Pseudomonas aeruginosa
Authors: Shigeki Fujitani, M.D., Kathryn S. Moffett, M.D., Victor L. Yu, M.D.
Infection caused by Pseudomonas aeruginosa (P. aeruginosa) is common, with the burden of infection in hospitalized patients. The National Nosocomial Infections Surveillance (NNIS) System reports P. aeruginosa to be the second most common organism isolated in nosocomial pneumonia (17% of cases), the third most common organism isolated in both urinary tract infection (UTI) and surgical site infection (11% of cases), and the fifth most common organism isolated from all sites of nosocomial infection (9% of cases) (179). P. aeruginosa is an opportunistic pathogen which rarely causes disease in healthy persons. This organism is commonly considered in the differential diagnosis of a number of gram-negative infections. It is associated with nosocomial infections, often severe and life-threatening, especially in immunocompromised hosts. Chronic infection leads to progressive lung disease in cystic fibrosis, frequently complicated by antimicrobial resistance. If present in large enough numbers of inocula, or there is trauma with a break in epithelium, P. aeruginosa can cause infection in a healthy host. While animal models suggest that both humoral and cell-mediated immunity are involved in host defense against P. aeruginosa, the most important host defense is the neutrophil.
MICROBIOLOGY
P. aeruginosa is an aerobic gram-negative bacterium and P. aeruginosa is typified by motile, non-spore forming rods that are oxidase positive and lactose nonfermenters. P. aeruginosa is a member of the genus Pseudomonas, colloquially called the pseudomonads. The water-soluble pigments, pyocyanin and pyoverdin, give P. aeruginosa its distinctive blue-green color on solid media. P. aeruginosa produces indophenol oxidase, an enzyme that renders them positive in the “oxidase” test, which distinguishes them from other gram-negative bacteria. The presence of polar flagella and pili gives P. aeruginosa motility.
Like many environmental bacteria, P. aeruginosa live in slime-enclosed biofilms which allow for survival and replication within human tissues and medical devices. Associated with the production of a biofilm protects P. aeruginosa from host-produced antibodies and phagocytes contributing to antibiotic resistance of this organism.
The P. aeruginosa organism thrives in moist environments such as soil and water. It can be found in large numbers on fresh fruits and vegetables. Human colonization begins within the gastrointestinal tract, with subsequent spread to moist cutaneous sites such as the perineum and axilla. It forms smooth fluorescent green colonies at 42oC, with a characteristic sweet (grape-like) odor, making it easy to recognize on solid media in the laboratory.
As a group, pseudomonads have minimal nutritional requirements. Many are capable of using a wide variety of environmental sources for nutrition; P. aeruginosa often only needs acetate and ammonia as the source of carbon and nitrogen, respectively. In additionP. aeruginosa can grow anaerobically, and does not carry out fermentation, rather obtaining energy from the oxidation of sugars. The flexible nutritional requirement permits its growth in marginal environments. They are difficult organisms to eradicate from areas that become contaminated, such as operating rooms, hospital rooms, clinics, and medical equipment (59).
EPIDEMIOLOGY
P. aeruginosa, first isolated in 1882 by Gessard from green pus. The ubiquitous life-style of P. aeruginosa allows this bacterium to contribute to frequent infections in humans. It is a highly adaptable bacterium, with soil being the primary habitat; howeverP. aeruginosa also survives in aquatic environments. Its nutritional diversity allows for P. aeruginosa to survive toxic waste degradation.
P. aeruginosa is an important plant pathogen, affecting lettuce, tomatoes, and tobacco plants. It can be found in fresh water environments (streams, lakes, and rivers), as well as sinks, showers, respiratory equipment, even contaminating distilled water (68). Human beings can ingest the P. aeruginosa from such sources; however it does not adhere well to normal intact epithelium. Therefore it may be found as part of normal intestinal flora, and with a healthy immune system, P. aeruginosa does not cause infection (59).
The temperature in hot tubs favors Pseudomonas reproduction, with a hot tub containing up to 100 million organisms per milliliter. P. aeruginosa is adaptable; it finds the hospital and intensive care unit environments accommodating, with reservoirs of P. aeruginosa developing in the water in respiratory equipment. Due to its intrinsic and acquired resistance to many common antimicrobial agents, P. aeruginosa can be cultured from hand creams, hand-washing sinks, and certain cleaning solutions. Respiratory therapy equipment and dialysis tubing, both of which require a wet, body-temperature environment, are particularly susceptible to contamination by P. aeruginosa. Multi-use vials of respiratory medications have been implicated in spread of P. aeruginosa due to contamination with the organism. Artificial fingernails or extenders are not recommended for use by healthcare workers due to the frequent finding of P. aeruginosa colonization of the fingernails. Pseudomonads can even survive in some antiseptic solutions used to disinfect endoscopes and surgical instruments (35, 59, 68, 123, 228).
In a recent analysis of 24,179 adults with nosocomial bloodstream infections in the United States from 1995 to 2002, P. aeruginosa accounted for 4% of cases, and was the third leading cause of gram-negative infection (230). In children in the pediatric intensive care (PICU), the incidence of nosocomial infection was 1.5 per 100 patient-days. Patients with cardiac surgery had the highest nosocomial infection rate, 2.3 per 100 patient-days. Bacteremia (51.7%), respiratory infection (19.0%) and urinary tract infection (17.2%) were the most frequent nosocomial infections observed, and these were associated with use of invasive devices. Coagulase-negative staphylococci (39%) and P. aeruginosa (24%) were the most common organisms isolated (232).
P. aeruginosa is involved in a variety of human infections ranging from neonatal sepsis, to burn sepsis, and acute and chronic lung infections. This organism is a common opportunistic pathogen, leading to infections in patients with defects in host defenses, such as chronic neutropenias and defects of neurtrophil function, hematologic cancers, human immunodeficiency (HIV)/ acquired immunodeficiency syndrome (AIDS), and diabetes mellitus. In addition chronic pulmonary disease is common in patients with cystic fibrosis.
Neutropenia
The role of P. aeruginosa in febrile, neutropenic patients is a very real threat. Despite a diminished role of P. aeruginosa as a cause of sepsis since the 1980’s, P. aeruginosa may account for one-third to one-half of gram-negative bacteremia in these patients. The decrease in infections due to P. aeruginosa may be attributed to the change in clinical practice over the past twenty five years, with aggressive early use of anti-Pseudomonal antimicrobials in the febrile neutropenic patient. However when P. aeruginosa infection occurs, mortality is as high as 50-70%.
Neutropenia remains an important predisposing factor to serious P. aeruginosa infections. Patients with human immunodeficiency virus infection or solid-organ transplant recipients receiving myelosuppressive therapies such as ganciclovir should be carefully monitored for decreases in neutrophil count. Patients who become neutropenic should receive granulocyte colony stimulating factors to enhance neutrophil counts. In general, immunosuppressed patients who develop signs or symptoms of serious gram-negative infections should receive high doses of anti-Pseudomonal antibiotics.
HIV/AIDS
P. aeruginosa has been increasingly recognized as nosocomial and community-acquired infections among both adults and pediatric patients with HIV/AIDS (73, 147, 200, 213), with low CD4 lymphocyte counts (3, 220). In a study of 4825 patients with HIV, P. aeruginosa infections were diagnosed in 1.5% (72) patients. Respiratory infection accounted for 47% (34/72), with an overall incidence was 0.07%. Risk factors for a higher rate of P. aeruginosa infection include prior hospitalization and receipt of both dapsone and trimethoprim/sulfamethoxazole (TMP/SMX). Azithromycin use decreased the risk of infection by nearly 70%.
Cystic Fibrosis
The cystic fibrosis (CF) patient is not immunocompromised in the typical sense; the genetic defect is on chromosome 7, leading to the abnormal production of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) protein, consequently with malfunction of the chloride channel on the cell surface. Chronic airway infection is the most important cause of morbidity and mortality in cystic fibrosis, and P. aeruginosa is the most significant pathogen in cystic fibrosis (28, 50). This organism infects approximately 60 percent of cystic fibrosis patients overall, with an 80% prevalence in the group of patients 18 years of age and older (51). It is believed in cystic fibrosis patients that the organism is acquired from environmental sources, however cystic fibrosis-patient-to- cystic fibrosis-patient spread of P. aeruginosa has occurred.
Both the presence of P. aeruginosa and its phenotype (e.g., developing a mucoid biofilm due to production of alginate as well as high-level resistance to multiple antibiotics) have been shown to correlate with severity of patient illness (55). This mucoid property predicts chronic infection that cannot be cleared. CF patients with P. aeruginosa have a decreased life expectancy of 30 years, compared with 40 years in non-colonized patients. They also experience a more rapid decline in pulmonary function and more frequent hospitalizations (72). On the other hand, anti-Pseudomonal therapy that decreases sputum colonization is associated with improved pulmonary function and improvement in clinical scores (174) [See chapter on Cystic Fibrosis & P. aeruginosa.] P. aeruginosa in the paranasal sinuses in cystic fibrosis patients frequently leads to acute and chronic infections, commonly complicated by nasal polyps. P. aeruginosa is otherwise an extremely rare finding in the sinuses in the non-cystic fibrosis population.
