
Tizanidine
A potentially dangerous interaction was recently found between ciprofloxacin and tizanidine, which is used for the treatment of muscle pain associated with spasticity or muscle tension (100). In healthy volunteers, compared to placebo, concentrations of tizanidine in blood were increased 10-fold after receipt of 500mg of ciprofloxacin twice daily for 3 days. The interaction resulted in a severe decrease in blood pressure and enhanced central nervous system effects. The proposed mechanism is an inhibition of the liver metabolism of tizanidine. The combination of these is now contraindicated.
Probenecid
Probenecid administration increases peak plasma concentrations and prolongs the half-life of quinolones primarily excreted by the renal route, such as ciprofloxacin, ofloxacin, levofloxacin, gatifloxacin, and gemifloxacin. The mechanism of this effect is inhibition of renal tubular secretion, most likely secondary to inhibition of renal transport proteins by probenecid. Accordingly, trovafloxacin and moxifloxacin are less affected, since they are excreted primarily by hepatic clearance mechanisms.
CLINICAL INDICATIONS
The spectrum of activity and potency of the fluoroquinolone class of antimicrobials has lead to a wide range of clinical indications and broad use of these agents for both gram-positive and gram-negative infections. While the older agents demonstrated reliable activity predominantly against gram-negative pathogens, the enhanced gram-positive spectrum of the newer agents has expanded their role in the treatment of community-acquired respiratory infections and skin and soft tissue infections. While concentration-dependent killing activity makes the fluoroquinolones desirable agents for the treatment of serious infections, the rise in resistance rates, particularly in P. aeruginosa has caused concern among practitioners, that overuse may threaten the future usefulness of the class. (178) Similarly, an increase in prevalence of first-step resistance mutations within S. pneumoniae (149, 230) has prompted recommendations for more prudent use of the fluoroquinolones for community-acquired respiratory tract infections; in essence, these drugs should be used only when alternatives are precluded by lack of pathogen susceptibility or patient allergy or intolerance (5).
When selecting fluoroquinolone, there are numerous considerations to take into account, among which are clinical efficacy and FDA labeled indications. These data will be reviewed for the most frequently used fluoroquinolones, ciprofloxacin, levofloxacin, moxifloxacin and the most recently approved agent gemifloxacin.
While intravenous therapy may be preferred for patients with severe infections, comorbid conditions, or gastrointestinal malabsorption, oral fluoroquinolones are readily bioavailable in the absence of drug interactions or the aforementioned conditions. When possible, oral administration is preferred over intravenous administration because of ease of administration, reduced risk of adverse drug events and lower cost (190).
Urinary Tract Infections
When the first fluoroquinolones including nalidixic acid, ciprofloxacin, norfloxacin, and ofloxacin were investigated, they were noted to have had excellent gram-negative activity including most uropathogens and were excreted in large amounts in the urine. Of the currently available agents, ciprofloxacin and levofloxacin have demonstrated efficacy and are FDA approved for the treatment of complicated and uncomplicated urinary tract infections and chronic bacterial prostatitis.
The quinolones have been proven effective in simple and complicated urinary tract infections. In both situations, they are as effective as trimethoprim-sulfamethoxazole (T/S) and more effective than β-lactam antibiotics (114,131,196,226). Though effective in simple cystitis, many effective narrower-spectrum and less expensive drugs are available. In general, these antibiotics should be used first-line, leaving the quinolones for the treatment of more difficult cases. It has been suggested that tissue rather than serum levels are a greater determinant of efficacy in pyelonephritis; however, norfloxacin which achieved high urine, but not tissue concentrations, still provides effective treatment for pyelonephritis as well as cystitis. It is proposed that back diffusion of antibiotics from the tubular solution into the renal interstitium may contribute to efficacy. Because norfloxacin does not achieve systemic concentrations, there is controversy over its use for the treatment of pyelonephritis; however, published studies exist demonstrating clinical success with norfloxacin in patients with uncomplicated pyelonephritis (224, 226). Because of its minimal renal excretion and relatively low urinary concentrations, moxifloxacin is not recommended for the treatment of urinary tract infections.
Bacterial Prostatitis
Because fluoroquinolones offer excellent penetration into prostatic tissue, they are frequently cited as drugs of choice for complicated infections in males which frequently involve the prostate. Although penetration of various fluoroquinolones into the prostate may vary, prostate concentrations are generally 20-50% of serum concentrations. Of note, ciprofloxacin is reported to produce higher prostate concentrations than ofloxacin (and presumably levofloxacin).
Both ciprofloxacin, ofloxacin and levofloxacin have been effective in chronic bacterial prostatitis due to Enterobacteriaceae(34, 37, 39, 207, 224, 268). Cure rates of 60 to 80% are possible with 4 to 6 weeks of therapy. This compares favorably to trimethoprim-sulfamethoxazole. Infections due to P. aeruginosa and enterococci are much more difficult to eradicate.
Respiratory Tract Infections
The quinolones have been extensively studied in the treatment of community and nosocomially acquired pneumonia, bronchitis, sinusitis, and infectious complications of cystic fibrosis. Some of the indications along with quinolones of choice and dosages are listed in Table 4. In general, the fluoroquinolones have been found equivalent or superior to comparator drugs such as β-lactams or β-lactam/macrolide combinations (74,154,227). The activities of levofloxacin, moxifloxacin, and gemifloxacin against common respiratory pathogens makes these drugs useful for community-acquired infections (162). Modest literature supports the use of these drugs for the treatment of atypical pneumonia, including Legionella infection (74,113,276). Most published studies have used a treatment duration of 7 to 10 days; however, levofloxacin 750 mg once daily for 5 days has been shown to be as effective as a 10 day course for the treatment of mild to severe community-acquired pneumonia (58).
Due to its effective antipseudomonal activity, ciprofloxacin is most suitable for nosocomial infections and infections in patients with of cystic fibrosis (123, 155, 195, 228, 236, 237). Levofloxacin, at the higher dose of 750mg daily has also demonstrated efficacy in nosocomial pneumonia (271). If lower respiratory tract infection with P. aeruginosa is suspected, ciprofloxacin may be the preferred quinolone, and for seriously ill patients the appropriate intravenous dose is 400 mg three times daily (163, 261). Synergy or additive effects between the quinolones and β-lactams such as piperacillin and ceftazidime against, P. aeruginosaand other Gram-negative organisms argues in favor of combination therapy in certain clinical situations. S. aureus and P. aeruginosa often develop resistance to quinolones used in therapy, particularly in patients who are intubated or have cystic fibrosis (237). These events are typically associated with low serum concentration:MIC ratios (195), low AUC:MICs (258), or both.
Skin and Soft-Tissue Infections
Ofloxacin, ciprofloxacin, and moxifloxacin have been extensively studied as therapy for soft tissue infections. When compared with third- generation cephalosporins in difficult-to-treat infections, antibiotics from both classes have traditionally fared well (67, 85, 88). The newer fluoroquinolones such as moxifloxacin or levofloxacin are often preferable to ciprofloxacin because of their improved Gram-positive activity. Quinolone monotherapy is not ideal for all patients. Fluoroquinolones may be useful as a component of combination therapy for serious polymicrobial skin and soft tissue infections such as diabetic foot infections or decubitus ulcers. Frequently, an additional agent should be considered for treatment of anaerobic organisms. However, because of the enhanced anaerobic activity of moxifloxacin, monotherapy with this agents may be considered. With all fluoroquinolones, lack of reliable activity against MRSA precludes their use for infections involving this organism.
Osteomyelitis
Ciprofloxacin and ofloxacin are effective therapy for osteomyelitis due to Gram-negative organisms (84, 86, 87). Cure rates of 70 to 80% at least in the short term can be expected. The fluoroquinolones have also been successful in the treatment of osteomyelitis due to S. aureus; however, in general, higher doses should be used. Ofloxacin in combination with rifampin was used successfully in one study of infected hip prostheses (49). Ciprofloxacin is effective in the treatment of malignant otitis externa (105,146). In open trials of ciprofloxacin and ofloxacin for osteomyelitis predominantly caused by Enterobacteriaceae or P. aeruginosa, clinical improvement rates ranged from 65-100% (42, 89, 182, 238). Published data on trovafloxacin, gatifloxacin, and other fluoroquinolones with improved Gram-positive activity are very limited. Newer fluoroquinolones could be effective against osteomyelitis due to Gram-positive pathogens; however, further studies are needed to clarify the role of quinolones for the treatment of S. aureus or streptococcal osteomyelitis.
Gastrointestinal Infections
Gastroenteritis has been a major area of quinolone usage. Many of the major pathogens - Salmonella, Shigella, E. coli, Aeromonas, - are generally very susceptible to quinolone antibiotics; however increasing resistance among Campylobacterspecies may limit their usefulness against this organism. Ciprofloxacin, levofloxacin and ofloxacin given for 3 to 5 days with or without loperamide are drugs of choice for travelers' diarrhea (59, 65, 255). Typhoid fever has been successfully treated with 10- to 14-day courses of ciprofloxacin, pefloxacin, or ofloxacin (101, 209). Norfloxacin, while marginally effective in the treatment of typhoid fever, can be given as a 4-week course to eradicate S. typhi in chronic carriers (99). Campylobacter infections are more problematic, as resistance may develop during treatment (241).
Intra-abdominal Infections
Hepatobiliary infections and a variety of intra-abdominal infections can also be treated with ciprofloxacin or ofloxacin, often in combination with a drug with anti-anaerobic activity such as metronidazole or clindamycin. Sequential IV/PO ciprofloxacin plus metronidazole has been shown to be an equivalent, cost effective alternative to IV piperacillin/tazobactam or imipenem/cilastatin for the treatment of complicated intra-abdominal infections (38, 243, 266). Ciprofloxacin, levofloxacin or moxifloxacin in combination with metronidazole, are among the agents recommended by the Infectious Diseases Society of America for the treatment of intra-abdominal infections of mild to moderate severity, while only ciprofloxacin in combination with metronidazole is recommended for severe infections (242).
Sexually Transmitted Diseases
Most quinolones have good activity against N. gonorrhoeae, H. ducreyii, C. trachomatis, and the genital mycoplasmas and ureaplasmas. Excellent results have been obtained using single-dose therapy with ciprofloxacin, ofloxacin, or norfloxacin for gonococcal infections. (33, 157, 191). Widespread use of quinolones has lead to the emergence of fluoroquinolone-resistant N. gonorrhoeae. High levels of fluoroquinolone resistant N. gonorrhoeae have been reported in Asia and the Pacific (262, 290) and recently started to increase in other geographical areas, including the United States (4). As a result, the Center for Disease Control and Prevention (CDC) no longer recommends empiric use of fluoroquinolones for the treatment of gonococcal infections (4). The quinolone of choice for chlamydia infections is ofloxacin or levofloxacin (280), which achieves 90% cure rates with a 7-day course of therapy (211). Data are very limited in ureaplasma and mycoplasma infections. Ofloxacin appears similar in efficacy to erythromycin, about 80%. For chancroid, caused by Haemophilus ducreyi, quinolones appear to be less effective than ceftriaxone or azithromycin, both of which can be given in a single dose. When a quinolone is used, ciprofloxacin daily for 3 days is highly effective. Combinations of quinolones with agents effective against anaerobes have been very successful in the treatment of pelvic inflammatory disease (263); current treatment guidelines recommend the addition of metronidazole to ofloxacin or levofloxacin (280).
Mycobacterial Infections
Many fluoroquinolones have good activity against M. tuberculosis, especially levofloxacin and moxifloxacin. Experience with levofloxacin has been the most extensive (91). Results have been favorable, and the drugs are preferred second-line agents, when first-line agents cannot be used because of resistance or intolerance.(2) When used for tuberculosis, fluoroquinolones are best given as a single daily dose (e.g., levofloxacin 500-750 mg moxifloxacin 400 mg) in the morning. Resistance has been described particularly when the quinolone is the only active drug in a regimen or when extensive cavitary disease is present (176). The quinolones may be used with other antimycobacterial agents to treat pulmonary disease due to a number of atypical mycobacterial pathogens such as Mycobacterium fortuitum and M. chelonae (283).
Inhalational Anthrax (Post-Exposure)
Ciprofloxacin and levofloxacin are FDA approved for the prophylaxis of inhalational Bacillus anthracis infection. During the recent bioterrorist attacks in the US, the Centers for Disease Control and Prevention included ciprofloxacin among recommended agents for post-exposure prophylaxis. The FDA approved dose in adults is 500 mg orally twice daily. The optimal duration of prophylaxis is uncertain, but currently 60 days is the recommended duration based on animal studies (1,6). Ciprofloxacin is also first-line therapy for treatment of inhalational and cutaneous anthrax (32). Because ciprofloxacin is superior to penicillin and amoxicillin in terms of its ability to kill germinating spores in macrophages, ciprofloxacin is among the recommended agents for treatment and prophylaxis of B. anthracis in children, despite the potential for adverse effects (3).
Neutropenia
The older fluoroquinolones, because of their excellent Gram-negative and poor anaerobic activity, are effective in eradicating Gram-negative bacteria from the intestinal flora without disrupting colonization resistance to new organisms. They are thus attractive candidates for prophylaxis of infection in the neutropenic host (177). Norfloxacin, ciprofloxacin, and ofloxacin have all been effective in this situation (46, 147, 177, 275). The newer agents with enhanced Gram-positive activity have not been thoroughly evaluated for prophylaxis in afebrile neutropenic patients.