Contaminated Medical Devices/Equipment
Clusters of P. aeruginosa bacteremia/ sepsis following procedures such as endoscopic retrograde cholangiopancreatography (ERCP), endoscopy, ophthtalmic devices, and transrectal ultrasound (TRUS) have been related to inadequate cleaning or disinfection of reusable medical devices. One cluster of postoperative P. aeruginosa ocular infections occurred when an internal tubing system of automated cataract surgical equipment became contaminated (35, 49, 122).
Neonatal Intensive Care Unit
Sepsis is a frequent infection in premature infants. P. aeruginosa is isolated in approximately 1% of culture-proven causes of early onset sepsis (infection occurring <7 days of life) in very low birth weight preterm infants; mortality from infections with P. aeruginosa is 37% (205). In late-onset sepsis (occurring after 7 days of age) in very low birth weight infants, P. aeruginosa accounts for 2.7% of all infections, with mortality as high as 74.4% (204). Infection by P. aeruginosa in all premature infants was associated with 52.3% mortality, significantly higher than the 13.7% to 23.8% fatality from other gram-negative bacilli (87).
CLINICAL MANIFESTATIONS
Infections in humans due to P. aeruginosa can be divided into opportunistic infections and those infections occurring in a healthy host. P. aeruginosa infections occur when the immune system is breached, either locally or systemically. Human hands infrequently are colonized with P. aeruginosa; however hospitalization increases the likelihood of finding the bacteria on intact skin. Therefore skin colonization can lead to bacteremia from catheter-related infection, or gastrointestinal colonization can lead to aspiration and pneumonia. Splashing of water from a contaminated sink, or droplets suctioned from a colonized endotrachial tube, can easily facilitate spread of P. aeruginosa. In the normal host, P. aeruginosa, in the proper setting can cause locally invasive disease. Large inocula of the bacteria can overwhelm normal defenses and lead to infection.
Urinary Tract Infections
P. aeruginosa, a common cause of nosocomial urinary tract infections (UTI’s), represents 7% of nosocomially-acquired UTIs in North America and Europe These infections are associated with an indwelling catheter, instrumentation of the urinary system, chronic prostatitis, nephrolithiasis, as well as prior antibiotic therapy. Community-acquired UTI’s are rarely caused by P. aeruginosa (97). In those persons with a neurogenic bladder, or in children with frequent infection from vesicoureteral reflux (VUR), P. aeruginosa UTI infection may occur. P. aeruginosa should be suspected in break-through infections in the urinary tract, especially in a host recently and/ or currently receiving antimicrobial agents, due to its nature to be resistant to many common antibiotics. In the elderly, urosepsis from P. aeruginosa may also follow acquisition of a simple UTI. Treatment regimens depend upon the presence of structural abnormalities or indwelling catheter(s), evidence of systemic sepsis, as well as the site of involvement, and prior antibiotic use. Eradication of the organism remains challenging and requires elimination of the predisposing factors in addition to antibiotic therapy.
Community-Acquired Pneumonia (CAP)
Infection due to P. aeruginosa is generally a rare cause of CAP (100), mainly occurs in HIV-infected patients, solid organ or bone marrow transplant recipients, or patients with neutropenia. However in a recent series of community-acquired bacterial pneumonia in adults, P. aeruginosa caused nearly 7% of the lower respiratory track infections. Nearly all of these patients had a preexisting risk factor for P. aeruginosa infection (9). Sicker adult patients have a high prevalence of severe CAP caused P. aeruginosa as well as Legionella pneumophilia (175).
Ventilator-Associated Pneumonia (VAP)
P. aeruginosa ranks as the leading cause of ventilator-associated intensive care unit (ICU)-acquired pneumonia, accounting for nearly 21% of cases (179). Spread/aspiration of bacteria to the lower respiratory track leads to a ventilator-associated pneumonia (VAP) by P. aeruginosa (161, 162, 221). Infection is common in those who have chronic disease, require respiratory/ventilatory assistance, have cystic fibrosis, or are immunocompromised, such as with cancer and neutropenia and/or hypogammaglobulinemia, (9, 12, 57).
Occasionally, P. aeruginosa, is accompanied by bloodstream infection, septic shock, and acute respiratory distress syndrome (ARDS) as a complication. The dramatic onset of septic shock, followed by death within hours that occurs in some immunocompromised patients with P. aeruginosa bacteremia, is a memorable experience for any observer. However, apart from the appearance of ecthyma gangrenosum, P. aeruginosa bacteremia is usually indistinguishable clinically from other forms of gram-negative bacteremia.
Near universal colonization with P. aeruginosa occurs in adults intubated > 5 days; colonization of tracheotomy sites in adults and children too is common. Risk factors for adults developing VAP include: antibiotic exposure, use of H2-receptor blockers, advanced age, reintubation, and transport from the ICU while intubated. In children in the pediatric intensive care unit three independent risk factors for pediatric VAP include: immunodeficiency, immunosuppression, and neuromuscular blockade (232). In patients with tracheostomy receiving short-term mechanical ventilation, P. aeruginosa can become a common pathogen (177).
Endocarditis
Infective endocarditis (IE) due to P. aeruginosa is uncommon, occurring primarily in patients with injection drug use (IDU), with regional epidemics in midwestern U. S. cities (110, 111). Clusters of cases of IE occur particularly in abusers of pentazocine and tripelennamine, likely due to mixing of drugs with contaminated water. IE in patients with IDU frequently have no preceeding valvular or heart disease. The specific heart valve involved is important with respect to clinical manifestations, therapy, and prognoses (88). Infection of the tricuspid valve may have a more subacute presentation, likely due to lower bacterial densities from lower oxygen tension on the right side of the heart. Infection of the mitral valve may present more acutely, with more severe systemic sepsis-like manifestations. In addition, valvular dysfunction may lead to congestive heart failure, and may dictate need for surgical intervention.
Other factors predisposing to Pseudomonas endocarditis include the presence of a prosthetic valve or other intravascular foreign body, underlying malignancy, chemotherapy, and prolonged neutropenia. Increasing reports of Pseudomonas endocarditis following prolonged hospitalization associated with prosthetic endovascular devices (i.e. pacemakers) have been noted (8, 89, 130,180).
Meningitis
Central nervous system (CNS) infection due to P. aeruginosa meningitis is extremely rare, unless there has been penetrating trauma to the head, placement of a CNS shunt (such as a ventriculoperitoneal (VP) shunt), or postneurosurgical procedures (36, 144, 145). Often meningitis or ventriculitis associated with CNS shunts is caused by a mixed bacterial infection, including multiple aerobic gram-negative bacteria, including P. aeruginosa. Gram-negative bacterial infection must be considered, especially when erosion or perforation of the bowel has occurred from a VP shunt catheter, leading to an ascending CNS infection.
In a case series of gram-negative bacterial meningitis, 21% (14 cases) were due to P. aeruginosa. Eight of these 14 cases were postneurosurgical and the overall mortality of Pseudomonas meningitis was 35.7% (144). Mortality is highest if infection results from bacteremia in an immunosuppressed host, underlying infective endocarditis, or malignant otitis externa. Cure is more likely if the meningitis results from neurosurgical procedures involving hardware, such as VP shunts, drains, or reservoirs. An additional group of patients at risk for meningitis secondary to P. aeruginosa bacteremia is those receiving hemodialysis (38).
Ocular Infections
P. aeruginosa is frequently a cause of infection associated with contaminated contact lens solutions. In addition, eye trauma and recent ophthalmic surgery, as well as contact lens use, are risk factors for sight-threatening infections from P. aeruginosa, leading to corneal ulcerations, keratitis, and endophthalmitis. In addition, cases of orbital cellulitis and endophthalmitis due to P. aeruginosa have resulted as a complication of sepsis in neonates, patients with hematologic malignancy, and HIV/AIDS (23, 148, 173, 228).
Ear Infections
There are three major ear infections caused by P. aeruginosa.
Perichondritis of the Ear
With the recent fashion of ear piercing, perichondritis of the auricle due to P. aeruginosa infections has become more common. The pinna may be markedly swollen, red and tender, with infection progressing to necrosis of the cartilage. It is felt that the decreased oxygen tension in the auricle may be more conducive to infection by P. aeruginosa.
Otitis Externa
Often known as swimmers’ ear, otitis media externa from P. aeruginosa causes a local infection of the external ear canal. Simple otitis media external is associated with warm, humid atmospheric conditions, aural water exposure, and ear canal trauma which may occur with frequent swimming, or when maceration or trauma occurs to the ear canal epithelium. Pain is found in 97.2% (13). Inflammation can be secondary to dermatitis only or it can be caused by active bacteria. Acute otitis externa can occur acutely and become painful. Wax in the ear can swell and block the canal and dampen hearing to varying degrees, creating a temporary conductive hearing loss. In more severe or untreated cases, the infection can rarely spread to the adjacent soft tissues, such as parotid gland and the jaw joint, making chewing painful.