The frequent use of fluoroquinolones for prophylaxis limits their potential use as initial therapy in febrile neutropenia. Ciprofloxacin has been used to treat febrile episodes in neutropenic patients, either alone or in combination with an aminoglycoside or an antipseudomonal penicillin (194). Used at doses of 400 mg every 8 to 12 h, ciprofloxacin has proven to be as effective as comparator β-lactam-aminoglycoside regimens (133, 150, 197). Gram-positive superinfections may occur (168) and resistance may develop in Pseudomonas and some other Gram-negative organisms if dosages are too low or baseline MICs are elevated. Oral ciprofloxacin in combination with amoxicillin-clavulanate has been used effectively in low-risk neutropenic patients. Levofloxacin and moxifloxacin are not currently recommended in the treatment of febrile neutropenia (133, 150, 194).
Nasal Carriage of Neisseria Meningitides
Ciprofloxacin achieves concentrations of 0.1 to 0.4 ug/mL in nasal secretions which are well above the 0.004 ug/mL concentration required to inhibit N. meningitides. Single doses of 750 mg or 500 mg twice daily are very effective in eradicating meningococcal carriage (60, 206, 210).
Eye Infections
Norfloxacin, ciprofloxacin, moxifloxacin, and ofloxacin ophthalmic solutions contain 3 to 5 mg/mL of the quinolone. They have been used very successfully to treat conjunctivitis and keratitis due to S. aureus, H. influenzae, S. pneumoniae, and P. aeruginosa (121, 142, 143). Systemic norfloxacin (1200 mg as a single dose) has proven effective in the treatment of gonococcal conjunctivitis (134).
Pediatric Indications
Historically, risk of bone and cartilage adverse effects have limited the study of fluoroquinolones for pediatric infections. Increasingly, however, data are becoming available in this population, particularly in cystic fibrosis patients with resistant pseudomonal respiratory tract infections. Because of its enhanced activity against Pseudomonas spp., ciprofloxacin has been the most studied agent in this population. Ciprofloxacin received FDA approval for use in pediatric patients, age 1 to 18 years, for the treatment of complicated urinary tract infections and pyelonephritis due to E. coli (199). Ciprofloxacin is not a drug of first choice in the pediatric population due to higher incidence of adverse effects compared to controls, but should be reserved for cases when use of other antibiotics is limited by pathogen antibiotic resistance or patient allergy or intolerance.
References
1. From the Centers for Disease Control and Prevention. Update: Investigation of bioterrorism-related anthrax and interim guidelines for exposure management and antimicrobial therapy, October 2001. JAMA. 2001;286(18):2226-32. [PubMed]
2. Treatment of Tuberculosis. American Thoracic Society, Centers for Deisease Control and Prevention, and Infectious Diseases Society of America. MMWR Recomm Rep. 2003;52(RR11):1-77.
3. ASHP News. Tequin to Exit Markets Worldwide. http://www.ashp.org/s_ashp/article_news.asp?CID=167&DID=2024&id=15156. Accessed July 2007.
4. Update to CDC's sexually transmitted diseases treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56(14):332-336. [PubMed]
5. Assessment of national reporting of drug-resistant Streptococcus pneumoniae--United States, 1995-1996. MMWR Morb Mortal Wkly Rep. 1996;45(43):947-9. [PubMed]
6. From the Centers for Disease Control and Prevention. Recommendations for antimicrobial prophylaxis for children and breastfeeding mothers and treatment of children with anthrax. JAMA. 2001;286(21):2663-4. [PubMed]
7. Ackermann G, Schaumann R, Pless B et al. Comparative activity of moxifloxacin in vitro against obligately anaerobic bacteria. Eur J Clin Microbiol Infect Dis. 2000;19(3):228-32. [PubMed]
8. Alghasham AA, Nahata MC. Clinical use of fluoroquinolones in children. Ann Pharmacother. 2000;34(3):347-59. [PubMed]
9. Allen A, Bygate E, Vousden M et al. Multiple-dose pharmacokinetics and tolerability of gemifloxacin administered orally to healthy volunteers. Antimicrob Agents Chemother. 2001;45(2):540-545. [PubMed]
10. Alonso R, Pelaez T, Gonzalez-Abad MJ et al. In vitro activity of new quinolones against Clostridium difficile. J Antimicrob Chemother. 2001;47(2):195-7. [PubMed]
11. Alvirez-Freites EJ, Carter JL, Cynamon MH. In vitro and in vivo activities of gatifloxacin against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2002;46(4):1022-5. [PubMed]
12. Ambrose PG, Grasela DM, Grasela TH et al. Pharmacodynamics of fluoroquinolones against Streptococcus pneumoniae in patients with community-acquired respiratory tract infections. Antimicrob Agents Chemother. 2001;45(10):2793-7. [PubMed]
13. Anderson ME, Mazur A, Yang T et al. Potassium current antagonist properties and proarrhythmic consequences of quinolone antibiotics. J Pharmacol Exp Ther. 2001;296(3):806-10. [PubMed]
14. Arcieri GM, Becker N, Esposito B et al. Safety of intravenous ciprofloxacin. A review. Am J Med. 1989;87(5A):92S-97S. [PubMed]
15. Artymowicz RJ, Cino BJ, Rossi JG et al. Possible interaction between gatifloxacin and warfarin. Am J Health Syst Pharm. 2002;59(12):1205-6. [PubMed]
16. Baker SE, Hangii MC. Possible gatifloxacin-induced hypoglycemia. Ann Pharmacother. 2002;36(11):1722-6. [PubMed]
17. Ball P. Future of the quinolones. Semin Respir Infect. 2001;16(3):215-24. [PubMed]
18. Ball P. Quinolone generations: natural history or natural selection? J Antimicrob Chemother. 2000;46 Suppl T1:17-24.[PubMed]
19. Ball P. Quinolone-induced QT interval prolongation: a not-so-unexpected class effect. J Antimicrob Chemother. 2000;45(5):557-9. [PubMed]
20. Ball P. Safety of the new fluoroquinolones compared with ciprofloxacin. J Chemother. 2000;12(Suppl 1):8-11. [PubMed]
21. Ball P, Tillotson G. Tolerability of fluoroquinolone antibiotics. Past, present and future. Drug Saf. 1995;13(6):343-58. [PubMed]
22. Barriere SL, Ely E, Kapusnik JE et al. Analysis of a new method for assessing activity of combinations of antimicrobials: area under the bactericidal activity curve. J Antimicrob Chemother. 1985;16(1):49-59. [PubMed]
23. Bassetti M, Dembry LM, Farrel PA et al. Antimicrobial activities of BMS-284756 compared with those of fluoroquinolones and beta-lactams against gram-positive clinical isolates. Antimicrob Agents Chemother. 2002;46(1):234-8. [PubMed]
24. Behra-Miellet J, Dubreuil L, Jumas-Bilak E. Antianaerobic activity of moxifloxacin compared with that of ofloxacin, ciprofloxacin, clindamycin, metronidazole and beta-lactams. Int J Antimicrob Agents. 2002;20(5):366-74. [PubMed]
25. Bermudez LE, Inderlied CB, Kolonoski P et al. Activity of moxifloxacin by itself and in combination with ethambutol, rifabutin, and azithromycin in vitro and in vivo against Mycobacterium avium. Antimicrob Agents Chemother. 2001;45(1):217-22. [PubMed]
26. Bertino JS, Jr., Owens RC, Jr., Carnes TD et al. Gatifloxacin-associated corrected QT interval prolongation, torsades de pointes, and ventricular fibrillation in patients with known risk factors. Clin Infect Dis. 2002;34(6):861-3. [PubMed]
27. Biedenbach DJ, Stephen JM, Jones RN. Antimicrobial susceptibility profile among beta-haemolytic Streptococcus spp. collected in the SENTRY Antimicrobial Surveillance Program--North America, 2001. Diagn Microbiol Infect Dis. 2003;46(4):291-4.[PubMed]
28. Biggs WS. Hypoglycemia and hyperglycemia associated with gatifloxacin use in elderly patients. J Am Board Fam Pract. 2003;16(5):455-7. [PubMed]
29. Blaser J, Stone BB, Groner MC et al. Comparative study with enoxacin and netilmicin in a pharmacodynamic model to determine importance of ratio of antibiotic peak concentration to MIC for bactericidal activity and emergence of resistance. Antimicrob Agents Chemother. 1987;31(7):1054-60. [PubMed]
30. Blondeau JM, Hansen G, Metzler KL et al. In vitro susceptibility of 4903 bacterial isolates to gemifloxacin--an advanced fluoroquinolone. Int J Antimicrob Agents. 2003;22(2):147-54. [PubMed]
31. Brogard JM, Jehl F, Monteil H et al. Comparison of high-pressure liquid chromatography and microbiological assay for the determination of biliary elimination of ciprofloxacin in humans. Antimicrob Agents Chemother. 1985;28(2):311-4. [PubMed]
32. Brook I. The prophylaxis and treatment of anthrax. Int J Antimicrob Agents. 2002;20(5):320-5. [PubMed]
33. Bryan JP, Hira SK, Brady W et al. Oral ciprofloxacin versus ceftriaxone for the treatment of urethritis from resistant Neisseria gonorrhoeae in Zambia. Antimicrob Agents Chemother. 1990;34(5):819-22. [PubMed]
34. Bundrick W, Heron SP, Ray P et al. Levofloxacin versus ciprofloxacin in the treatment of chronic bacterial prostatitis: a randomized double-blind multicenter study. Urology. 2003;62(3):537-541. [PubMed]
35. Byrd DC, Gaskins SE, Parrish AM et al. Warfarin and ciprofloxacin interaction: case report and controversy. J Am Board Fam Pract. 1999;12(6):486-8. [PubMed]
36. Child J, Mortiboy D, Andrews JM et al. Open-label crossover study to determine pharmacokinetics and penetration of two dose regimens of levofloxacin into inflammatory fluid. Antimicrob Agents Chemother. 1995;39(12):2749-51. [PubMed]
37. Childs SJ. Ciprofloxacin in treatment of chronic bacterial prostatitis. Urology. 1990;35(1 Suppl):15-18. [PubMed]
38. Cohn SM, Lipsett PA, Buchman TG et al. Comparison of intravenous/oral ciprofloxacin plus metronidazole versus piperacillin/tazobactam in the treatment of complicated intraabdominal infections. Ann Surg. 2000;232(2):254-62. [PubMed]
39. Corrado ML. Worldwide clinical experience with ofloxacin in urologic cases. Urology. 1991;37(3 Suppl):28-32. [PubMed]
40. Critchley IA, Jones ME, Heinze PD et al. In vitro activity of levofloxacin against contemporary clinical isolates of Legionella pneumophila, Mycoplasma pneumoniae and Chlamydia pneumoniae from North America and Europe. Clin Microbiol Infect. 2002;8(4):214-21. [PubMed]
41. Dalvie DK, Khosla N, Vincent J. Excretion and metabolism of trovafloxacin in humans. Drug Metab Dispos. 1997;25(4):423-7.[PubMed]
42. Dan M, Torossian K, Weissberg D et al. The penetration of ciprofloxacin into bronchial mucosa, lung parenchyma, and pleural tissue after intravenous administration. Eur J Clin Pharmacol. 1993;44(1):101-2. [PubMed]
43. Davis R, Bryson HM. Levofloxacin. A review of its antibacterial activity, pharmacokinetics and therapeutic efficacy. Drugs. 1994;47(4):677-700. [PubMed]
44. Davis RL, Kelly HW, Quenzer RW et al. Effect of norfloxacin on theophylline metabolism. Antimicrob Agents Chemother. 1989;33(2):212-4. [PubMed]
45. Davy M, Bird N, Rost KL et al. Lack of effect of gemifloxacin on the steady-state pharmacodynamics of warfarin in healthy volunteers. Chemotherapy. 1999;45(6):491-5. [PubMed]
46. Dekker AW, Rozenberg-Arska M, Verhoef J. Infection prophylaxis in acute leukemia: a comparison of ciprofloxacin with trimethoprim-sulfamethoxazole and colistin. Ann Intern Med. 1987;106(1):7-11. [PubMed]
47. DeSarro A, DeSarro G. Adverse reactions to fluoroquinolones, an overview on mechanistic aspects. Curr Med Chem. 2001;8:371-384. [PubMed]
48. Domagala JM. Structure-activity and structure-side-effect relationships for the quinolone antibacterials. J Antimicrob Chemother. 1994;33(4):685-706. [PubMed]
49. Drancourt M, Stein A, Argenson JN et al. Oral rifampin plus ofloxacin for treatment of Staphylococcus-infected orthopedic implants. Antimicrob Agents Chemother. 1993;37(6):1214-8. [PubMed]
50. Drlica K, Malik M. Fluoroquinolones: action and resistance. Curr Top Med Chem. 2003;3(3):249-82. [PubMed]
51. Drusano GL. Role of pharmacokinetics in the outcome of infections. Antimicrob Agents Chemother. 1988;32(3):289-97.[PubMed]
52. Dubois J, St-Pierre C. In vitro activity of gatifloxacin, compared with ciprofloxacin, clarithromycin, erythromycin, and rifampin, against Legionella species. Diagn Microbiol Infect Dis. 1999;33(4):261-5. [PubMed]
53. Dubois J, St-Pierre C. Comparative in vitro activity and post-antibiotic effect of gemifloxacin against Legionella spp. J Antimicrob Chemother. 2000;45 Suppl 1:41-6. [PubMed]
54. Dubois J, St-Pierre C. In vitro susceptibility study of BMS-284756 against Legionella species. Diagn Microbiol Infect Dis. 2001;41(1-2):79-82. [PubMed]
55. Dubreuil L, Behra-Miellet J, Neut C et al. In vitro activity of gatifloxacin, a new fluoroquinolone, against 204 anaerobes compared to seven other compounds. Clin Microbiol Infect. 2003;9(11):1133-8. [PubMed]
56. Dudley MN. Pharmacodynamics and pharmacokinetics of antibiotics with special reference to the fluoroquinolones. Am J Med. 1991;91(6A):45S-50S. [PubMed]
57. Dudley MN, Blaser J, Gilbert D et al. Bactericidal activity of ciprofloxacin against P. aeruginosa and other bacteria in an in vitro two compartment capillary model. Rev Infect Dis. 1988;10(Suppl):S34-S35.
58. Dunbar LM, Wunderink RG, Habib MP et al. High-dose, short-course levofloxacin for community-acquired pneumonia: a new treatment paradigm. Clin Infect Dis. 2003;37(6):752-60. [PubMed]
59. DuPont HL, Ericsson CD, Mathewson JJ et al. Five versus three days of ofloxacin therapy for traveler's diarrhea: a placebo-controlled study. Antimicrob Agents Chemother. 1992;36(1):87-91. [PubMed]
60. Dworzack DL, Sanders CC, Horowitz EA et al. Evaluation of single-dose ciprofloxacin in the eradication of Neisseria meningitidis from nasopharyngeal carriers. Antimicrob Agents Chemother. 1988;32(11):1740-1. [PubMed]
61. Incidence of phototoxicity to three fluoroquinolones(Abstract). Washington, DC, Jun 27-Jun12: 1993.
62. Edmiston CE, Jr., Suarez EC, Walker AP et al. Penetration of ciprofloxacin and fleroxacin into biliary tract. Antimicrob Agents Chemother. 1996;40(3):787-91. [PubMed]
63. Ednie LM, Rattan A, Jacobs MR et al. Antianaerobe activity of RBX 7644 (ranbezolid), a new oxazolidinone, compared with those of eight other agents. Antimicrob Agents Chemother. 2003;47(3):1143-7. [PubMed]
64. Ellis RJ, Mayo MS, Bodensteiner DM. Ciprofloxacin-warfarin coagulopathy: a case series. Am J Hematol. 2000;63(1):28-31.[PubMed]
65. Ericsson CD, Johnson PC, DuPont HL et al. Ciprofloxacin or trimethoprim-sulfamethoxazole as initial therapy for travelers' diarrhea. A placebo-controlled, randomized trial. Ann Intern Med. 1987;106(2):216-20. [PubMed]
65a. Falagas ME, Rafailidis PI, Rosmarakis ES. Arrhythmias associated with fluoroquinolone therapy. Int J Antimicrob Agents 2007;29:374-379.[PubMed]
66. Famularo G, De Simone C. Nephrotoxicity and purpura associated with levofloxacin. Ann Pharmacother. 2002;36(9):1380-2.[PubMed]
67. Fass RJ, Plouffe JF, Russell JA. Intravenous/oral ciprofloxacin versus ceftazidime in the treatment of serious infections. Am J Med. 1989;87(5A):164S-168S. [PubMed]
68. Fernandez-Roblas R, Cabria F, Esteban J et al. In vitro activity of gemifloxacin (SB-265805) compared with 14 other antimicrobials against intestinal pathogens. J Antimicrob Chemother. 2000;46(6):1023-7. [PubMed]
69. Fillastre JP, Leroy A, Moulin B et al. Pharmacokinetics of quinolones in renal insufficiency. J Antimicrob Chemother. 1990;26(Suppl B):51-60. [PubMed]
70. Fish DN, Chow AT. The clinical pharmacokinetics of levofloxacin. Clin Pharmacokinet. 1997;32(2):101-19. [PubMed]
71. Fix AM, Pfaller MA, Biedenbach DJ et al. Comparative antimicrobial spectrum and activity of BMS284756 (T-3811, a desfluoroquinolone) tested against 656 Enterobacteriaceae, including preliminary in vitro susceptibility test comparisons and development. Int J Antimicrob Agents. 2001;18(2):141-5. [PubMed]
72. Forrest A, Ballow CH, Nix DE et al. Development of a population pharmacokinetic model and optimal sampling strategies for intravenous ciprofloxacin. Antimicrob Agents Chemother. 1993;37(5):1065-72. [PubMed]
73. Forrest A, Nix DE, Ballow CH et al. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob Agents Chemother. 1993;37(5):1073-81. [PubMed]
74. Forsberg P, Maller R, Nilsson LG. Comparative study of oral ofloxacin and erythromycin in the treatment of pneumonia. Rev Infect Dis. 1989;11(SuppI5):S1229-S1230.
75. Fraise AP, Smith SP. Ciprofloxacin in combined renal and hepatic impairment. J Antimicrob Chemother. 1990;25(2):297-8.[PubMed]
76. Frothingham R. Rates of torsades de pointes associated with ciprofloxacin, ofloxacin, levofloxacin, gatifloxacin, and moxifloxacin. Pharmacotherapy. 2001;21(12):1468-72. [PubMed]
77.Fuchs PC, Barry AL, Brown SD. In vitro activity of gemifloxacin against contemporary clinical bacterial isolates from eleven North American medical centers, and assessment of disk diffusion test interpretive criteria. Diagn Microbiol Infect Dis. 2000;38(4):243-53. [PubMed]
78. Fung-Tomc J, Minassian B, Kolek B et al. In vitro antibacterial spectrum of a new broad-spectrum 8-methoxy fluoroquinolone, gatifloxacin. J Antimicrob Chemother. 2000;45(4):437-46. [PubMed]
79. Gajjar DA, LaCreta FP, Kollia GD et al. Effect of multiple-dose gatifloxacin or ciprofloxacin on glucose homeostasis and insulin production in patients with noninsulin-dependent diabetes mellitus maintained with diet and exercise. Pharmacotherapy. 2000;20(6 Pt 2):76S-86S. [PubMed]
80. Galante D, Esposito S, Barba D et al. Ciprofloxacin in the treatment of urinary and respiratory tract infections in patients with chronic liver disease. Chemioterapia. 1986;5(5):322-6. [PubMed]
81. Gales AC, Sader HS, Mendes RE et al. Salmonella spp. isolates causing bloodstream infections in Latin America: report of antimicrobial activity from the SENTRY Antimicrobial Surveillance Program (1997-2000). Diagn Microbiol Infect Dis. 2002;44(3):313-8. [PubMed]
82. Gee T, Andrews JM, Ashby JP et al. Pharmacokinetics and tissue penetration of gemifloxacin following a single oral dose. J Antimicrob Chemother. 2001;47(4):431-434. [PubMed]
83. Gehanno P, Darantiere S, Dubreuil C et al. A prospective, multicentre study of moxifloxacin concentrations in the sinus mucosa tissue of patients undergoing elective surgery of the sinus. J Antimicrob Chemother. 2002;49(5):821-6. [PubMed]
84. Gentry LO. Oral antimicrobial therapy for osteomyelitis. Ann Intern Med. 1991;114(11):986-7. [PubMed]
85. Gentry LO, Ramirez-Ronda CH, Rodriguez-Noriega E et al. Oral ciprofloxacin vs parenteral cefotaxime in the treatment of difficult skin and skin structure infections. A multicenter trial. Arch Intern Med. 1989;149(11):2579-83. [PubMed]
86. Gentry LO, Rodriguez GG. Oral ciprofloxacin compared with parenteral antibiotics in the treatment of osteomyelitis. Antimicrob Agents Chemother. 1990;34(1):40-3. [PubMed]
87. Gentry LO, Rodriguez-Gomez G. Ofloxacin versus parenteral therapy for chronic osteomyelitis. Antimicrob Agents Chemother. 1991;35(3):538-41. [PubMed]
88. Gentry LO, Rodriguez-Gomez G, Zeluff BJ et al. A comparative evaluation of oral ofloxacin versus intravenous cefotaxime therapy for serious skin and skin structure infections. Am J Med. 1989;87(6C):57S-60S. [PubMed]
89. Gilbert DN, Tice AD, Marsh PK et al. Oral ciprofloxacin therapy for chronic contiguous osteomyelitis caused by aerobic gram-negative bacilli. Am J Med. 1987;82(4A):254-8. [PubMed]
90. Gillespie SH, Billington O. Activity of moxifloxacin against mycobacteria. J Antimicrob Chemother. 1999;44(3):393-5. [PubMed]
91. Gillespie SH, Kennedy N. Fluoroquinolones: a new treatment for tuberculosis? Int J Tuberc Lung Dis. 1998;2(4):265-271.[PubMed]
92. Goldstein EJ. Review of the in vitro activity of gemifloxacin against gram-positive and gram-negative anaerobic pathogens. J Antimicrob Chemother. 2000;45 Suppl 1:55-65. [PubMed]
93. Goldstein EJ, Citron DM, Merriam CV et al. Activity of gatifloxacin compared to those of five other quinolones versus aerobic and anaerobic isolates from skin and soft tissue samples of human and animal bite wound infections. Antimicrob Agents Chemother. 1999;43(6):1475-9. [PubMed]
94. Goldstein EJ, Citron DM, Warren Y et al. In vitro activity of gemifloxacin (SB 265805) against anaerobes. Antimicrob Agents Chemother. 1999;43(9):2231-5. [PubMed]
95. Goldstein EJ, Conrads G, Citron DM et al. In vitro activity of gemifloxacin compared to seven other oral antimicrobial agents against aerobic and anaerobic pathogens isolated from antral sinus puncture specimens from patients with sinusitis. Diagn Microbiol Infect Dis. 2002;42(2):113-8. [PubMed]
96. Gootz TD, Brighty KE. Fluoroquinolone antibacterials: SAR mechanism of action, resistance, and clinical aspects. Med Res Rev. 1996;16(5):433-86. [PubMed]
97. Gordon KA, Sader HS, Jones RN. Contemporary re-evaluation of the activity and spectrum of grepafloxacin tested against isolates in the United States. Diagn Microbiol Infect Dis. 2003;47(1):377-83. [PubMed]
98. Goss TF, Forrest A, Nix DE et al. Mathematical examination of dual individualization principles (II): The rate of bacterial eradication at the same area under the inhibitory curve is more rapid for ciprofloxacin than for cefmenoxime. Ann Pharmacother. 1994;28(7-8):863-8. [PubMed]
99. Gotuzzo E, Guerra JG, Benavente L et al. Use of norfloxacin to treat chronic typhoid carriers. J Infect Dis. 1988;157(6):1221-5.[PubMed]
100. Granfors MT, Backman JT, Neuvonen M et al. Ciprofloxacin greatly increases concentrations and hypotensive effect of tizanidine by inhibiting its cytochrome P450 1A2-mediated presystemic metabolism. Clin Pharmacol Ther. 2004;76(6):598-606.[PubMed]
101. Hajji M, el Mdaghri N, Benbachir M et al. Prospective randomized comparative trial of pefloxacin versus cotrimoxazole in the treatment of typhoid fever in adults. Eur J Clin Microbiol Infect Dis. 1988;7(3):361-3. [PubMed]
102. Hammerschlag MR. Activity of gemifloxacin and other new quinolones against Chlamydia pneumoniae: a review. J Antimicrob Chemother. 2000;45 Suppl 1:35-9. [PubMed]
103. Healy DP, Brodbeck MC, Clendening CE. Ciprofloxacin absorption is impaired in patients given enteral feedings orally and via gastrostomy and jejunostomy tubes. Antimicrob Agents Chemother. 1996;40(1):6-10. [PubMed]
104. Hecht DW, Osmolski JR. Activities of garenoxacin (BMS-284756) and other agents against anaerobic clinical isolates. Antimicrob Agents Chemother. 2003;47(3):910-6. [PubMed]
105. Hickey SA, Ford GR, O'Connor AF et al. Treating malignant otitis with oral ciprofloxacin. BMJ. 1989;299(6698):550-1.[PubMed]
106. Hoban D, Waites K, Felmingham D. Antimicrobial susceptibility of community-acquired respiratory tract pathogens in North America in 1999-2000: findings of the PROTEKT surveillance study. Diagn Microbiol Infect Dis. 2003;45(4):251-9. [PubMed]
107. Hoban DJ, Biedenbach DJ, Mutnick AH et al. Pathogen of occurrence and susceptibility patterns associated with pneumonia in hospitalized patients in North America: results of the SENTRY Antimicrobial Surveillance Study (2000). Diagn Microbiol Infect Dis. 2003;45(4):279-85. [PubMed]
108. Hoban DJ, Bouchillon SK, Johnson JL et al. Comparative in vitro potency of gemifloxacin and fluoroquinolones against recent European clinical isolates from a global surveillance study. Eur J Clin Microbiol Infect Dis. 2001;20(11):814-9. [PubMed]
109. Hoban DJ, Bouchillon SK, Johnson JL et al. Comparative in vitro activity of gemifloxacin, ciprofloxacin, levofloxacin and ofloxacin in a North American surveillance study. Diagn Microbiol Infect Dis. 2001;40(1-2):51-7. [PubMed]
110. Hoellman DB, Kelly LM, Jacobs MR et al. Comparative antianaerobic activity of BMS 284756. Antimicrob Agents Chemother. 2001;45(2):589-92. [PubMed]
111. Hoffken G, Lode H, Prinzing C et al. Pharmacokinetics of ciprofloxacin after oral and parenteral administration. Antimicrob Agents Chemother. 1985;27(3):375-9. [PubMed]
112. Hoogkamp-Korstanje JA, Roelofs-Willemse J. Comparative in vitro activity of moxifloxacin against Gram-positive clinical isolates. J Antimicrob Chemother. 2000;45(1):31-9. [PubMed]
113. Hooper TL, Gould FK, Swinburn CR et al. Ciprofloxacin: a preferred treatment for legionella infections in patients receiving cyclosporin A. J Antimicrob Chemother. 1988;22(6):952-3. [PubMed]
114. Hooton TM, Latham RH, Wong ES et al. Ofloxacin versus trimethoprim-sulfamethoxazole for treatment of acute cystitis. Antimicrob Agents Chemother. 1989;33(8):1308-12. [PubMed]
115. Horn R, Robson HG. Susceptibility of the Bacteroides fragilis group to newer quinolones and other standard anti-anaerobic agents. J Antimicrob Chemother. 2001;48(1):127-30. [PubMed]
116. Howard W, Biedenbach DJ, Jones RN. Comparative antimicrobial spectrum and activity of the desfluoroquinolone BMS284756 (T-3811) tested against non-fermentative Gram-negative bacilli. Clin Microbiol Infect. 2002;8(6):340-4. [PubMed]
117. Hyatt JM, McKinnon PS, Zimmer GS et al. The importance of pharmacokinetic/pharmacodynamic surrogate markers to outcome. Focus on antibacterial agents. Clin Pharmacokinet. 1995;28(2):143-60. [PubMed]
118. Hyatt JM, Nix DE, Schentag JJ. Pharmacokinetic and pharmacodynamic activities of ciprofloxacin against strains of Streptococcus pneumoniae, Staphylococcus aureus, and Pseudomonas aeruginosa for which MICs are similar. Antimicrob Agents Chemother. 1994;38(12):2730-7. [PubMed]