Malignant External Otitis
Malignant (necrotizing) otitis media external is a subset of osteomyelitis caused by P. aeruginosa in which the temporal bone and skull base is involved (92). Patients with diabetes mellitus and advanced age (above 60 years) are at risk for necrotizing otitis externa, perhaps due to a higher pH in diabetic cerumen, and microangiopathy in the ear canal. Otitis externa may also complicate myringotomy tube placement, leading to a chronic otorrhea and subsequent removal of the ear tubes (183). Malignant otitis media external has also been reported in patients with HIV/AIDS.
In malignant external otitis, classic signs of infection including fever, leukocytosis, and systemic toxicity are notably absent, thus making diagnosis difficult. Otalgia followed by severe and often excruciating headache is the most common presenting symptom. Cranial nerve dysfunction, especially facial nerve palsy, is a late complication. Diagnosis rests on the findings of bony erosion and soft tissue abnormalities of the temporal bone and infratemporal fossa on computed tomographic (CT) scan or magnetic resonance imaging (MRI), elevated erythrocyte sedimentation rate (ESR), and isolation of P. aeruginosa from the external auditory canal or mastoid in a patient with a recalcitrant headache (91). In a 1-year prospective comparison, MRI was found to be slightly better than CT at distinguishing medial skull base disease, by delineating changes in the fat content of the marrow (91, 108).
Skin and Soft Tissue Infections
There are seven major skin and soft tissue infections caused by P. aeruginosa.
Hot Tub Folliculitis
Hot tub follculitis is an infection involving the hair follicles can occur as a result of bathing in a contaminated tub. If there inadequate chlorination, P. aeruginosa thrives at the higher temperature environment of hot tubs. Normal hosts with intact skin are affected predominantly in body sites covered by bathing suits, although areas of skin that have abrasion or have recently been shaved, may be at increased risk of infection (34). With a high enough inoculum of P. aeruginosa in the water, normal host defenses are overwhelmed, resulting in infection in immunocompetent hosts (47). Exposure of the head and external auditory canal may result in facial folliculitis or otitis media externa.
Puncture Wounds/Osteomyelitis
Puncture wounds, especially a nail or sharp object through a tennis shoe/sneaker into the plantar aspect of the foot, and lacerations and trauma occurring in fresh water streams and lakes, may lead to infection caused by P. aeruginosa. Soft-tissue infection, as well as osteocondritis, septic arthritis, and/or osteomyelitis of the traumatized bone or joint may occur.Hematogenous osteomyelitis/septic arthritis due to P. aeruginosa in children is extremely rare. More frequently osteomyelitis is found in intravenous drug users with P. aeruginosa bacteremia. Osteomyelitis does occur frequently as a polymicrobial infection in diabetics with foot ulcers, from local trauma and direct inoculation of the organisms(s).
Foot Infections
The moist interdigital areas of the feet are ideal sites for colonization with P. aeruginosa. When these areas become infected with dermatophytes, or suffer other trauma to the dermal barrier, secondary bacterial invasion can readily occur.
Green Nail Syndrome
Patients with chronic onycholytic nails who have prolonged immersion exposure to fresh water may develop the green nail syndrome. This characteristic green discoloration is almost always a complication of onycholysis or a chronic paronychia and is usually restricted to one or two nails. Fungi and P. aeruginosa are frequently isolated from the nail. The green color is due to the pigment pyocyanin adhering to the undersurface of the nail plate with accumulated debris below the nail (93).
Ecthyma Gangrenosum
Ecthyma gangrenosum is a cutaneous manifestation of serious infection due to P. aeruginosa usually associated with bacteremia and sepsis. The lesions of ecthyma gangrenosum involve skin or mucous membranes, resulting from perivascular bacterial invasion of the adventicia of arteries and veins, leading to secondary ischemic necrosis. The painless skin lesions of ecthyma ganrenosum may be multiple, with rapid evolution through stages of macules, nodules, vesicles, and ulcerative eschars; the erythematous nodular lesion evolves into a hemorrhagic, ulcerative and necrotic area. Although not pathognomonic for P. aeruginosainfection, the finding of ecthyma raises the probability of P. aeruginosa systemic infection. Occasionally other skin lesions may occur with dissemination of systemic P. aeruginosa infection. These include macular papular lesions, clusters of pustules, cellulitis that mimics erysipelas, and soft-tissue abscesses. Many lesions become necrotic over time. The lesions contain little, if any, pus. In children the lesions may be more likely to be present on the perineum and buttocks.
Necrotizing Fasciitis
Necrotizing fasciitis has been well-described in neutropenic and diabetic hosts (129). Cellulitis due to P. aeruginosa occurs at sites of damage to the dermal barrier, such as puncture sites or surgical wounds. Pyoderma occurs when a preexisting lesion of the skin becomes colonized and subsequently invaded by P. aeruginosa. In patients with facial cellulitis, the mucous membrane of the mouth is often the initial site of infection with subsequent spread to subcutaneous tissue and blood. Rapid progression to gangrene ![]() mandates vigorous surgery including extensive debridement and resection (129, 223). Exfoliative skin diseases, venous stasis ulcers, and eczema may predispose to infection. Rare skin disorders such as epidermolysis bullosa may be susceptible to chronic skin infection from P. aeruginosa. Severity of infection may range from a simple localized, nonnecrotic cellulitis to a necrotizing, gangrenous process with associated bacteremia in an immunocompromised host. P. aeruginosa can be cultured from the purulent discharge, but clinical manifestations of erythema, tenderness or warmth should differentiate infection from colonization. Infection may be acute and invasive, or may follow a chronic indolent course.
mandates vigorous surgery including extensive debridement and resection (129, 223). Exfoliative skin diseases, venous stasis ulcers, and eczema may predispose to infection. Rare skin disorders such as epidermolysis bullosa may be susceptible to chronic skin infection from P. aeruginosa. Severity of infection may range from a simple localized, nonnecrotic cellulitis to a necrotizing, gangrenous process with associated bacteremia in an immunocompromised host. P. aeruginosa can be cultured from the purulent discharge, but clinical manifestations of erythema, tenderness or warmth should differentiate infection from colonization. Infection may be acute and invasive, or may follow a chronic indolent course.
Burns
Pseudomonas colonization in a patient with severe burn wounds is acquired when normal skin/respiratory/ gastrointestinal flora is replaced by hospital flora; typical infections from P. aeruginosa occur several weeks after the initial burn. Colonization may be abetted by broad spectrum topical and/or systemic antibiotics. A trend toward a reduction in infection in the burn patient due to P. aeruginosa in the last two decades has been seen. Culture of the burn wound may not differentiate wound colonization from true invasive disease, necessitating biopsy and quantitative cultures to differentiate colonization (227). The fruity or grape-like odor associated with P. aeruginosa can be observed in wounds heavily colonized with the organism. Burn sepsis is typically associated with positive blood cultures and significant morbidity and mortality.
LABORATORY DIAGNOSIS
P. aeruginosa is easily cultured in the microbiology laboratory and is easily recognized by on a variety of media, with its spreading, flat colonies with serrated edges and metallic sheen. The characteristic: (1) fruity, sweet-grape smell, sometimes corn/taco-like odor, (2) elaboration of green pigment which is a combination of the yellow (pyoverdin) and blue (pyocyanin) pigments, giving the culture a characteristic bright green color, and (3) nonfermentive, oxidase-positive properties, help to easily confirm its presence on culture plates. The mucoid appearance of P. aeruginosa strains is pathopneumonic for chronic colonization/infection of these bacteria in cystic fibrosis respiratory specimens. Infrequently a rotten-potato odor or the lack of pigment may delay identification of the organism.P. aeruginosa can grow easily between 20oC and 43oC. Growth at higher temperatures may help to distinguish P. aeruginosa from other gram-negative organisms and other pseudomonads.
P. aeruginosa must be differentiated from other lactose non-fermenting gram-negative rods. These species includeBurkholderia, Stenotrophomonas, Comamonas, Shewanella, Ralstonia, Methylobacterium, Sphingomonas, Acidovorax and Brevundimonas. Within the Pseudomonas genus these bacteria include P. aeruginosa, as well as P. uorescens, P. putida, P. stutzeri, andP. mendocina. Members of the genus Pseudomonas can be differentiated from other genera on the basis of cellular fatty acid composition. The different Pseudomonas species can be distinguished biochemically. Biochemically, P. aeruginosa can be confidently identified on the basis of (1) a positive oxidase test, (2) a triple sugar iron agar reaction of alkaline over no change, and (3) growth at 42oC. Other key biochemical characteristics include oxidation of glucose but not disaccharide, hydrolysis of acetamide, and reduction of nitrates to nitrogen gas.
PATHOGENESIS
P. aeruginosa is typically an extracellular pathogen. The growth in tissue depends on its ability to resist ingestion by neutrophils. P. aeruginosa produces a wide variety of virulence factors, and employs survival techniques to survive, which include:
- polar flagella, which are critical for motility in initial stages of pulmonary infection, activate IL-8 production by binding to toll-like receptor on the surface of airway epithelial cells, and facilitate adherence to epithelial and eukaryotic cells with pile/ non-piling adhesions (polar pili) (2, 189);
- lipopolysaccharide (LPS) adhesion moiety, which acts as a signal through toll-like receptors and has 3 parts:
- lipid A (also known as endotoxin, interacts with host cell receptor to initiate the inflammatory response),
- core oligosaccharide (interacts with the CFTR on epithelial cells, leading to bacterial innate immune resistance to the pathogen)
- O-antigen side chains (responsible for resistance to normal human serum, detergents, and some antibiotics) (10, 98,181);
- exotoxin A which is an ADP-driven toxin, causes direct tissue damage and necrosis;
- extracellular proteases called LasA, LasB, and alkaline protease, which all degrade elastin;
- type III secretion system that allows direct manipulation of host cell function by injecting virulent proteins directly into the cell (189); • leukocidin which destroys neutrophils;
- innate antibiotic resistance due to limited permeability of the outer membrane, as well as numerous multidrug resistanceefflux pumps; and
- acquisition of antibiotic resistance genes readily from other bacteria by transformation, conjugation, and transduction (Navon).
In chronic infections of P. aeruginosa additional strategies are employed by the bacteria that include:
- biofilm formation, which prevents host defenses and antibiotics from reaching the bacteria;
- quorum sensing, which is the production of autoinducers, to activate transcription of a number of genes that faciliattae cell-to-cell communication; and
- alginate production of a mucopolysaccharide or mucoid appearance, with an altered LPS and lipid A, which serve to hide the organism from the immune system (pathopneumonic of chronic infection in cystic fibrosis respiratory isolates of P. aeruginosa).
Several strategies are employed by P. aeruginosa to obtain scarce nutrients during infection. These include:
- production of siderophores, pyochelin, pyoverdin, and pyocyanin which chelate iron, to support bacterial metabolic processes and control the expression of other P. aeruginosa virulence factors, such as exotoxin A, endoprotease and pyoverdine (131,149, 209, 218); and
- hemolysins such as phospholipase C and lecithinase, which hydrolyze phospholipids from the host cell membrane to release phosphate in an available form (189).
All these mechanisms induce local inflammation and tissue destruction, enabling P. aeruginosa to get the nutrients it needs, and invade the host. The antimicrobial resistance of P. aeruginosa is a predisposing factor in treatment failure and the biofilm mode of growth provides protection from antibiotics (59).
SUSCEPTIBILITY IN VITRO AND IN VIVO
Numerous antibacterial agents have potent in vitro activity against P. aeruginosa including the third generation cephalosporins (cefoperazone, cefsulodin and ceftazidime, but not cefotaxime or ceftriaxone), fourth generation cephalosporins (cefepime, cefpirome, cefclidin), extended spectrum penicillins (ticarcillin, piperacillin, azlocillin), monobactams (aztreonam); carbapenems (imipenem, meropenem), quinolones (ciprofloxacin, levofloxacin, gatifloxacin, moxifloxacin), and aminoglycocides (gentamycin, amikacin, tobramycin, colimycin). The addition of beta-lactamase inhibitors to extended spectrum penicillins (ticarcillin/clavulanate and piperacillin/tazobactam) does not enhanced anti-Pseudomonal activity. The comparative in vitro susceptibilities of anti-Pseudomonal agents are given in Table 1.
P. aeruginosa has notable intrinsic mechanisms of resistance and is capable of acquiring multiple mechanisms of antibiotic resistance. Three mechanisms of resistance predominate: production of beta-lactamases, loss of outer membrane proteins, and upregulation of efflux pumps. During treatment of an invasive infection from P. aeruginosa, there is concern about development of resistance with a single antimicrobial agent (169). Multiple cases of beta-lactam antibiotic resistance developing during therapy have been documented in human and in in vivo models. Resistance is believed to arise from selection of resistant mutants as well as the induction of beta-lactamases (14, 15, 115, 134). In the hospital setting, multidrug-resistant P. aeruginosa are common, often with simultaneous resistance to ciprofloxacin, imipenem, ceftazidime and piperecillin (137). Synergy between enhanced production of beta-lactamases and decreased outer membrane permeability may occur in species of P. aeruginosa.
The intrinsic resistance of P. aeruginosa to a number of antibiotics, including first-, second-, and many third-generation cephalosporin, penicillins, and macrolides, in addition to the acquisition of resistance during therapy, assists in bacterial virulence. Rates of multidrug resistance (resistance to >= 3 antimicrobial agents), known as multi-drug resistant P. aeruginosa (MDR- P. aeruginosa), increased in the United States from 7.2% in 2001 to 9.9% in 2003 (121, 157). Risks factors for MDR- P. aeruginosa infection include prolonged hospitalization, exposure to antimicrobial therapy, and immunosuppressed states such as HIV/AIDS (81). Delay in initiation of proper anti-Pseudomonal antibiotics is associated with a worse outcome. Knowledge of the local and regional drug resistance patterns is important when choosing empiric therapy for treatment of P. aeruginosa infections.
Antibiotic resistance is currently one of the most important problems faced by caregivers, especially in cystic fibrosis patients. Multiply antibiotic-resistant P. aeruginosa were identified in 11.6% of cystic fibrosis patients in a large multicenter study of patients with moderate disease (28, 191) and even testing for synergistic combination of agents in these resistant organisms failed to identify potential therapy for 17% (191). The presence of antibiotic-resistant P. aeruginosa not only limits potential antimicrobial treatment, but can also preclude patients from eligibility for lung transplantation and other potentially life-saving modalities. An additional concern is that the introduction of new antimicrobial agents is contributing to the emergence of other, intrinsically antibiotic resistant pathogens that may be associated with increased morbidity in cystic fibrosis.
A significant concern about the emergence of resistance during therapy with prolonged intermittent inhaled tobramycin, in treatment of chronic P. aeruginosa infection in cystic fibrosis patients, has also been raised. Microbiology data at completion of the phase 3 trials of inhaled tobramycin demonstrated some decrease in susceptibility of P. aeruginosa isolates in association with treatment, with no increase in the isolation of B. cepacia, S. maltophilia, or A. xylosoxidans. The number of strains defined as resistant according to standard criteria increased in the tobramycin group, although there is no evidence of selection of the most resistant isolates to become the most prevalent. Although low level resistance may not impact the efficacy of inhaled tobramycin, it could significantly decrease the activity of parenteral drug alone or in synergistic combinations (29). Current antimicrobial strategies may also be contributing to the increased rate of isolation of other pathogens, including intrinsically antibiotic-resistant organisms. Currently studies are monitoring resistance patterns of bacteria isolated in cystic fibrosis sputum to determine the impact of years of chronic intermittent inhaled tobramycin.
Antibiotic testing, for sensitivity and resistance is determined according to guidelines set by the Clinical and Laboratory Standards Institute,(41) formerly known as National Committee for Clinical Laboratory Standards (NCCLS). Minimum inhibitory concentrations (MIC) values are defined for each antibiotic tested, and is characterized by determining the MIC50 and the MIC90 for allP. aeruginosa isolates. The MIC50 is be defined as the median MIC value; the MIC90 is defined as the concentration that inhibits 90% of isolates (41). MIC values are clinically relevant when tissue/ fluid concentrations of the antibiotic at the site of infection are able to be measured, as in CSF concentrations in the treatment of a CNS shunt infection (217).
Mechanisms of Resistance
Beta-lactamase Production
The major mechanism of resistance by P. aeruginosa to beta-lactam antibiotics is beta-lactamase production. Both chromosomally-mediated and plasmid-mediated beta-lactamases characteristically produce AmpC-type which mediate resistance to the third generation cephalosporins and the monobactam, aztreonam. Examples of other beta-lactamases produced include TEM, SHV, PER, PSE and OXA, including some with expression as an extended-spectrum beta-lactamase (ESBL).
Recent appearance of transferable beta lactamases, metalloenzymes, may hydrolyze cephalosporin and carbapenem antibiotics (164, 199). Two major variants are IMP and VIM-type enzymes, originally discovered in Japan, and now found worldwide (212). The proportion of P. aeruginosa isolates from the United States which are resistant to imipenem is increasing. In the year 2000, 17.7% of P. aeruginosa isolates from patients in ICUs in the United States tested as part of the National Nosocomial Infections Surveillance (NNIS) program were resistant to imipenem.
Resistant to the carbapenems by P. aeruginosa can be associated with lowered permeability of the bacterial outer membrane due to loss of the D2 porin protein (170). This porin protein facilitates the passage of only carbapenems, but not other beta-lactams, therefore resistance to other antimicrobial agents does not occur (214). Alteration in penicillin-binding proteins is a very rare cause of resistance which has been seen to emerge with therapy for P. aeruginosa in patients with cystic fibrosis (85).
Upregulated Efflux Pumps
Efflux pumps are active transport mechanisms which move or pump antibiotics out of the bacterial cell, mediating antibiotic resistance. The most relevant efflux pump, MexAB-OprM, facilitates resistance. Upregulation of the MexAB-OprM efflux pump system results in diminished susceptibility to quinolones, anti-Pseudomonal penicillins, and cephalosporins (139). The regulation of efflux pumps appears to occur in tandem with the regulation of outer membrane protein proteins. A single operon (mexA, mexB, oprK) facilitates resistance to quinolones, beta-lactams, tetracycline, and chloramphenicol by way of drug efflux (169). Outer membrane proteins (OprK) may be involved in this energy-dependent process.