119. Isenmann R, Friess H, Schlegel P et al. Penetration of ciprofloxacin into the human pancreas. Infection. 1994;22(5):343-346.[PubMed]
120. Jacobs MR, Felmingham D, Appelbaum PC et al. The Alexander Project 1998-2000: susceptibility of pathogens isolated from community-acquired respiratory tract infection to commonly used antimicrobial agents. J Antimicrob Chemother. 2003;52(2):229-46. [PubMed]
121. Jacobson JA, Call NB, Kasworm EM et al. Safety and efficacy of topical norfloxacin versus tobramycin in the treatment of external ocular infections. Antimicrob Agents Chemother. 1988;32(12):1820-4. [PubMed]
122. Jacobus RB, Brouwers J. Drug Interactions with quinolone antibiotics. Drug Safety. 1992;7:268-281. [PubMed]
123. Jensen T, Pedersen SS, Hoiby N et al. Efficacy of oral fluoroquinolones versus conventional intravenous antipseudomonal chemotherapy in treatment of cystic fibrosis. Eur J Clin Microbiol. 1987;6(6):618-22. [PubMed]
124. Ji B, Lounis N, Maslo C et al. In vitro and in vivo activities of moxifloxacin and clinafloxacin against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1998;42(8):2066-9. [PubMed]
125. Johnson DM, Sader HS, Fritsche TR et al. Susceptibility trends of haemophilus influenzae and Moraxella catarrhalis against orally administered antimicrobial agents: five-year report from the SENTRY Antimicrobial Surveillance Program. Diagn Microbiol Infect Dis. 2003;47(1):373-6. [PubMed]
126. Jones CB, Fugate SE. Levofloxacin and warfarin interaction. Ann Pharmacother. 2002;36(10):1554-7. [PubMed]
127. Jones ME, Blosser-Middleton RS, Critchley IA et al. In vitro susceptibility of Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis: a European multicenter study during 2000-2001. Clin Microbiol Infect. 2003;9(7):590-9.[PubMed]
128. Jones RN. Microbiology of newer fluoroquinolones: focus on respiratory pathogens. Diagn Microbiol Infect Dis. 2002;44(3):213-20. [PubMed]
129. Jones RN, Johnson DM, Erwin ME et al. Comparative antimicrobial activity of gatifloxacin tested against Streptococcus spp. including quality control guidelines and etest method validation. Quality Control Study Group. Diagn Microbiol Infect Dis. 1999;34(2):91-8. [PubMed]
130. Jones RN, Sader HS, Beach ML. Contemporary in vitro spectrum of activity summary for antimicrobial agents tested against 18569 strains non-fermentative Gram-negative bacilli isolated in the SENTRY Antimicrobial Surveillance Program (1997-2001). Int J Antimicrob Agents. 2003;22(6):551-6. [PubMed]
131. Jonsson M, Englund G, Norgard K. Norfloxacin vs. pivmecillinam in the treatment of uncomplicated lower urinary tract infections in hospitalized elderly patients. Scand J Infect Dis. 1990;22(3):339-44. [PubMed]
132. Karlowsky JA, Draghi DC, Jones ME et al. Surveillance for antimicrobial susceptibility among clinical isolates of Pseudomonas aeruginosa and Acinetobacter baumannii from hospitalized patients in the United States, 1998 to 2001. Antimicrob Agents Chemother. 2003;47(5):1681-8. [PubMed]
133. Kelsey SM, Wood ME, Shaw E et al. A comparative study of intravenous ciprofloxacin and benzylpenicillin versus netilmicin and piperacillin for the empirical treatment of fever in neutropenic patients. J Antimicrob Chemother. 1990;25(1):149-57. [PubMed]
134. Kestelyn P, Bogaerts J, Stevens AM et al. Treatment of adult gonococcal keratoconjunctivitis with oral norfloxacin. Am J Ophthalmol. 1989;108(5):516-23. [PubMed]
135. Khaliq Y, Zhanel GG. Fluoroquinolone-associated tendinopathy: a critical review of the literature. Clin Infect Dis. 2003;36(11):1404-10. [PubMed]
136. King A, May J, French G et al. Comparative in vitro activity of gemifloxacin. J Antimicrob Chemother. 2000;45 Suppl 1:1-12.[PubMed]
137. Kleinkauf N, Ackermann G, Schaumann R et al. Comparative in vitro activities of gemifloxacin, other quinolones, and nonquinolone antimicrobials against obligately anaerobic bacteria. Antimicrob Agents Chemother. 2001;45(6):1896-9. [PubMed]
138. Kohler T, Pechere JC. Bacterial resistance to quinolones: mechanisms and clinical implications. In: Andriole VT, editor. The Quinolones. San Diego: Academic Press; 2000:139-167.
139. LeBel M. Ciprofloxacin: chemistry, mechanism of action, resistance, antimicrobial spectrum, pharmacokinetics, clinical trials, and adverse reactions. Pharmacotherapy. 1988;8(1):3-33. [PubMed]
140. LeBel M, Vallee F, Bergeron MG. Tissue penetration of ciprofloxacin after single and multiple doses. Antimicrob Agents Chemother. 1986;29(3):501-5. [PubMed]
141. Ledergerber B, Bettex JD, Joos B et al. Effect of standard breakfast on drug absorption and multiple-dose pharmacokinetics of ciprofloxacin. Antimicrob Agents Chemother. 1985;27(3):350-2. [PubMed]
142. Leibowitz HM. Clinical evaluation of ciprofloxacin 0.3% ophthalmic solution for treatment of bacterial keratitis. Am J Ophthalmol. 1991;112(4 Suppl):34S-47S. [PubMed]
143. Leibowitz HM. Antibacterial effectiveness of ciprofloxacin 0.3% ophthalmic solution in the treatment of bacterial conjunctivitis. Am J Ophthalmol. 1991;112(4 Suppl):29S-33S. [PubMed]
144. Lesher GY, Froelich EJ, Gruett MD et al. 1,8-Naphthyridine Derivatives. A New Class of Chemotherapeutic Agents. J Med Pharm Chem. 1962;91:1063-5.
145. Lettieri JT, Rogge MC, Kaiser L et al. Pharmacokinetic profiles of ciprofloxacin after single intravenous and oral doses. Antimicrob Agents Chemother. 1992;36(5):993-6. [PubMed]
146. Levenson MJ, Parisier SC, Dolitsky J et al. Ciprofloxacin: drug of choice in the treatment of malignant external otitis (MEO). Laryngoscope. 1991;101(8):821-824. [PubMed]
147. Liang RH, Yung RW, Chan TK et al. Ofloxacin versus co-trimoxazole for prevention of infection in neutropenic patients following cytotoxic chemotherapy. Antimicrob Agents Chemother. 1990;34(2):215-8. [PubMed]
148. Lim S, Alam MG. Ciprofloxacin-induced acute interstitial nephritis and autoimmune hemolytic anemia. Ren Fail. 2003;25(4):647-51. [PubMed]
149. Lim S, Bast D, McGeer A et al. Antimicrobial susceptibility breakpoints and first-step parC mutations in Streptococcus pneumoniae: redefining fluoroquinolone resistance. Emerg Infect Dis. 2003;9(7):833-7. [PubMed]
150. Lim SH, Smith MP, Goldstone AH et al. A randomized prospective study of ceftazidime and ciprofloxacin with or without teicoplanin as an empiric antibiotic regimen for febrile neutropenic patients. Br J Haematol. 1990;76(Suppl 2):41-4. [PubMed]
151. Lipsky BA, Baker CA. Fluoroquinolone toxicity profiles: a review focusing on newer agents. Clin Infect Dis. 1999;28(2):352-64.[PubMed]
152. Lister PD. Pharmacodynamics of gatifloxacin against Streptococcus pneumoniae in an in vitro pharmacokinetic model: impact of area under the curve/MIC ratios on eradication. Antimicrob Agents Chemother. 2002;46(1):69-74. [PubMed]
153. Lister PD, Sanders CC. Pharmacodynamics of trovafloxacin, ofloxacin, and ciprofloxacin against Streptococcus pneumoniae in an in vitro pharmacokinetic model. Antimicrob Agents Chemother. 1999;43(5):1118-23. [PubMed]
154. Lode H, Garau J, Grassi C et al. Treatment of community-acquired pneumonia: a randomized comparison of sparfloxacin, amoxycillin-clavulanic acid and erythromycin. Eur Respir J. 1995;8(12):1999-2007. [PubMed]
155. Lode H, Wiley R, Hoffken G et al. Prospective randomized controlled study of ciprofloxacin versus imipenem-cilastatin in severe clinical infections. Antimicrob Agents Chemother. 1987;31(10):1491-6. [PubMed]
156. Lodise T, Graves J, Miller C et al. Effects of gatifloxacin and levofloxacin on rates of hypoglycemia and hyperglycemia among elderly hospitalized patients. Pharmacotherapy. 2007;27(11):1498-1505. [PubMed]
157. Lutz FB, Jr. Single-dose efficacy of ofloxacin in uncomplicated gonorrhea. Am J Med. 1989;87(6C):69S-74S. [PubMed]
158. Madaras-Kelly KJ, Moody J, Larsson A et al. Characterization of synergy between ofloxacin, ceftazidime, and tobramycin against Pseudomonas aeruginosa. Chemotherapy. 1997;43(2):108-17. [PubMed]
159. Madaras-Kelly KJ, Ostergaard BE, Hovde LB et al. Twenty-four-hour area under the concentration-time curve/MIC ratio as a generic predictor of fluoroquinolone antimicrobial effect by using three strains of Pseudomonas aeruginosa and an in vitro pharmacodynamic model. Antimicrob Agents Chemother. 1996;40(3):627-32. [PubMed]
160. Maeda N, Tamagawa T, Niki I et al. Increase in insulin release from rat pancreatic islets by quinolone antibiotics. Br J Pharmacol. 1996;117(2):372-6. [PubMed]
161. Malay S, Roblin PM, Reznik T et al. In vitro activities of BMS-284756 against Chlamydia trachomatis and recent clinical isolates of Chlamydia pneumoniae. Antimicrob Agents Chemother. 2002;46(2):517-8. [PubMed]
162. Mandell LA, Bartlett JG, Dowell SF et al. Update of practice guidelines for the management of community-acquired pneumonia in immunocompetent adults. Clin Infect Dis. 2003;37(11):1405-33. [PubMed]
163. Mandell LA, Wunderink RG, Anzueto A et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2:S27-S72. [PubMed]
164. Marco F, Almela M, Nolla-Salas J et al. In vitro activities of 22 antimicrobial agents against Listeria monocytogenes strains isolated in Barcelona, Spain. The Collaborative Study Group of Listeriosis of Barcelona. Diagn Microbiol Infect Dis. 2000;38(4):259-61. [PubMed]
165. Martinez-Martinez L, Joyanes P, Suarez AI et al. Activities of gemifloxacin and five other antimicrobial agents against Listeria monocytogenes and coryneform bacteria isolated from clinical samples. Antimicrob Agents Chemother. 2001;45(8):2390-2.[PubMed]
166. Melnik G, Schwesinger WH, Teng R et al. Hepatobiliary elimination of trovafloxacin and metabolites following single oral doses in healthy volunteers. Eur J Clin Microbiol Infect Dis. 1998;17(6):424-426. [PubMed]
167. Mertes PM, Jehl F, Burtin P et al. Penetration of ofloxacin into heart valves, myocardium, mediastinal fat, and sternal bone marrow in humans. Antimicrob Agents Chemother. 1992;36(11):2493-6. [PubMed]
168. Meunier F, Zinner SH, Gaya H et al. Prospective randomized evaluation of ciprofloxacin versus piperacillin plus amikacin for empiric antibiotic therapy of febrile granulocytopenic cancer patients with lymphomas and solid tumors. The European Organization for Research on Treatment of Cancer International Antimicrobial Therapy Cooperative Group. Antimicrob Agents Chemother. 1991;35(5):873-8. [PubMed]
169. Michea-Hamzehpour M, Auckenthaler R, Regamey P et al. Resistance occurring after fluoroquinolone therapy of experimental Pseudomonas aeruginosa peritonitis. Antimicrob Agents Chemother. 1987;31(11):1803-8. [PubMed]
170. Milazzo I, Blandino G, Musumeci R et al. Antibacterial activity of moxifloxacin against periodontal anaerobic pathogens involved in systemic infections. Int J Antimicrob Agents. 2002;20(6):451-6. [PubMed]
171. Montay G, Bruno R, Vergniol JC et al. Pharmacokinetics of sparfloxacin in humans after single oral administration at doses of 200, 400, 600, and 800 mg. J Clin Pharmacol. 1994;34(11):1071-6. [PubMed]
172. Montay G, Goueffon Y, Roquet F. Absorption, distribution, metabolic fate, and elimination of pefloxacin mesylate in mice, rats, dogs, monkeys, and humans. Antimicrob Agents Chemother. 1984;25(4):463-72. [PubMed]
173. Montay G, Jacquot C, Bariety J et al. Pharmacokinetics of pefloxacin in renal insufficiency. Eur J Clin Pharmacol. 1985;29(3):345-9. [PubMed]
174. Morita M, Nakagawa H, Suzuki K. [Ciprofloxacin concentration in human prostatic tissue following 3 days' administration]. Hinyokika Kiyo. 1991;37(5):563-6. [PubMed]
175. Muller M, Stass H, Brunner M et al. Penetration of moxifloxacin into peripheral compartments in humans. Antimicrob Agents Chemother. 1999;43(10):2345-9. [PubMed]
176. Nakae I, Nakatani K, Inoue S et al. [Therapeutic effect of ofloxacin on intractable pulmonary tuberculosis and ofloxacin resistance of tubercle bacilli isolated from the patients. Chest DIsease Cooperative Study Unit of National Sanatoriums in Kinki District]. Kekkaku. 1991;66(4):299-307. [PubMed]
177. NCCN. Prevention and Treatment of Cancer-Related Infections. http://www.nccn.org/professionals/physician_gls/PDF/infections.pdf. Accessed September 1, 2007. 2007.