Aminoglycoside-Modifying Enzymes
Aminoglycoside resistance in P. aeruginosa is most commonly due to aminoglycoside-modifying enzymes (aminoglycoside-inactivating enzymes) which are coded by genes on plasmids of the chromosome. The aminoglycosides can be inactivated by acetylation of an amino group by acetyltransferases, by adenylation of a hydroxyl group by adenyltransferases, or by phosphorylation of a hydroxyl group by phosphotransferases. At least 14 different aminoglycoside modifying enzymes can be produced by P. aeruginosa, the most common of which is acetyltransferase (188). Modification of the aminoglycoside results in poor binding to the ribosome, the site of action of this class of drug.
Reduced uptake of the aminoglycoside into the bacteria has also been described as a mode of resistance in P. aeruginosa. In some cases this has been the mechanism of emergence of resistance during therapy. Cross-resistance for all aminoglycosides generally results, but the level of resistance is less than that resulting from enzymatic modification. Intergeneric lateral gene transfer of 16S rRNA methylase gene (rmtA gene) from some aminoglycoside-producing microorganisms to P. aeruginosa occurs (234).
Other Mechanisms
Fluoroquinolones act by inhibiting the activity of DNA gyrase enzymes, which consist of two subunits, A and B. Resistance of P. aeruginosa to quinolones is a chromosomally mediated process, without plasmid-mediated resistance found. Alterations in Gyr B have been found in other bacteria but not P. aeruginosa. Infrequently, resistance occurs from decreased penetration of outer membrane, due to mutations in the genes nal B, nfx B or nfx C (104).
A unique relationship between P. aeruginosa and the macrolide antibiotics (erythromycin, clarithromycin, and azithromycin) exists. Intrisically resistant to P. aeruginosa, macrolides appear to have benefit in modulating chronic infection. Macrolides may exert their effect on the host by modulation of immune response or by disturbing/interrupting the biofilm. A multicenter, randomized, double-blind, placebo-controlled trial demonstrated that azithromycin treatment was associated with clinical improvement in patients with cystic fibrosis infected chronically with P. aeruginosa (69, 82, 118, 192, 233). An animal study suggested that a combination of the action of polymorphonuclear leukocytes (PMNs), quorum sensing inhibitors, and conventional antibiotics may eliminate the biofilm-forming bacteria before a chronic infection was established (19).
ANTIMICROBIAL THERAPY
Drugs of Choice
The choices for treatment for P. aeruginosa infections include the following antimicrobial agents, with the fluroquinolones being the only oral options:
• Aminogylcosides (amikacin, tobramycin, gentamicin)
• Carbapenems (imipenem, meropenem, doripenem)
• Cephalosporins, third-generation (cefoperazone, cefsulodin, ceftazidime, but not cefotaxime or ceftriaxone)
• Cephalosporins, fourth-generation (cefepime, cefpirome, cefclidin)
• Fluoroquinolones (ciprofloxacin, levofloxacin)
• Monobactam (aztreonam)
• Extended-spectrum penicillins (ticarcillin and/or ticarcillin-clavulanate, piperacillin and/or piperacillin–tazobactam,azlocillin).
• Polymyxin B/Colistin
The fluoroquinolones are not approved for use in children < 18 years of age, due to concerns about growth plate issues in the puppy animal model. Experience with use of the fluoroquinolones in cystic fibrosis in children, as well as other infectious diseases, has demonstrated safety. Consensus guidelines for fluoroquinolone use in children by the American Academy of Pediatrics (AAP) were published in 2006, giving validity to their use in the cystic fibrosis pediatric population, as a way to treat P. aeruginosa in the ambulatory setting (43). Consultation with a pediatric infectious disease specialist may be warranted in these situations. Choice of anti-Pseudomonal therapy and dosage (see Table 2) will depend on the site and severity of the infection as well as the underlying condition of the patient.
Polymyxin B/Colistin
The development of better-tolerated anti-Pseudomonal agents since the 1970s has largely contributed to the diminished use of systemic colistin. Infection by P. aeruginosa resistant to all commercially available antibiotics is now commonplace, with colimycin offering an option for salvage therapy. The polymyxins were originally isolated from Bacillus spp. with colistin (also known as polymyxin E) coming from B. colistinus in 1950. The polymyxins act primarily on the bacterial cell wall, leading to rapid permeability changes in the cytoplasmic membrane. Entry into the cell is not necessary. The polymyxins may also have antiendotoxin activity. In desperate situations, combination therapy with colistin may be attempted. Parenteral colistin has been used successfully for MDR-P. aeruginosa (135, 137, 151, 203).
Most pharmacokinetic studies on colistin were performed more than 30 years ago using intramuscular administration of the drug. Currently recommended dosing of colistin are: creatinine clearance > 80 mL/min. - 2.5 mg/kg every 12 hours; creatinine clearance 30-80 mL/ minute loading dose of 3 mg/kg on day 1 then 1.25-1.9 mg/kg every 12 hours and creatinine clearance < 30 mL/min loading dose of 3 mg/kg on day 1 then 1.25 mg/kg every 12 hours (62). Colistin resulted in a positive clinical outcome in 58% of patients treated for MDR P. aeruginosa and Acinetobacter strains (135). In patients with normal renal function, initial doses were 2.5-5 mg/kg/day divided into two or three doses, up to a maximum of 300 mg daily. More recent pharmacodynamic studies in P. aeruginosaisolates from cystic fibrosis patients suggest that doses higher than 2-3 mg/ kg every 12 hours may be required for effective therapy (136). In a study of 12 patients with cystic fibrosis, 160 mg three times per day was found to be safe (44). Toxicity concerns with the use of colistin include nephrotoxicity and neurotoxicity. The drug may also produce drug fevers.
Combination Therapy
In the face of systemic infection with shock/sepsis, antimicrobial therapy should consist of two intravenous (IV) antimicrobial agents, with one of these being an aminogylcoside (20). Dose and duration may also depend on severity of illness, as well as renal function of the patient, and presence of cystic fibrosis. In cystic fibrosis patients, combination therapy is recommended due to concerns about development of antibiotic resistance with single-agent therapy (50, 191). The most frequently used combinations include a β-lactam antibiotic plus an aminoglycoside (153). Because these classes of drugs have different bacterial targets, as well as different modes of entry into the bacterial cell, they often mutually enhance antimicrobial activity. Appropriate initial antimicrobial treatment appears critically important for optimal cure of P. aeruginosa bacteremia (101, 119, 141, 150). There are 3 potential advantages to using combination therapy: (1) an increased likelihood that the infecting pathogen will be susceptible to at least one of the antimicrobes; (2) prevention of emergence of resistance; and (3) additive or even synergistic effect of the combination.
There is an ongoing debate over the role of combination antimicrobial therapy. Combination therapy had been considered the mainstay of therapy for many years, although proponents of monotherapy have emerged:
- In a prospective observational study of 200 consecutive patients with P. aeruginosa bacteremia, combination therapy was found to be significantly better than monotherapy in improving outcome (101). Mortality was significantly higher in patients given monotherapy (47%) than in patients given combination therapy (27%). It should be noted that the most common combination used was piperacillin or ticarcillin combined with tobramycin or gentamicin. The monotherapy group was dominated by patients given an aminoglycoside alone. Few patients received cephalosporins, aztreonam, carbapenems or quinolones.
- A prospective observational study evaluated monotherapy versus beta-lactam/ aminoglycoside combination therapy for gram-negative bacteremia (133). 16% of the 2165 patients in the study had P. aeruginosa. 34% (21/61) patients with P. aeruginosabacteremia died on beta-lactam monotherapy while 28% (11/39) patients died after receipt of combination therapy. This corresponded to an odds ratio of 0.7 with 95% confidence intervals of 0.3-1.8.
- An additional prospective study evaluated therapy in 170 bacteremic patients; 48% received monotherapy while 52% received combination (78 out of 88 of these received a beta-lactam plus aminoglycoside). The authors did not find statistically significant differences in mortality between these groups (219).
- A randomized trial comparing ciprofloxacin plus piperacillin versus tobramycin plus piperacillin for empirical therapy in 543 febrile neutropenic patients was performed; only 4 of 543 febrile episodes were due to P. aeruginosa bacteremia (166).
- In a meta-analysis of studies of immunocompetent patients with severe sepsis, 64 trials and 7586 patients were reviewed. No advantage of combination therapy over monotherapy could be seen for mortality and clinical outcome among patients with all gram-negative infections (1835 patients). Nephrotoxicity was more common with combination therapy in nearly all studies. Analysis of onlyP. aeruginosa bacteremias (426 patients) showed a significant mortality benefit (OR 0.50, 95% CI 0.30-0.79) (190).
- In a second meta-analysis of gram negative bacteremia, 17 (5 prospective, 2 prospective randomized, and 10 retrospective) cohort studies were evaluated. Five studies focused on P. aeruginosa bacteremia; 2 studies used extended-spectrum beta-lactam and aminoglycoside antibiotics; 2 studies used extended-spectrum beta-lactam and aminoglycoside or fluoroquinolones; one study used extended-spectrum beta-lactam and fluoroquinolones. Subgroup analysis of bacteremias due to P. aeruginosa demonstrated that mortality was reduced in the combination therapy (27%) versus monotherapy therapy (47%) group (p < 0.02); this significant relationship held true for patients with malignancy, nosocomial infection, and infection site. The monotherapy group in this meta-analysis was dominated by patients given an aminoglycoside alone resulting in potential bias from the adverse outcome (101).