178. Neuhauser MM, Weinstein RA, Rydman R et al. Antibiotic resistance among gram-negative bacilli in US intensive care units: implications for fluoroquinolone use. JAMA. 2003;289(7):885-8. [PubMed]
179. Neuman M. Clinical pharmacokinetics of the newer antibacterial 4-quinolones. Clin Pharmacokinet. 1988;14(2):96-121.[PubMed]
180. Niki Y, Hashiguchi K, Miyashita N et al. Influence of gatifloxacin, a new quinolone antibacterial, on pharmacokinetics of theophylline. J Infect Chemother. 1999;5(3):156-162. [PubMed]
181. Nitsche D, Schulze C, Oesser S et al. Impact of different classes antimicrobial agents on plasma endotoxin activity. Arch Surg. 1996;131(2):192-199. [PubMed]
182. Nix DE, Cumbo TJ, Kuritzky P et al. Oral ciprofloxacin in the treatment of serious soft tissue and bone infections. Efficacy, safety, and pharmacokinetics. Am J Med. 1987;82(4A):146-53. [PubMed]
183. Nix DE, DeVito JM, Whitbread MA et al. Effect of multiple dose oral ciprofloxacin on the pharmacokinetics of theophylline and indocyanine green. J Antimicrob Chemother. 1987;19(2):263-9. [PubMed]
184. Nix DE, Schentag JJ. The quinolones: an overview and comparative appraisal of their pharmacokinetics and pharmacodynamics. J Clin Pharmacol. 1988;28(2):169-78. [PubMed]
185. Norrby SR. Side-effects of quinolones: comparisons between quinolones and other antibiotics. Eur J Clin Microbiol Infect Dis. 1991;10(4):378-83. [PubMed]
186. O'Connor KA, O'Mahony D. The interaction of moxifloxacin and warfarin in three elderly patients. Eur J Intern Med. 2003;14(4):255-257. [PubMed]
187. Okezaki E, Terasaki T, Nakamura M et al. Serum protein binding of lomefloxacin, a new antimicrobial agent, and its related quinolones. J Pharm Sci. 1989;78(6):504-7. [PubMed]
188. Otani T, Tanaka M, Ito E et al. In vitro and in vivo antibacterial activities of DK-507k, a novel fluoroquinolone. Antimicrob Agents Chemother. 2003;47(12):3750-9. [PubMed]
189. Owens RC, Jr., Ambrose PG. Torsades de pointes associated with fluoroquinolones. Pharmacotherapy. 2002;22(5):663-8.[PubMed]
190. Paladino JA, Sperry HE, Backes JM et al. Clinical and economic evaluation of oral ciprofloxacin after an abbreviated course of intravenous antibiotics. Am J Med. 1991;91(5):462-470. [PubMed]
191. Panikabutra K, Lee CT, Ho B et al. Single dose oral norfloxacin or intramuscular spectinomycin to treat gonorrhoea (PPNG and non-PPNG infections): analysis of efficacy and patient preference. Genitourin Med. 1988;64(4):235-40. [PubMed]
192. Pappalardo G, Bianchi A, Scire Scappuzzo G et al. Preliminary pharmacokinetic and clinical evaluation of ofloxacin in dental and oral cavity diseases. Int J Clin Pharmacol Res. 1989;9(3):229-32. [PubMed]
193. Patmore L, Fraser S, Mair D et al. Effects of sparfloxacin, grepafloxacin, moxifloxacin, and ciprofloxacin on cardiac action potential duration. Eur J Pharmacol. 2000;406(3):449-52. [PubMed]
194. Peacock JE, Herrington DA, Wade JC et al. Ciprofloxacin plus piperacillin compared with tobramycin plus piperacillin as empirical therapy in febrile neutropenic patients. A randomized, double-blind trial. Ann Intern Med. 2002;137(2):77-87. [PubMed]
195. Peloquin CA, Cumbo TJ, Nix DE et al. Evaluation of intravenous ciprofloxacin in patients with nosocomial lower respiratory tract infections. Impact of plasma concentrations, organism, minimum inhibitory concentration, and clinical condition on bacterial eradication. Arch Intern Med. 1989;149(10):2269-73. [PubMed]
196. Peters HJ. Comparison of intravenous ciprofloxacin and mez- locillin in treatment of complicated urinary tract infections. In: Neu HC, Reeves DS, editors. Current topics. 1986:124-126. [PubMed]
197. Philpott-Howard JN, Barker KF, Wade JJ et al. Randomized multicentre study of ciprofloxacin and azlocillin versus gentamicin and azlocillin in the treatment of febrile neutropenic patients. J Antimicrob Chemother. 1990;26(Suppl F):89-99.[PubMed]
198. Preston SL, Drusano GL, Berman AL et al. Pharmacodynamics of levofloxacin: a new paradigm for early clinical trials. JAMA. 1998;279(2):125-9. [PubMed]
199. Product Information. Cipro(R), ciprofloxacin. Bayer Corporation, West Haven, CT, PI revised 3/25/2004.
200. Product Information. Factive(R), gemifloxacin mesylate tablets. Genesoft Pharmaceuticals, San Francisco, CA, 07/2003.
201. Product Information. Floxin(R), ofloxacin. Ortho-McNeil Pharmaceutical, Inc., aritan, NJ, PI revised 02/2000.
202. Product Information. Levaquin(R), levofloxacin. Ortho-McNeil Pharmaceutical, Inc., Raritan, New Jersey, PI revised 10/2003.
203. Product Information. Noroxin(R), norfloxacin. Merck & Co, Inc, West Point, PA, PI revised 08/2002.
204. Product Information. Tequin(R), gatifloxacin. Bristol-Myers Squibb Company, Princeton, NJ, PI revised 10/2003.
205. Product Information. Avelox(TM), moxifloxacin hydrochloride. Bayer Corporation, West Haven, CT, PI revised 10/2003.
206. Pugsley MP, Dworzack DL, Roccaforte JS et al. An open study of the efficacy of a single dose of ciprofloxacin in eliminating the chronic nasopharyngeal carriage of Neisseria meningitidis. J Infect Dis. 1988;157(4):852-3. [PubMed]
207. Pust RA, Ackenheil-Koppe HR, Weidner W et al. Clinical efficacy and tolerance of fleroxacin in patients with urethritis caused by Chlamydia trachomatis. J Antimicrob Chemother. 1988;22(Suppl D):227-30. [PubMed]
208. Ramalakshmi S, Bastacky S, Johnson JP. Levofloxacin-induced granulomatous interstitial nephritis. Am J Kidney Dis. 2003;41(2):E7. [PubMed]
209. Ramirez CA, Bran JL, Mejia CR et al. Open, prospective study of the clinical efficacy of ciprofloxacin. Antimicrob Agents Chemother. 1985;28(1):128-32. [PubMed]
210. Renkonen OV, Sivonen A, Visakorpi R. Effect of ciprofloxacin on carrier rate of Neisseria meningitidis in army recruits in Finland. Antimicrob Agents Chemother. 1987;31(6):962-3. [PubMed]
211. Richmond SJ, Bhattacharyya MN, Maiti H et al. The efficacy of ofloxacin against infection caused by Neisseria gonorrhoeae and Chlamydia trachomatis. J Antimicrob Chemother. 1988;22(Suppl C):149-53. [PubMed]
212. Ritz M, Lode H, Fassbender M et al. Multiple-dose pharmacokinetics of sparfloxacin and its influence on fecal flora. Antimicrob Agents Chemother. 1994;38(3):455-9. [PubMed]
213. Roblin PM, Reznik T, Kutlin A et al. In vitro activities of rifamycin derivatives ABI-1648 (Rifalazil, KRM-1648), ABI-1657, and ABI-1131 against Chlamydia trachomatis and recent clinical isolates of Chlamydia pneumoniae. Antimicrob Agents Chemother. 2003;47(3):1135-6. [PubMed]
214. Roblin PM, Reznik T, Kutlin A et al. In vitro activities of gemifloxacin (SB 265805, LB20304) against recent clinical isolates of Chlamydia pneumoniae. Antimicrob Agents Chemother. 1999;43(11):2806-7. [PubMed]
215. Rodriguez Diaz JC, Lopez M, Ruiz M et al. In vitro activity of new fluoroquinolones and linezolid against non-tuberculous mycobacteria. Int J Antimicrob Agents. 2003;21(6):585-8. [PubMed]
216. Rodriguez JC, Ruiz M, Climent A et al. In vitro activity of four fluoroquinolones against Mycobacterium tuberculosis. Int J Antimicrob Agents. 2001;17(3):229-31. [PubMed]
217. Rodriguez JC, Ruiz M, Lopez M et al. In vitro activity of moxifloxacin, levofloxacin, gatifloxacin and linezolid against Mycobacterium tuberculosis. Int J Antimicrob Agents. 2002;20(6):464-7. [PubMed]
218. Rodvold KA, Neuhauser M. Pharmacokinetics and pharmacodynamics of fluoroquinolones. Pharmacotherapy. 2001;21(10 Pt 2):233S-252S. [PubMed]
219. Rohwedder RW, Bergan T, Thorsteinsson SB et al. Transintestinal elimination of ciprofloxacin. Diagn Microbiol Infect Dis. 1990;13(2):127-33. [PubMed]
220. Rolston KV, Frisbee-Hume S, LeBlanc BM et al. Antimicrobial activity of a novel des-fluoro (6) quinolone, garenoxacin (BMS-284756), compared to other quinolones, against clinical isolates from cancer patients. Diagn Microbiol Infect Dis. 2002;44(2):187-94. [PubMed]
221. Rosen T. The fluoroquinolone antibacterial agents. Prog Med Chem. 1990;27:235-95. [PubMed]
222. Ruiz J, Marco F, Sierra JM et al. In vitro activity of gemifloxacin against clinical isolates of Neisseria gonorrhoeae with and without mutations in the gyrA gene. Int J Antimicrob Agents. 2003;22(1):73-6. [PubMed]
223. Ruiz-Serrano MJ, Alcala L, Martinez L et al. In vitro activities of six fluoroquinolones against 250 clinical isolates of Mycobacterium tuberculosis susceptible or resistant to first-line antituberculosis drugs. Antimicrob Agents Chemother. 2000;44(9):2567-8. [PubMed]
224. Sabbaj J, Hoagland VL, Shih WJ. Multiclinic comparative study of norfloxacin and trimethoprim-sulfamethoxazole for treatment of urinary tract infections. Antimicrob Agents Chemother. 1985;27(3):297-301. [PubMed]
225. Sader HS, Biedenbach DJ, Jones RN. Global patterns of susceptibility for 21 commonly utilized antimicrobial agents tested against 48,440 Enterobacteriaceae in the SENTRY Antimicrobial Surveillance Program (1997-2001). Diagn Microbiol Infect Dis. 2003;47(1):361-4. [PubMed]
226. Sandberg T, Englund G, Lincoln K et al. Randomised double-blind study of norfloxacin and cefadroxil in the treatment of acute pyelonephritis. Eur J Clin Microbiol Infect Dis. 1990;9(5):317-23. [PubMed]
227. Sanders WE, Jr., Morris JF, Alessi P et al. Oral ofloxacin for the treatment of acute bacterial pneumonia: use of a nontraditional protocol to compare experimental therapy with "usual care" in a multicenter clinical trial. Am J Med. 1991;91(3):261-6. [PubMed]
228. Schaad UB, Wedgwood-Krucko J, Guenin K et al. Antipseudomonal therapy in cystic fibrosis: aztreonam and amikacin versus ceftazidime and amikacin administered intravenously followed by oral ciprofloxacin. Eur J Clin Microbiol Infect Dis. 1989;8(10):858-65. [PubMed]
229. Schaumann R, Ackermann G, Pless B et al. In vitro activities of gatifloxacin, two other quinolones, and five nonquinolone antimicrobials against obligately anaerobic bacteria. Antimicrob Agents Chemother. 1999;43(11):2783-6. [PubMed]
230. Scheld WM. Maintaining fluoroquinolone class efficacy: review of influencing factors. Emerg Infect Dis. 2003;9(1):1-9.[PubMed]
231. Schentag JJ. Pharmacokinetic and pharmacodynamic predictors of antimicrobial efficacy: moxifloxacin and Streptococcus pneumoniae. J Chemother. 2002;14(Suppl 2):13-21. [PubMed]
232. Schentag JJ, Nix DE, Adelman MH. Mathematical examination of dual individualization principles (I): Relationships between AUC above MIC and area under the inhibitory curve for cefmenoxime, ciprofloxacin, and tobramycin. DICP. 1991;25(10):1050-7.[PubMed]
233. Schentag JJ, Nix DE, Forrest A. Pharmacodynamics of the fluoroquinolones. Quinolone Antimicrobial Agents. Washington, DC: American Society for Microbiology; 1993.