- Current recommendations for gram-negative sepsis by the Cochrane library is monotherapy (165).
- Animal models have been used to compare the efficacy of monotherapy versus combination antibiotic therapy for pulmonary infections due to P. aeruginosa pneumonia. Four studies demonstrated improved survival with combination therapy compared with monotherapy; two studies demonstrated a synergistic effect with the combination therapies of ceftazidime/clarithromycin and a beta-lactam/ aminoglycoside. (25, 86, 120, 159, 168, 186).
To definitively show that combination therapy is superior to monotherapy would require a randomized controlled trial of several hundred patients. It is not likely that such a study will be performed in the near future. The demonstration of in vitro synergy between anti-Pseudomonal beta-lactam antibiotics and aminoglycosides, and the development of resistance with monotherapy, prompts us to continue to recommend combination antibiotic therapy for invasive P. aeruginosa infections in severely-ill patients. Combinations of beta-lactams and quinolones are increasingly used but the clinical data to support such combinations is sparse. We do not recommend combinations of two beta-lactams. Double beta-lactam therapy has proved inferior to the beta-lactam-aminoglycoside combination in animal models (116). One study in humans showed emergence of resistance in 40% (two of five) of cases in one series of P. aeruginosainfection treated with double beta-lactams (229).
Synergy Testing
Debate continues as to the clinical relevance of in vitro synergy testing as a guide for antimicrobial therapy in the clinical setting. Detection of synergy using different methods (checkerboard vs. time-kill methodology) may have some utility in choice of an antibiotic combination. Results from the time-kill methodology have been shown to be more accurate in predicting clinical outcome than results from checkerboard methodology (101). In a prospective observational study, when the combination was synergistic in vitrosurvival was higher than when it was not (p= 0.10) (101).
Synergy has observed between beta-lactams and aminoglycosides, beta-lactam antibiotics and quinolones, and fluoroquinolones and aminoglycosides. Antagonism is rarely observed between currently used anti-Pseudomonal agents, although double beta-lactam therapy is discouraged. Synergy was noted more frequently (42%) with a piperacillin/tazobactam and amikacin combination than with piperacillin/tazobactam and ciprofloxacin (8%) (27). Predominantly, additive effects have been found with the combinations of levofloxacin or ciprofloxacin and piperacillin, ceftazidime or aztreonam (167). In contrast, recent studies with gatifloxacin have shown that synergy occurred in combination with an anti-Pseudomonal beta-lactam or aminoglycoside (90). Beta lactam agents may be synergistic in combination with ciprofloxacin against multi-drug resistant P. aeruginosa (60, 61).
A number of in vitro studies have suggested that use of colistin as part of combination therapy may result in greater killing ofP. aeruginosa than monotherapy. Colistin has been evaluated in combination with ceftazidime and rifampin (83, 211), amikacin (210) , ceftazidime (96), aztreonam, meropenem, and ciprofloxacin (187). Caution with use of IV colistin must be used, due to potential and rapid development of renal insufficiency with the parenteral administration of this drug.
Dosing of Anti-Pseudomonal Antibiotics
Dosing of antimicrobial agents for treatment of Pseudomonas infections should be aggressive (See Table 2 & 3). For ciprofloxacin, an intravenous (IV) dose of 400 mg every 8 hr should be considered instead of the standard 400 mg every 12 hr. For oral therapy in cystic fibrosis, a dose of 750 mg twice a day is frequently employed, instead of the usual 500 mg. For children, the dosage for ciprofloxacin is 30 mg/ kg/ day, and for levofloxacin 10 mg/kg/day. The dose of levofloxacin at 750 mg per day, rather than 500 mg per day, should be considered (26).
For the class of beta-lactam antibiotics, the rate of bactericidal activity of beta-lactams does not increase substantially once concentrations exceed four times the MIC (46). Beta-lactams do not exhibit a post-antibiotic effect against P. aeruginosa with the notable exception of the carbapenems. Thus, high drug concentrations do not kill P. aeruginosa any faster than low concentrations, and bacterial regrowth will begin very soon after serum and tissue levels fall below the MIC. Beta-lactam agents exhibit time-dependent killing, i.e. the duration of time that serum levels exceed the MIC is the pharmacokinetic/pharmacodynamic parameter that best correlates with in vivo efficacy of the beta-lactams.
Continuous Infusion/Once-Daily Dosing
Continuous infusion of anti-Pseudomonal beta-lactams is theoretically attractive with killing in a time-dependent manner (140,143); however the stability of drug should be considered and this approach remains to be validated in large clinical studies. Strong consideration of once-daily dosing of aminoglycosides, even when in combination therapy, should be considered when used against P. aeruginosa. Aminoglycosides exhibit concentration-dependent bactericidal activity, and also produce prolonged postantibiotic effects. Thus, high drug concentrations kill P. aeruginosa faster than low concentrations, supporting this practice of once daily dosing (SeeTable 4). Whether aminoglycosides need to be continued for the full treatment course is debatable. Aminoglycosides duration may be dictated by potential toxicity and clinical response. Except for treatment of P. aeruginosa infections in the cystic fibrosis patient when combination therapy with beta-lactam antibiotics is standard for the entire duration, most caregivers would discontinue aminoglycosides within a week of their onset.
Due to the increased frequency of multi-drug resistant strains, empiric antibiotic regimens should be based on local resistance patterns with definitive therapy guided by susceptibility testing. Significant geographic variability in fluoroquinolone resistance has been reported, with 55% of urinary isolates resistant to ciprofloxocin in Latin America, 41% in Europe, and 29% in North America (117). Globally, urinary P. aeruginosa isolates were more susceptible to amikacin, carbapenems, cefepime, ceftazidime, and piperacillin, with resistance to tobramycin and gentamycin reported in 26% and 31%, respectively (117).
Special Situations
Urinary Tract Infection
Asymptomatic individuals with indwelling urinary catheters should have an attempt at removal of the catheter without antibiotic treatment. Symptomatic uncomplicated urinary tract infections may be treated with 3 to 5 days of therapy, with removal of the catheter. Therapy is with an oral fluoroquinolone or appropriate intravenous agent for 3-5 days. Urinary tract infections with sepsis should be treated for 10-14 days with a combination beta-lactam and aminoglycoside. Pyelonephritis should be treated for 14-21 days, while a perinephric or intrarenal abscess may require a longer duration of therapy. Prostatitis with P. aeruginosa may require 6 to 12 weeks of oral quinolones. Unfortunately, long-term therapy with quinolones has been associated with the induction of resistance inPseudomonas strains (105).
Community-Acquired Pneumonia (CAP)
Consensus guidelines recommend that since P. aeruginosa as a cause of CAP is rare, empiric therapy for immunocompetent patients does not need to be considered except in special situations (155). Infectious Disease Society of America (IDSA) /American Thoracic Society (ATS) guidelines for CAP only recommend anti-Pseudomonal agents if one or more of the following risk factors are present: chronic oral steroid administration, severe underlying bronchopulmonary disease, hematologic malignancy with neutropenia, hypogammaglobulinemia, HIV/AIDS, alcoholism, or frequent antibiotic therapy (146).
Ventilator-Associated Pneumonia (VAP)
Empiric treatment of VAP should include treatment for P. aeruginosa, recognizing that often it is difficult to distinguish true infection from colonization when cultured from respiratory tract samples of intubated patients. Consideration of colonization versus true infection should take the following into consideration:
- Success may be better if appropriate antimicrobial therapy is initiated initially against P. aeruginosa.
- Patients with a clinical pulmonary infection score (CPIS) of 6 or less with P. aeruginosa isolated from endotracheal aspirates or sputum did not deteriorate with monotherapy even in the face of in vitro resistance of the organism. This is strong circumstantial evidence that P. aeruginosa is a common colonizer of the respiratory tract, for which aggressive combination therapy for prolonged duration is unnecessary (72a, 73a 235, 238). A clinical strategy for VAP, demonstrated that a CPIS of 6 or less for 3 days, is an objective criterion to select patients at low risk for 3 day monotherapy of ICU pneumonia. Antibiotic resistance, superinfection, and 30-day mortality was lower for the 3-day monotherapy group, than for those patients receiving combination therapy of prolonged duration (201, 235). One likely explanation is that pulmonary infiltrates in these patients were not due to infection.
- A large study enrolled 413 patients in a labor-intensive, multicenter, randomized trial of VAP. A non-invasive management strategy was compared to an invasive management strategy of direct examination of bronchoalveolar lavage (BAL) or protected specimen brush specimens with quantitative culture. Patients randomized to the invasive strategy group experienced significantly lower mortality at 14 days, earlier attenuation of organ dysfunction, and decreased antibiotic use as compared to patients randomized to the noninvasive strategy group (63, 65).