234. Schmitz FJ, Verhoef J, Fluit AC. Comparative activities of six different fluoroquinolones against 9,682 clinical bacterial isolates from 20 European university hospitals participating in the European SENTRY surveillance programme. The SENTRY participants group. Int J Antimicrob Agents. 1999;12(4):311-7. [PubMed]
235. Schuler P, Zemper K, Borner K et al. Penetration of sparfloxacin and ciprofloxacin into alveolar macrophages, epithelial lining fluid, and polymorphonuclear leucocytes. Eur Respir J. 1997;10(5):1130-6. [PubMed]
236. Scully BE, Nakatomi M, Ores C et al. Ciprofloxacin therapy in cystic fibrosis. Am J Med. 1987;82(4A):196-201. [PubMed]
237. Scully BE, Neu HC, Parry MF et al. Oral ciprofloxacin therapy of infections due to Pseudomonas aeruginosa. Lancet. 1986;1(8485):819-22. [PubMed]
238. Seibold R, Betz A. Treatment of posttraumatic osteitis with intravenous ofloxacin. Clin Ther. 1991;13(4):457-9. [PubMed]
239. Sharma AK, Khosla R, Kela AK et al. The fluoroquinolones: antimicrobial agents of the 1990s. Can J Pharmacol. 1994;26:249-261.
240. Shimada J, Nogita T, Ishibashi Y. Clinical pharmacokinetics of sparfloxacin. Clin Pharmacokinet. 1993;25(5):358-69.[PubMed]
241. Smith KE, Besser JM, Hedberg CW et al. Quinolone-resistant Campylobacter jejuni infections in Minnesota, 1992-1998. Investigation Team. N Engl J Med. 1999;340(20):1525-32. [PubMed]
242. Solomkin JS, Mazuski JE, Baron EJ et al. Guidelines for the selection of anti-infective agents for complicated intra-abdominal infections. Clin Infect Dis. 2003;37(8):997-1005. [PubMed]
243. Solomkin JS, Reinhart HH, Dellinger EP et al. Results of a randomized trial comparing sequential intravenous/oral treatment with ciprofloxacin plus metronidazole to imipenem/cilastatin for intra-abdominal infections. The Intra-Abdominal Infection Study Group. Ann Surg. 1996;223(3):303-15. [PubMed]
244. Soman A, Honeybourne D, Andrews J et al. Concentrations of moxifloxacin in serum and pulmonary compartments following a single 400 mg oral dose in patients undergoing fibre-optic bronchoscopy. J Antimicrob Chemother. 1999;44(6):835-838.[PubMed]
245. Sorgel F, Naber KG, Jaehde U et al. Gastrointestinal secretion of ciprofloxacin. Evaluation of the charcoal model for investigations in healthy volunteers. Am J Med. 1989;87(5A):62S-65S. [PubMed]
246. Speciale A, Musumeci R, Blandino G et al. Minimal inhibitory concentrations and time-kill determination of moxifloxacin against aerobic and anaerobic isolates. Int J Antimicrob Agents. 2002;19(2):111-8. [PubMed]
247. Stahlmann R. Clinical toxicological aspects of fluoroquinolones. Toxicol Lett. 2002;127(1-3):269-77. [PubMed]
248. Stass H, Bottcher MF, Ochmann K. Evaluation of the influence of antacids and H2 antagonists on the absorption of moxifloxacin after oral administration of a 400mg dose to healthy volunteers. Clin Pharmacokinet. 2001;40(Suppl 1):39-48.[PubMed]
249. Stass H, Kubitza D. Pharmacokinetics and elimination of moxifloxacin after oral and intravenous administration in man. J Antimicrob Chemother. 1999;43(Suppl B):83-90. [PubMed]
250. Stass H, Kubitza D, Schuhly U. Pharmacokinetics, safety and tolerability of moxifloxacin, a novel 8-methoxyfluoroquinolone, after repeated oral administration. Clin Pharmacokinet. 2001;40(Suppl 1):1-9. [PubMed]
251. Stein GE. Drug interactions with fluoroquinolones. Am J Med. 1991;91(6A):81S-86S. [PubMed]
252. Takahata M, Mitsuyama J, Yamashiro Y et al. In vitro and in vivo antimicrobial activities of T-3811ME, a novel des-F(6)-quinolone. Antimicrob Agents Chemother. 1999;43(5):1077-84. [PubMed]
253. Takei M, Fukuda H, Yasue T et al. Inhibitory activities of gatifloxacin (AM-1155), a newly developed fluoroquinolone, against bacterial and mammalian type II topoisomerases. Antimicrob Agents Chemother. 1998;42(10):2678-2681. [PubMed]
254. Tanimura H, Ohnishi H, Okamura T et al. [Chemotherapy of biliary tract infections (XXXVII). Excretion into bile and gallbladder tissue levels of levofloxacin and its clinical effect in biliary tract infections]. Jpn J Antibiot. 1992;45(5):557-68. [PubMed]
255. Taylor DN, Sanchez JL, Candler W et al. Treatment of travelers' diarrhea: ciprofloxacin plus loperamide compared with ciprofloxacin alone. A placebo-controlled, randomized trial. Ann Intern Med. 1991;114(9):731-4. [PubMed]
256. Teng R, Harris SC, Nix DE et al. Pharmacokinetics and safety of trovafloxacin (CP-99,219), a new quinolone antibiotic, following administration of single oral doses to healthy male volunteers. J Antimicrob Chemother. 1995;36(2):385-94. [PubMed]
257. Teng R, Liston TE, Harris SC. Multiple-dose pharmacokinetics and safety of trovafloxacin in healthy volunteers. J Antimicrob Chemother. 1996;37(5):955-63. [PubMed]
258. Thomas JK, Forrest A, Bhavnani SM et al. Pharmacodynamic evaluation of factors associated with the development of bacterial resistance in acutely ill patients during therapy. Antimicrob Agents Chemother. 1998;42(3):521-7. [PubMed]
259. Tomioka H, Saito H, Sato K. Comparative antimycobacterial activities of the newly synthesized quinolone AM-1155, sparfloxacin, and ofloxacin. Antimicrob Agents Chemother. 1993;37(6):1259-63. [PubMed]
260. Tomioka H, Sato K, Akaki T et al. Comparative in vitro antimicrobial activities of the newly synthesized quinolone HSR-903, sitafloxacin (DU-6859a), gatifloxacin (AM-1155), and levofloxacin against Mycobacterium tuberculosis and Mycobacterium avium complex. Antimicrob Agents Chemother. 1999;43(12):3001-4. [PubMed]
261. Torres A. The new American Thoracic Society/Infectious Disease Society of North America guidelines for the management of hospital-acquired, ventilator-associated and healthcare-associated pneumonia: a current view and new complementary information. Curr Opin Crit Care. 2006;12(5):444-445. [PubMed]
261a. Tsikouris JP, Peeters MJ, Cox CD, et al. Effects of three fluoroquinolones on QT analysis after standard treatment courses. Ann Noninvasive Electrocardiol 2006;11:52-56. [PubMed]
262. Trees DL, Sirivongrangson P, Schultz AJ et al. Multiclonal increase in ciprofloxacin-resistant Neisseria gonorrhoeae, Thailand, 1998-1999. Sex Transm Dis. 2002;29(11):668-73. [PubMed]
263. Verhoest P, Fernandez H, Henry-Suchet J et al. [A new therapeutic strategy using a ofloxacin-amoxicillin-clavulanic acid combination in the treatment of upper gynecologic infections. Apropos of 123 cases]. J Gynecol Obstet Biol Reprod (Paris). 1994;23(1):39-46. [PubMed]
264. Vincent J, Teng R, Dogolo LC et al. Effect of trovafloxacin, a new fluoroquinolone antibiotic, on the steady-state pharmacokinetics of theophylline in healthy volunteers. J Antimicrob Chemother. 1997;39(Suppl B):81-6. [PubMed]
265. Wagai N, Tawara K. Possible reasons for differences in phototoxic potential of a 5 quinolone antibacterial agents: generation of toxic oxygen. Free Radic Res Commun. 1992;17(6):387-98. [PubMed]
266. Walters DJ, Solomkin JS, Paladino JA. Cost effectiveness of ciprofloxacin plus metronidazole versus imipenem-cilastatin in the treatment of intra-abdominal infections. Pharmacoeconomics. 1999;16(5 Pt 2):551-61. [PubMed]
267. Weidekamm E, Portmann R, Partos C et al. Single and multiple dose pharmacokinetics of fleroxacin. J Antimicrob Chemother. 1988;22(Suppl D):145-54. [PubMed]
268. Weidner W, Schiefer HG, Dalhoff A. Treatment of chronic bacterial prostatitis with ciprofloxacin. Results of a one-year follow-up study. Am J Med. 1987;82(4A):280-3. [PubMed]
269. Weiss K, Restieri C, De Carolis E et al. Comparative activity of new quinolones against 326 clinical isolates of Stenotrophomonas maltophilia. J Antimicrob Chemother. 2000;45(3):363-5. [PubMed]
270. Weller TM, Andrews JM, Jevons G et al. The in vitro activity of BMS-284756, a new des-fluorinated quinolone. J Antimicrob Chemother. 2002;49(1):177-84. [PubMed]
271. West M, Boulanger BR, Fogarty C et al. Levofloxacin compared with imipenem/cilastatin followed by ciprofloxacin in adult patients with nosocomial pneumonia: a multicenter, prospective, randomized, open-label study. Clin Ther. 2003;25(2):485-506.[PubMed]
272. Westphal JF, Brogard JM. Clinical pharmacokinetics of newer antibacterial agents in liver disease. Clin Pharmacokinet. 1993;24(1):46-58. [PubMed]
273. Wijnands WJ, Vree TB, Baars AM et al. The penetration of ofloxacin into lung tissue. J Antimicrob Chemother. 1988;22(Suppl C):85-9. [PubMed]
274. Wijnands WJ, Vree TB, van Herwaarden CL. The influence of quinolone derivatives on theophylline clearance. Br J Clin Pharmacol. 1986;22(6):677-83. [PubMed]
275. Winston DJ, Ho WG, Nakao SL et al. Norfloxacin versus vancomycin/polymyxin for prevention of infections in granulocytopenic patients. Am J Med. 1986;80(5):884-90. [PubMed]
276. Winter JH, McCartney C, Bingham J et al. Ciprofloxacin in the treatment of severe Legionnairres disease. Rev Infect Dis. 1988;10(Suppl1):218-219.
277. Wise R, Lister D, McNulty CA et al. The comparative pharmacokinetics of five quinolones. J Antimicrob Chemother. 1986;18(Suppl D):71-81. [PubMed]
278. Wise R, Mortiboy D, Child J et al. Pharmacokinetics and penetration into inflammatory fluid of trovafloxacin (CP-99,219). Antimicrob Agents Chemother. 1996;40(1):47-9. [PubMed]
279. Woodcock JM, Andrews JM, Boswell FJ et al. In vitro activity of BAY 12-8039, a new fluoroquinolone. Antimicrob Agents Chemother. 1997;41(1):101-6. [PubMed]
280. Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006;55(RR-11):1-94. [PubMed]
281. Yamamoto T, Kusajima H, Hosaka M et al. Uptake and intracellular activity of AM-1155 in phagocytic cells. Antimicrob Agents Chemother. 1996;40(12):2756-9. [PubMed]
282. Yasumoto R, Asakawa M. Comparison of enoxacin, ofloxacin, and norfloxadcin concentration in human benign prostatis tissue. [Japanese]. Hinyokika Kiyo. 1988;34:1519-1521. [PubMed]
283. Yew WW, Kwan SY, Ma WK et al. In-vitro activity of ofloxacin against Mycobacterium tuberculosis and its clinical efficacy in multiply resistant pulmonary tuberculosis. J Antimicrob Chemother. 1990;26(2):227-36. [PubMed]
284. Yip C, Lee AJ. Gatifloxacin-induced hyperglycemia: a case report and summary of the current literature. Clin Ther. 2006;28(11):1857-1866. [PubMed]
285. Yong DE, Cheong HJ, Kim YS et al. In vitro activity of gemifloxacin against recent clinical isolates of bacteria in Korea. J Korean Med Sci. 2002;17(6):737-42. [PubMed]
286. Yoshida T, Muratani T, Iyobe S et al. Mechanisms of high-level resistance to quinolones in urinary tract isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1994;38(7):1466-9. [PubMed]
287. Yuk JH, Nightingale CH, Sweeney KR et al. Relative bioavailability in healthy volunteers of ciprofloxacin administered through a nasogastric tube with and without enteral feeding. Antimicrob Agents Chemother. 1989;33(7):1118-20. [PubMed]
288. Zervos MJ, Hershberger E, Nicolau DP et al. Relationship between fluoroquinolone use and changes in susceptibility to fluoroquinolones of selected pathogens in 10 United States teaching hospitals, 1991-2000. Clin Infect Dis. 2003;37(12):1643-1648. [PubMed]
289. Zhanel GG, Palatnick L, Nichol KA et al. Antimicrobial resistance in Haemophilus influenzae and Moraxella catarrhalis respiratory tract isolates: results of the Canadian Respiratory Organism Susceptibility Study, 1997 to 2002. Antimicrob Agents Chemother. 2003;47(6):1875-81. [PubMed]
290. Zheng HP, Cao WL, Wu XZ et al. Antimicrobial susceptibility of Neisseria gonorrhoeae strains isolated in Guangzhou, China, 1996-2001. Sex Transm Infect. 2003;79(5):399-402. [PubMed]
Tables
Table 1. In Vitro Activity Profiles of the Fluoroquinolones (μg/mL).
| Ciprofloxacin | Levofloxacin | Gatifloxacin | Moxifloxacin | Gemifloxacin | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Organism | MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 |
| Gram-Negatives | ||||||||||
| Acinetobacter baumannii | 0.5-32 | >16 | 0.25-4 | 8-16 | 0.12-4 | 8-16 | 0.12-8 | 16-32 | 0.12-8 | 16 - >16 |
| Enterobacter cloacae | 0.008-0.03 | 0.016-1 | £0.03 - 0.06 | 0.03-1 | 0.03-0.06 | 0.03-1 | 0.06 | 0.125-0.25 | 0.016-0.03 | 0.03-0.5 |
| Escherichia coli | 0.008- 0.03 | 0.03-1 | 0.015- 0.03 | 0.06-0.5 | 0.016-0.3 | £0.03-0.5 | 0.03-0.06 | 0.06-1 | 0.008- 0.016 | 0.03-0.5 |
| Haemophilus influenzae | 0.008-0.015 | 0.015-0.03 | 0.015 | 0.015-0.06 | 0.008-0.015 | 0.015-0.03 | 0.015-0.03 | 0.06-0.03 | 0.002-0.004 | 0.008-0.03 |
| Klebsiella pneumoniae | 0.016- 0.06 | 0.06-2 | ≤0.03-0.06 | 0.25-2 | 0.03-0.06 | 0.06-1 | 0.06- 0.25 | 0.125-1 | 0.03-0.06 | 0.125-1 |
| Moraxella catarrhalis | 0.03-0.06 | 0.03-0.06 | 0.03-0.06 | 0.06 | 0.03 | 0.03-0.06 | 0.03-0.12 | 0.06-0.12 | 0.008-0.015 | 0.008-0.03 |
| Morganella morganii | 0.008-0.016 | 0.015-2 | 0.031 | 0.125- 0.25 | 0.25 | 0.25 | 0.12-0.25 | 0.25-0.5 | 0.06 | 0.125-4 |
| Neisseria gonorrhoeae | 0.004-0.5 | 0.004-0.5 | 0.008-0.5 | 0.12-0.5 | 0.12 | 0.25 | 0.008-0.25 | 0.015-0.5 | 0.004-0.12 | 0.06-0.5 |
| Neisseria meningitidis | ≤0.002-0.008 | 0.004-0.008 | 0.008 | 0.008 | 0.004-0.008 | 0.008 | 0.008 | 0.008-0.015 | 0.004-0.008 | 0.008 |
| Proteus mirabilis | 0.03-0.06 | 0.03- 2 | 0.03-0.5 | 0.12-2 | 0.12-0.25 | 2-4 | 0.25-1 | 0.25-8 | 0.12-0.25 | 0.25-8 |
| Pseudomonas aeruginosa | ≤0.25-1 | 4 - >16 | 1-4 | >4 - 16 | 1-4 | >4 | 2-16 | 8 | 0.5-4 | >4 - >16 |
| Serratia marcescens | 0.06-0.25 | 0.5-4 | 0.125 | 0.25-2 | 0.25 | 2 | 0.25 | 4 | 0.125-0.25 | 1-2 |
| Salmonella spp. | 0.016-0.12 | 0.03- 0.25 | £0.03-0.06 | 0.25 - 0.5 | £0.03- 0.06 | 0.06-0.25 | 0.12 | 1 | 0.016-0.03 | 0.016- 0.12 |
| Stenotrophomonas maltophilia | 2-4 | 8-16 | 0.5-1 | 4-8 | 0.5-1 | 4-8 | 0.5-1 | 2-4 | 0.5-2 | 4-8 |
| Gram-Positives | ||||||||||
| Staphylococcus aureus | 0.5-1 | 0.5 - >4 | 0.125-0.25 | 0.25 - >4 | 0.06-0.12 | 0.12 - >4 | 0.06 | 0.06-2 | NA | 0.06-8 |
| MS S. aureus | 0.25-1 | 0.5-4 | 0.12-0.25 | 0.25-2 | 0.06-0.12 | 0.125-0.5 | 0.03-0.12 | 0.06-0.25 | 0.015-0.06 | 0.03-0.12 |
| MR S. aureus | 1 - >16 | 16 - >128 | 0.25-32 | 8 - >128 | 0.125-8 | 4 - >128 | 0.06 - 2 | 2-128 | 0.03-4 | 2 - >128 |
| Streptococcus pneumoniae | 1-2 | 2-16 | 0.5-1 | 1-2 | 0.25-0.5 | 0.5 | 0.03-0.25 | 0.12-0.25 | 0.015-0.03 | 0.03-0.06 |
| Penicillin susceptible | 1-2 | 2-4 | 0.5-1 | 1-2 | 0.25 | 0.5 | 0.125-0.25 | 0.25 | 0.015-0.03 | 0.03-0.06 |
| Penicillin intermediate | 1-2 | 2-4 | 0.5-1 | 1-2 | 0.25 | 0.5 | 0.125-0.25 | 0.12-0.25 | 0.015-0.03 | 0.03-0.06 |
| Penicillin resistant | 1-2 | 2-4 | 0.5-1 | 1-2 | 0.25-0.5 | 0.25-0.5 | 0.125-0.25 | 0.12-0.25 | 0.015-0.03 | 0.03-0.06 |
| Viridans Streptococcus | 1-2 | 4 | 1 | 1-2 | 0.25-0.5 | 0.5-1 | 0.25 | 0.25-0.5 | 0.03-0.06 | 0.06-0.25 |
| S. agalactiae | 0.25-1 | 1-2 | 0.5 | 0.5-1 | 0.25-0.5 | 0.25-0.5 | 0.125 – 0.25 | 0.25 | 0.03-0.06 | 0.03-0.25 |
| S. pyogenes | 0.25-1 | 1 | 0.25-0.5 | 0.5-1 | 0.12-0.25 | 0.25-0.5 | 0.06-0.25 | 0.25-0.5 | 0.016-0.06 | 0.03-0.25 |
| Enterococcus faecalis | 0.5-2 | 1 - >16 | 1 | ³8 - >16 | 0.5-1 | 1-16 | 0.12-0.5 | 0.25 - ³4 | 0.06-0.25 | 2 - ³4 |
| Enterococcus faecium | 2 - >16 | 4 - >128 | ³8->16 | ³8->16 | 2 - ³16 | ³16 | 2 - 32 | 2 - >32 | ³4 - >16 | ³4 - >16 |
| Anaerobes | ||||||||||
| Bacteroides fragilis | 4 | 32 | 1-2 | 4-16 | 0.25-1 | 1-4 | 0.125-1 | 1-4 | 0.5 - 1 | 2 - 16 |
| Clostridium difficile | 8-16 | 16-64 | 4 | 4 - >16 | 1 | 2 | 1 | 2 | 1-2 | 2 - >16 |
| Fusobacterium spp. | 1- 4 | 2-16 | 1 | 1-8 | 0.25-1 | 0.25-8 | 0.25-0.5 | 2-4 | ≤0.25 - 4 | 0.5-8 |
| Peptostreptococcusspp. | 1 | 2 | 0.5-4 | 4 | 0.5 | 1 | 0.06-0.5 | 0.12-1 | 0.03-0.12 | 0.06-2 |
| Porphyromonas spp. | 0.5 | 2 | 0.25 | 1 | 0.125 | 0.25 | £0.03-0.25 | £0.03-0.25 | 0.06 | 0.125 |
| Prevotella spp. | 1-4 | 2-64 | 0.5-1 | 1-8 | 0.25-0.5 | 0.5-4 | 0.125-0.5 | 0.25-2 | 1-4 | 2-16 |
| Miscellaneous | ||||||||||
| Mycobacterium tuberculosis | 0.125-2 | 0.125-4 | 0.125-0.5 | 0.125-1 | 0.03-0.12 | 0.03-0.25 | 0.25-0.6 | 0.125-1 | 4 | 8 |
| Mycoplasma pneumoniae | 1 | 1-2 | 0.25-0.5 | 0.5-1 | 0.06 | 0.12 | 0.06 | 0.06-0.12 | NA | 0.25 |
| Chlamydia pneumoniae | 0.5 | 2 | 0.25-0.5 | 0.5-1 | 0.06-0.25 | 0.125-0.25 | 0.06-1 | 0.5-1 | 0.25 | 0.25 |
| Legionella pneumophila | 0.016-0.03 | 0.016-0.06 | 0.008-0.016 | 0.008-0.016 | 0.008-0.016 | 0.016-0.03 | 0.008-0.016 | 0.008-0.03 | 0.008-0.03 | 0.016-0.03 |
NA: not available
Complied from references (7,10,23,24,30,35,40,52,53,54,55,63,68,71,77,78,81,92,93,94,95,97,102,104,106-110,116,120,124,128-130,137,161,170,188,199,213,214,216,217,220,222,223,229,234,246,252,269,270,279,285)
Table 2. Pharmacokinetic Parameters of the Fluoroquinolones.
| Parameter | Norfloxacin | Ciprofloxacin | Oflox/Levofloxacin | Gatifloxacin | Trovafloxacin | Moxifloxacin | Gemifloxacin |
|---|---|---|---|---|---|---|---|
| Dose (mg) | 400 | 750 | 400/500 | 400 | 300 | 400 | 320 |
| Peak (mg/mL) | 1.5 | 3.5 | 4.0/6.0 | 3.4 | 4.0 | 4.5 | 1.6 |
| Peak / 100 mg dose | 0.38 | 0.46 | 1.0/1.2 | 0.85 | 1.32 | 1.12 | 0.5 |
| Protein bound (%) | 15 | 25 | 25 | 18 | 70 | 50 | 60 |
| Vdss (L/kg) | 1.7 | 3.2 | 1.45 | 1.7 | 1.1 | 2.7 | 4.18 |
| t ½ (h) (CrCl 100 mL/min) | 3.3 | 4.0 | 6.0 | 8.4 | 10.0 | 12.7 | 6.1 |
| t ½ (h) (CrCl 10 mL/min) | 8.0 | 10.0 | 30.0 | >40 | 12 | 14.5 | NA |
| AUC (mg∙h/mL) | 13.6 | 29 | 38/58 | 32 | 41.4 | 48.0 | 9.1 |
| AUC / 100 mg dose | 1.7 | 1.9 | 11.7 | 8 | 13.8 | 12 | 2.8 |
| Clrenal (mL/min) | 234 | 250 | 190 | NA | 10 | 43 | 134 |
| % Nonrenal | 60 | 40 | 5 | 10 | 90 | 80 | 64 |
| % Renal | 40 | 60 | 95 | 90 | 10 | 20 | 22 |
| Bioavailability (%) | 40 | 70 | 99 | 96 | 88 | 86 | 71 |
Compiled from references: (9,31,43,70,96,111,139,141,145,178,179,184,187,218,219,239,245,249,256,267,277,287)
Table 3. Tissue Penetration of the Fluoroquinolones.