- A multi-center, randomized trial comparing the use of BAL with quanitative cultures or endotracheal aspiration with nonquantitative culture for diagnosing VAP, reported no significant difference in the 28-day mortality rate, or the rate of antibiotic modification. The limitations of this study were that the exclusion criteria were strict, especially excluding patients with methicillin-resistant Staphylococcus aureus (MRSA) infection and P. aeruginosa colonization. Therefore, at least 40% of the screened patients who were excluded had risk factors for colonization or infection with potentially multi-drug resistant (MDR) pathogens (30).
Adherence to de-escalation strategy (initiation of broad-spectrum antibiotics, with narrowing/discontinuation of medications after 2-3 days) is a practical strategy, and may be a way to curb the unnecessary use of antibiotics in the intensive care setting (64). However broad-spectrum empiric antibiotic therapy, especially in the ICU, is warranted, since initial therapy is often inadequate for P. aeruginosa pneumonia (7, 102, 126, 176). Frustratingly, P. aeruginosa infection is associated with a high rate of mortality, even among patients who received appropriate antimicrobial therapy (48, 215). In general, it is recommended to use combination therapy empirically rather than monotherapy for VAP until the microbial etiology is unknown.
A number of randomized controlled trials have been performed for study of which combinations are the most appropriate antibiotic treatment of pneumonia:
- Previous data supporting the use of combination therapy for Pseudomonas pneumonia has been derived from bacteremic patients only (101).
- A comparison of the clinical efficacy of piperacillin/tazobactam and amikacin versus ceftazidime and amikacin in patients with VAP, showed that 25% of 170 cases accounted for P. aeruginosa VAP. Clinical cure was observed in 51% of those patients treated with piperacillin/tazobactam and amikacin versus 36% treated with ceftazidime and amikacin. This trend was not statistically significant (24).
- Comparison of cefepime and amikacin versus ceftazidime and amikacin in 275 mechanically ventilated patients with pneumonia, demonstrated superior clinical outcome for cefepime/amikacin-treated patients (53.3%) compared to ceftazidime/amikacin (39.3%) (p= 0.05)(16).
- A study of nosocomial pneumonia compared piperacillin/tazobactam monotherapy with imipenem monotherapy, with 18% of cases due to P. aeruginosa. Clinical success was observed in 83% of patients treated with piperacillin/tazobactam and 71% treated with imipenem. There was a trend towards superiority of piperacillin/tazobactam, although this did not attain statistical significance. However, in the subset of patients with confirmed P. aeruginosa pneumonia, piperacillin/ tazobactam was significantly superior (109).
- Disturbingly, 25% of imipenem treated-patients, in the piperacillin/tazobactam versus imipenem monotherapy study, developed resistance to imipenem during therapy, compared to 5% of piperacillin/tazobactam-treated patients developing resistance to piperacillin/tazobactam during therapy (109). In an earlier study by the same group, combination therapy with imipenem and an aminoglycoside did not prevent emergence of resistance during therapy (42).
- In the largest study of nosocomial pneumonia yet performed, 405 patients with severe, hospital-acquired pneumonia received ciprofloxacin (400mg every 8 hours) compared to imipenem (1 gram every 8 hours) (30). 20% of evaluable patients had pneumonia due to P. aeruginosa. Cure was observed in significantly more patients treated with ciprofloxacin (69%) compared to imipenem (56%) (p= 0.02). 28% of ciprofloxacin-treated patients compared with 50% of imipenem-treated patients developed resistance during treatment to the antibiotic used (70).
- A multi-center, prospective, randomized double-blind trial of 401 patients compared the effectiveness between 8 days versus 15 days of antibiotic therapy for VAP. Patients with VAP caused by nonfermenting gram-negative bacilli, including P. aeruginosa treated 8 days did not have a more unfavorable outcome, however, recurrence of pneumonia was significantly higher in the 8-day group compared to those receiving 15 days of treatment. The authors concluded that 8 days of treatment was sufficient for all patients with VAP. Extreme vigilance be maintained after cessation of antimicrobial therapy especially for those in whom P. aeruginosawas isolated (37).
Development of resistance during therapy is common (32, 99, 215). Increased severity of illness and prior/ recent use of anti-Pseudomonal beta-lactam antibiotics, aminoglycosides, and fluoroquinolones were risk factors for infection by a multi-drug resistant (MDR) P. aeruginosa strain (99, 215). A single center study that investigated 54 cases of VAP with P. aeruginosa, demonstrated 39% isolates being MDR. Mortality rates did not differ between patients with MDR P. aeruginosa and pan-sensitive P. aeruginosa infections, although patients with VAP caused by MDR P. aeruginosa had longer ICU stays and were less likely to receive adequate, initial antibiotic therapy (225).
Given the necessity for adequate antimicrobial severity and the prominent role that P. aeruginosa plays in the ICU, empiric combination antibiotic therapy is often given. A pragmatic approach has been suggested for ICU pneumonia which takes into consideration the clinician's concern for P. aeruginosa as a cause and the appearance of multi-drug resistant (MDR) bacteria resistant to all commercially-available antibiotics (207a). Risk factors for MDR occur because the prior antibiotic use is so ubiquitous in ICU management (235a). This approach depicted in Figure 1 and Figure 2 is derived in part from the approach by Singh et al. (201) and Torres A., Approach to Pulmonary Infiltrates in the ICU Method of Antoni Torres.
Severity of illness is assessed by teh APACHE score or Pitt Bacteremia Score. If the patient is severely ill, empiric antibacterial therapy is given. If not, and the CPIS is less or equal to 6, monotherapy with an antipseudomonal agent is given (201). The CPIS is used as a screening tool to identify patients who can receive short-course monotherapy (Figure 1). Given that colonization is a distinct possibility in a stable patient, the use of the CPIS will minimize the possibility of overtreatment of P. aeruginosa found in an innocent bystander. At the end of three days the patient should be re-evaluated (Figure 2). For patients who were not severely ill at onset and received monotherapy, the antipseudomonal agent can be discontinued if the CPIS remains low and cultures do not confirm the presence of a pathogen.
Inhaled Antibiotics
Inhaled antibiotics in the face of acute or chronic lower respiratory track infection has the theoretical advantage of increasing drug levels in the bronchial secretions, without subjecting the patient to systemic side effects of the drug. Certainly concerns about long-term employment of aerosolized antibiotics include development of resistance to the micro-organisms. Most of the recent experience with inhaled antibiotics and P. aeruginosa infections comes from treatment of chronic infection in cystic fibrosis. Inhaled tobramycin (TOBI®) is given in the dosage of 300 mg vials, administered by nebulized treatment twice a day for 28 days, with 28 days off. This intermittent cycle is then repeated. TOBI® is a preservative-free preparation, developed and FDA-approved exclusively for suppressive, inhalation treatment (171).
This alternating cycle has shown to maintain or improved pulmonary function, is associated with weight gain, as well as diminished need for oral or parenteral antibiotics in adolescent patients with cystic fibrosis over a two year period of long-term intermittent therapy. Frequently patients notice an initial increase in their cough at the beginning of a TOBI® month, as well as a change in their voice (more husky or hoarse-sounding). Inhaled tobramycin has minimal side effects (10% with bronchospasm), decreases the risk of hospitalization and P. aeruginosa density in sputum, and is well tolerated.
Patients requiring tracheotomy tubes and chronic mechanical ventilation are frequently colonized with P. aeruginosa, making intermittent use of TOBI®, a reasonable consideration. The dose of 300 mg twice a day is the same regardless of age or size. Tobramycin levels are not recommended.
A double-blind, placebo-controlled crossover trial with 30 non-cystic fibrosis patients was conducted to determine the clinical effectiveness and safety of 6-month tobramycin inhalation therapy. Pulmonary function and quality of life were unaffected (56). Inhaled tobramycin has been successfully used for severe non-cystic fibrosis bronchiectasis with chronic bronchial P. aeruginosa infections (56,196). Eradication or presumed eradication of P. aeruginosa occurred in 22% (6/27) of evaluable non-cystic fibrosis patients (196). Therefore some patients with non-cystic fibrosis bronchiectasis may benefit from therapy with inhaled tobramycin.
Nebulized colistin (colimycin) also has been used in cystic fibrosis as long-term intermittent therapy, as well as part of combination parenteral therapy for nosocomial pneumonia caused by MDR P. aeruginosa (66). The dose used is 75-150 mg in 2-4 ml of normal saline twice a day. In cystic fibrosis patients, it is cycled every other 28 days, often alternating with TOBI® therapy. The disadvantage of colistin is the lack of a preservative-free form for nebulization (the IV solution is used). Some patients may dislike the foamy, sticky nature of the solution when reconstituted; the solution may be irritating to the airway.
Other antimicrobial agents, using the parenteral solution of the drug, have been attempted with limited success (i.e.gentamicin, ceftazidime) for inhalation. Lack of guidelines as to the frequency and dosage of these drugs are drawbacks to their clinical utility. Another major drawback to use of inhaled ceftazidime is the offensive taste and smell when this drug in placed into a nebulizer for inhalation. Currently a new powdsuer form of tobramycin for dry inhalation is in Phase 3 studies in cystic fibrosis, as well asaztreonam in a solution for inhalation; both drugs are being studied for long-term intermittent treatment of P. aeruginosa infection in cystic fibrosis patients.