| Site | Dose | Route | Frequency | Time to Ctissue | Conc. Tissue | Time to Cplasma | Conc. Plasma | Reference |
|---|---|---|---|---|---|---|---|---|
| Ciprofloxacin | ||||||||
| Bile | 500 mg | po | bid x 6 | 25-26 h | 4.5 mg/L | 4h | 2.5 | (62) |
| Blister, suction | 500 mg | 1 x | 84.70% | 2.26 | (140) | |||
| Lung, parenchyma | 200 mg | iv | 1 x | 3.4 mcg/g | 0.6 | (42) | ||
| Lung, pleura | 200 mg | iv | 1 x | 1.7 mcg/g | 0.6 | (42) | ||
| Muscle, heart valve | 750 mg | po | q 12h x 4 | 1-3 h | 8.3/3.1 mcg/g | 11.59/3.95 | (167) | |
| Muscle, myocardium | 400 mg | iv | 1 x | 1 h | 31.6/25 mcg/g | 6.19/1.73 | (167) | |
| Prostate, TURP | 200 mg | po | tid x 9 | 5.5 h | 1.32/0.64 | 5.5 h | 0.65/0.31 | (174) |
| Levofloxacin | ||||||||
| Bile | 100 mg | po | 1 x | 2-6 h | 0.49/5.63 | 2-6 h | 0.55/1.63 | (254) |
| Norfloxacin | ||||||||
| Prostate, TURP | 200 mg | po | tid x 5 | 4.42/1.94 (2.45/7.76) intraoperative | 2.89/2.34 (0.87/8.33) intraoperative | (282) | ||
| Ofloxacin | ||||||||
| Bone | 300 mg | po | 1 x | 1 h | 1.58/0.06 mcg/g | 1 h | 2.61/0/17 | (192) |
| Lung, parenchyma | 600 mg | po | 1 x | 2 h | 17.7/9.2 mcg/g | 2 h | 8.7/4.2 | (273) |
| Muscle, myocardium | 400 mg | iv | 1 x | 1 h | 8.89/2.16 | 15.9/2.5 | (167) | |
| Prostate, TURP | 200 mg | po | tid x 5 | 5.51/1.79 (3.62-7.19) intraoperative | 5.36/1.28 (3.12-8.24) 30 min preop | (282) | ||
| Moxifloxacin | ||||||||
| Alveolar macrophages | 400 mg | po | 1 x | 2.2 h | 56.7 | 2.2 h | 3.2 | (244) |
| Bronchial mucosa | 400 mg | po | 1 x | 2.2 h | 1.29 | 2.2 h | 3.2 | (244) |
| Epithelial lining fluid | 400 mg | po | 1 x | 2.2 h | 20.7 | 2.2 h | 3.2 | (244) |
| Maxillary sinus mucosa | 400 mg | po | qd x 5d | 3 h | 7.48 | 3 h | 3.58 | (83) |
| Blister | 400 mg | iv | 1 x | 5.6 h | 1.7 | 1 h | 3.7 | (175) |
| Skeletal muscle | 400 mg | iv | 1 x | 1.8 h | 1.2 | 1 h | 3.7 | (175) |
| Subcutaneous adipose | 400 mg | iv | 1 x | 2 h | 1.0 | 1 h | 3.7 | (175) |
| Gemifloxacin | ||||||||
| Alveolar macrophages | 320 mg | po | 1 x | 107 | 1.4 | (200) | ||
| Bronchial mucosa | 320 mg | po | 1 x | 9.52 | 1.4 | (200) | ||
| Epithelial lining fluid | 320 mg | po | 1 x | 2.69 | 1.4 | (200) | ||
| Blister | 320 mg | po | 1 x | 3.4 h | 0.74 | 1.2 h | 2.3 | (82) |
Abbreviations: bid = twice daily; iv = intravenously; po = orally; qd = once daily; tid = three times daily
Table 4. Clinical Indications for the Fluoroquinolones and Dosage Regimens.
| Indication | Agent | Regimen |
|---|---|---|
| Respiratory Infections | ||
| Acute community-acquired pneumonia | Moxifloxacin | 400 mg q24h iv/po x 7-14 days |
| Gemifloxacin | 320 mg q24h po x 7 days | |
| Ofloxacin | 400 mg q12h iv/po x 10 days | |
| Levofloxacin | 500 mg q24 iv/po x 7-14 days 750 mg q24 iv/po x 5 days | |
| Lower Respiratory Tract Infections | Ciprofloxacin | 500-750 mg q12h po / 400 mg q8-12h iv x 7-14 days |
| Acute Maxillary Sinusitis | Moxifloxacin | 400 mg q24 iv/po x 5 days |
| Ciprofloxacin | 500 mg po q12h po / 400 mg q12h iv x 10 days | |
| Levofloxacin | 500 mg q12h iv/po x 10-14 days | |
| Acute bacterial exacerbations of chronic bronchitis | Moxifloxacin | 400 mg q24h iv/po x 5 days |
| Gemifloxacin | 320 mg q24h po x 5 days | |
| Levofloxacin | 500 mg q24h iv/po x 7 days | |
| Ofloxacin | 400 mg q12h iv/po x 10 days | |
| Nosocomial pneumonia | Ciprofloxacin +/- another agent | 400 mg q8 iv x 10-14 days |
| Levofloxacin +/- another agent | 750 mg q24h iv/po x 7-14 days | |
| Urinary Tract Infections (UTI) | ||
| Acute Uncomplicated UTIs | Ciprofloxacin | 250 mg q12h po x 3 days |
| Levofloxacin | 250 mg q24h iv/po x 3 days | |
| Norfloxacin | 400 mg q12h po x 3-10 days | |
| Ofloxacin | 200 mg q12h iv/po x 3-7 days | |
| Complicated UTI and pyelonephritis | Levofloxacin | 250 mg q24h iv/po x 10 days |
| Ciprofloxacin | 500 mg q 12h po / 400 mg q12h iv x 7-14 days | |
| Ofloxacin | 200 mg q12h iv/po x 10 days | |
| Norfloxacin | 400 mg q12h po x 10-21 days | |
| Prostatitis | Levofloxacin | 500 mg q24h iv/po x 28 days |
| Ciprofloxacin | 500 mg q12 h po / 400 mg q12h iv x 28 days | |
| Norfloxacin | 400 mg q12h po x 28 days | |
| Ofloxacin | 300 mg q12h iv/po x 6 weeks | |
| Venereal Diseases | ||
| Uncomplicated gonococcal infections | Ciprofloxacin | 500 mg po single dose |
| (fluoroquinolones not first-line therapy) | Ofloxacin | 400 mg iv/po single dose |
| Levofloxacin | 250 mg iv/po single dose | |
| Norfloxacin | 400 mg po single dose | |
| Urethritis due to C. trachomatis | Ofloxacin | 300 mg q12h iv/po x 7 days |
| Levofloxacin | 500 mg q24h x 7 days | |
| Acute PID | Ofloxacin (+ metronidazole) | 400 mg q12h iv/po x 10-14 days |
| Levofloxacin (+ metronidazole) | 500 mg q24h iv/po x 10-14 days | |
| Chancroid | Ciprofloxacin | 500 mg q12h po x 3 days |
| Epididymitis due to enteric gram-negative organisms | Ofloxacin | 300 mg q12h iv/po x 10 days |
| Levofloxacin | 500 mg q24h iv/po x 10 days | |
| Skin and Bone | ||
| Uncomplicated skin and skin structure infection | Moxifloxacin | 400 mg q24h x 7 days |
| Ciprofloxacin | 500-750 mg q12h po x 7-14 days 400 mg q8-12h iv | |
| Levofloxacin | 500 mg q24h iv/po x 7-10 days | |
| Ofloxacin | 400 mg q12h iv/po x 10 days | |
| Complicated skin and skin structure infection | Levofloxacin | 750 mg q24h iv/po x 7-14 days |
| Bone and Joint infection | Ciprofloxacin | 500-750 mg q12h po x 4-6 weeks 400 mg q 8-12h iv |
| Abdominal infections | ||
| Intra-abdominal infection | Ciprofloxacin (+ metronidazole) | 500 mg q12h po x 7-14 days 400 mg q12h iv |
| Infectious diarrhea | Ciprofloxacin | 500 mg q12h x 3-5 days (current recommendations) 500 mg q12h po x 5-7 days (labeled) |
| Ofloxacin | 300 mg q 12h x 3-5 days | |
| Norfloxacin | 400 mg q12h x 3-5 days | |
| Typhoid Fever | Ciprofloxacin | 500 mg q12h po x 10 days |
| Inhalational anthrax (post-exposure) | Ciprofloxacin | 500 mg q 12h po x 60 days |
| Empiric therapy in febrile neutropenia | Ciprofloxacin (+ piperacillin) | 400 mg q8h iv |
Compiled from references (58, 199, 200, 201, 202, 203, 205)
Table 5. Side Effect and Drug Interaction Profile of the Fluoroquinolones.
| Parameter | Norfloxacin | Ciprofloxacin | Oflox/Levofloxacin | Gatifloxacin | Trovafloxacin | Moxifloxacin | Gemifloxacin |
|---|---|---|---|---|---|---|---|
| GI Upset | +2 | +2 | +1 | +3 | +2 | +3 | +2 |
| Photosensitivity | 0 | 0 | +1 | +0 | 0 | 0 | 0 |
| CNS Effects | +1 | +1 | +2 | +2 | +3 | +1 | +1 |
| QTc prolongation | +1 | +1 | +1 | +1 | +1 | +1 | +1 |
| Theophylline interaction | +1 | +1 | +1 | 0 | 0 | 0 | 0 |
| Cation interaction | +4 | +4 | +2 | +3 | +1 | +3 | +4 |
| Warfarin interaction | +2 | +1 | 0 | 0 | 0 | 0 | 0 |
For interactions: 0, none; +1, 1-20% change; +2, 20-40% change; +3, 40-60% change; +4, > 60% change. For GI upset,QTc prolongation, photosensitivity, and CNS effects: 0, none; +1, 1-3%; +2, 3-5%; +3, 5-10%; +4, > 10%.
Table 6. Dose Adjustments in Renal Insufficiency.
| Agent | Degree of Renal Insufficiency | Regimen |
|---|---|---|
| Ciprofloxacin | CrCl 5 – 29 mL/min | 200-400 mg IV q18-24h |
| Hemodialysis or CAPD | 200-400mg IV q24h* | |
| CVVH | 200mg IV q12h | |
| CVVHD or CVVHDF | 200-400mg IV q12h | |
| Gemifloxacin | CrCl < 40 mL/min | 160mg PO q24h |
| Hemodialysis or CAPD | 160mg PO q24h* | |
| Levofloxacin** | CrCl 20-49 mL/min | IV/PO: 500-750mg once, then 250mg q24h to 750mg q48 |
| CrCl 10-49 mL/min | IV/PO: 500-750mg once, then 250-500 q48h | |
| Hemodialysis or CAPD | IV/PO: 500-750mg once, then 250-500mg q48h* | |
| Moxifloxacin | Any creatinine clearance | No adjustments necessary |
| Hemodialysis or CAPD | No adjustments necessary* | |
| CVVH, CVVHD, or CVVHDF | 400mg IV q12h |
* Administer doses after dialysis. **Manufacturer’s dosage recommendations vary by indication within the ranges listed.
Abbreviations: CAPD = chronic ambulatory peritoneal dialysis; CVVH = continuous venovenous hemofiltration; CVVHD = continuous venovenous hemodialysis; CVVHDF = continuous venovenous hemodiafiltration; IV = intravenous; PO = oralNote: CVVH is mainly for fluid removal alone. Many institutions will employ more CVVHD or CVVHDF which combine dialysis with fluid removal.
Figure 1. Structure Activity Relationship of the Fluoroquinolones.
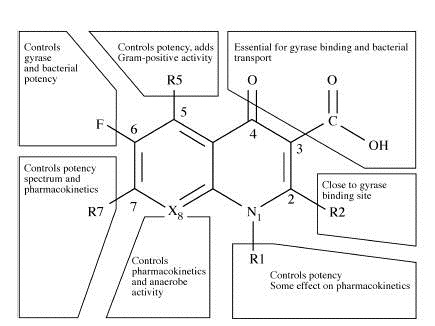
Figure 2. Structure of the Fluoroquinolones.

Figure 3. Time to Eradication Versus AUIC.

Relationship between the day of bacterial eradication and AUIC values above 250 for cefmenoxime (●) and cirprofloxacin (■).
At the same AUIC range above 250, ciprofloxacin eradicates bacteria earlier in therapy.
Figure 4. Relationship Between Serum Concentrations and Selected MIC Values.

Relationship between serum concentrations of ciprofloxacin and selected MIC values.
At each value of MIC, the AUIC and peak to MIC ratio is calculated.
Values are based on a 750-mg dose given twice daily.
What's New
Hurley M, et al. Fluoroquinolones in the treatment of bronchopulmonary disease in cystic fibrosis. Ther Adv Respir Dis 2012;6:363-73.
Chou HW, et al. Dysglycemia among diabetic patients receiving Levofloxacin, Ciprofloxacin, or Moxifloxacin. Clin Infect Dis 2013;57:971-980.
Chen et al. The role of fluoroquinolones in the management of urinary tract infections in areas with high rates of fluoroquinolone-resistant uropathogens. Eur J Clin Microbiol Infect Dis 2012;31:1699-1704.
Cooper MR. Single-agent, broad-spectrum fluoroquinolones for the outpatient treatment of low-risk febrile neutropenia. Ann Pharmacother. 2011 Sep;45(9):1094-102.
Sendzik J, et al. Quinolone-induced arthropathy: an update focusing on new mechanistic and clinical data. Int J Antimicrob Agents 2009;33:194-200.
Lee CL, et al. Tendon or joint disorders in children after treatment with fluoroquinolones or azithromycin. Pediatr Infect Dis J 2002;21:525-529.
GUIDED MEDLINE SEARCH FOR:
GUIDED MEDLINE SEARCH FOR RECENT REVIEWS:
Radl S. From Chloroquine to Antineoplastic Drugs? The Story of Antibacterial Quinolones. Arch Pharm 1996;329:115-119.
History
Table of Contents
- Class
- Antimicrobial Activity
- Pharmacodynamic Effects
- Mechanism of Action
- Mechanism of Resistance
- Pharmacokinetics
- Dosage
- Adverse Effects
- Drug Interactions
- Clinical Indications
- Urinary Tract Infections
- Bacterial Prostatitis
- Respiratory Tract Infections
- Skin and Soft Tissue Infections
- Osteomyelitis
- Gastrointestional Infections
- Intra-abdominal Infections
- Sexually Transmitted Diseases
- Mycobacterial Infections
- Inhalational Anthrax (Post-Exposure)
- Neutropenia
- Nasal Carriage of Neisseria Meningitides
- Eye Infections
- Pediatric Indications