Azithromycin
Macrolide antibiotics have recently been recognized to have a stabilizing effect on patients with chronic P. aeruginosainfection. Recent clinical trails in cystic fibrosis patients have demonstrated a benefit of chronic therapy with azithromycin or clarithromycin by maintaining or improving pulmonary function, improving weight gain, and diminishing need for oral or parenteral antibiotics in adolescent patients with cystic fibrosis over a 48-week period of long-term therapy. The benefit of the longer acting macrolide, such as azithromycin, over erythromycin, is less GI-toxicity, and ease of dosing. For patients the dose of either 250 mg (bodyweight < 40 kg) or 500 mg (weight > 40 kg) of oral azithromycin, administered 3 times a week (17, 192).
There is also evidence that chronic azithromycin therapy improves outcome in patients with bronchiolitis obliterans organizing pneumonia, now called cryptogenic organizing pneumonia, and radiation-related bronchiolitis obliterans organizing pneumonia. Macrolides may have anti-inflammatory effects in patients with these syndromes although the mechanism of action is not completely understood at this time (206).
Currently studies are being conducted in cystic fibrosis children without chronic colonization with P. aeruginosa and long-term azithromycin to determine the safety and impact on pulmonary function and time to acquisition of P. aeruginosa chronic infection. Very limited experience exists in the child less than 13 years of age with chronic macrolide therapy.
Endocarditis
Optimal treatment of infective endocarditis from P. aeruginosa involves combined antibiotic and surgical therapy. Infection of the aortic valve is severe, often with an acute and fulminant onset. Sepsis with multiple septic emboli, congestive heart failure, renal failure, and early death is common. Successful outcome requires rapid institution of aggressive antibiotic therapy combined with early replacement of the aortic valve. Despite aggressive medical and surgical therapy, survival is as low as 38%(111). Consideration for treatment of P. aeruginosa infective endocarditis should include:
- An aminoglycoside combined with an anti-Pseudomonal beta-lactam as the regimen of choice for most cases of infective endocarditis
- Maintenance of serum concentrations of beta-lactam agents above the MIC for the organism for longer periods of time, such as by use of continuous infusions to reduce the development of resistance (67)
- Concomitant aminoglycosides should be aggressively dosed (See Table 4) (208)
- Consideration of once-daily aminoglycoside dosing, due to theoretical advantages over a multiple dosing regimen
- Recognition that ototoxicity and nephrotoxicity are common during therapy with aminoglycosides, with the latter usually reversing with discontinuation of the drug. In patients with preexisting renal compromise, total aminoglycoside dosage and dosing intervals must be adjusted on the basis of serum concentrations
- Antibiotic therapy alone may have significantly higher mortality than those who also underwent valvulectomy (226). Thus, recommendations for left-sided infection include valve replacement plus a 6-week course of high-dose combined beta-lactam and aminoglycoside antimicrobials (127, 178, 226)
- Aggressive antibiotic therapy combined with surgical resection of the valve appears to be optimal therapy for infective endocarditis. Antibiotic therapy alone may have significantly higher mortality than those who also underwent valvulectomy.
- Theoretical pharmacodynamic considerations would dictate that continuous infusion of beta-lactam antibiotics (time-kill dependent) and once-daily aminoglycoside, as opposed to multiple–dosing (concentration-dependent killing) is preferred.
- Colistin/Polymyxin B is a therapeutic option for multidrug resistant P. aeruginosa. The parenteral toxicities and high failure rates associated with these compounds may limit their utility(78, 135, 164). An anecdotal report found that meropenem plus tobramycin was successful after failure with ceftazidime and piperacillin-tazobactam combined with tobramycin (80).
Meningitis
Antimicrobial choice for treatment of gram-negative meningitis must take into consideration penetration of the drug into the cerebrospinal fluid (CSF). Ceftazidime has been widely used because of its in vitro activity and ability to penetrate the CSF (74).Cefepime has been used in the management of non-pseudomonal gram-negative organisms causing meningitis (182), but no published cases exist of treatment of P. aeruginosa meningitis with this antibiotic. Carbapenems, with excellent in vitro activity against P. aeruginosa and ability to penetrate into the cerebrospinal fluid, are a useful alternative, especially when resistant organisms are present. However, the high doses of imipenem required may be associated with central nervous system toxicity, most notably seizures.Meropenem reaches high concentrations in the cerebrospinal fluid and has been successful in treating P. aeruginosa meningitis (39, 160,163). Meropenem should be considered the drug of choice in P. aeruginosa meningitis, in combination with aminoglycosides (intraventricular and/or intravenous) (45) (31).
Addition of an aminoglycoside can be justified on the basis of synergistic interaction or prevention of ceftazidime resistance. Anecdotal reports, however, have not clearly shown enhanced efficacy with addition of an aminoglycoside, due to poor penetration of the aminoglycosides into the CSF. Thus, intrathecal or intraventricular, as well as intravenous administration of aminoglycosides may be required for maximal effect. For dosage recommendations, see Table 5.
Ciprofloxacin has been successfully used in anecdotal reports (107, 152, 197, 231). However, given its relatively poor penetration into the CSF, ciprofloxacin usage should be confined to situations involving life-threatening beta-lactam drug allergies or the presence of documented beta-lactam-resistant organisms (156). In one case of recurrent meningitis, a ciprofloxacin dose of 800 mg every 8 h (49 mg/kg/day) was used successfully, achieving CSF concentration of 2.6 mcg/mL, while the MIC for this MDR organism was 0.5 to 1.0 mcg/mL(231). An additional case of Pseudomonas meningitis was treated with ciprofloxocin 400 mg intravenously every 8 hours in combination with ceftazidime, with peak serum and CSF ciprofloxocin concentrations of 10.29 µg/mL and 0.9 µg/mL, respectively, during treatment (138). These data suggest that higher intravenous doses of ciprofloxocin may be appropriate in pseudomonas meningitis.
Intraventricular Antimicrobial Administration
Direct installation of antimicrobial agents into the ventricles through either an external ventriculostomy or shunt reservoir is occasionally necessary in patients who have shunt infections that are difficult to eradicate or who cannot undergo the surgical components of therapy (See Table 4). No antimicrobial agent has been approved by the US Food and Drug Administration (FDA) for intraventricular use. Antimicrobial dosages have been used with dosing intervals based on the ability of the agent to achieve adequate CNS concentrations (217).
Ocular Infections
Eye infections with P. aeruginosa involve close collaboration with an ophthalmologist. Therapy frequently includes vitrectomy, topical fluoroquinolones, and intravitreal antibiotics, in addition to systemic antibiotics.
Corneal Ulceration and Keratitis
P. aeruginosa is a common cause of corneal ulceration, usually in contact lens wearers. The quinolones have proven useful for this infection (124, 216). Although ciprofloxacin is more active in vitro, ofloxacin has greater ocular tissue penetration. Fortification of the commercial preparation (0.3%, 3.0 mg/mL) of ofloxacin is possible by using the intravenous preparation (ciprofloxacin is near its saturation limit with the commercial preparation) (21, 237). Increasing quinolone resistance for P. aeruginosa is occurring (79, 128, 194), although the concentration of ciprofloxacin in eye drops far exceeds the in vitro MICs of the resistant P. aeruginosa (142).
Alternative therapy includes use of topical ticarcillin, piperacillin or aminoglycosides. Topical tobramycin is often preferred over gentamicin and amikacin because of superiority in vitro (which should be confirmed in the laboratory). Amikacin can be used for strains resistant in vitro to tobramycin. Both tobramycin and gentamicin are commercially available as a 0.3% solution (3 mg/mL). Fortification with the intravenous preparation (40 mg/mL) to 13.6 mg/mL may be advantageous because of increased diffusion into the tissues and anterior chamber. Multiple and frequent instillation topically is recommended for severe infections, especially for initial therapy (e.g., every 15-30 min). As an alternative, a collagen shield reconstituted with 0.3% tobramycin and placed onto the eye releases antibiotic over several hours (237).
Endophthalmitis
Endophthalmitis caused by P. aeruginosa is a medical emergency. The course of pseudomonas endophthalmitis is typically fulminant and is characterized by intense pain. Patients have decreased visual acuity, with the findings of panophthalmitis, including conjunctivitis with chemosis, lid edema, and anterior uveitis, developing over hours. Endophthalmitis is diagnosed clinically by the presence of these symptoms and the absence of a red reflex. The clinical diagnosis should be confirmed by aspiration of the anterior chamber or vitreous with cultures.
Seventy to eighty percent of these patients develop blindness. If treatment is delayed, return of retinal function is usually poor. Systemic therapy is required in addition to ocular administration. Ciprofloxacin, ceftazidime, and imipenem have better intraocular penetration than aminoglycosides. Oral levofloxocin may not achieve MIC 90 levels for P. aeruginosa in the aqueous and vitreous humor even if given at 500 mg twice daily (71). Direct injection of anti-Pseudomonal antibiotics into the anterior chamber and vitreous cavity is usually necessary. Retinal necrosis may occur if antibiotic concentrations are excessive.
A prospective trial of 30 patients received 2 gram IV dose of meropenem prior to undergoing cataract surgery; thirty minutes after administration, these patients were found to have a mean aqueous humor levels of 13.4 mg/L and mean vitreous levels of 8.94 mg/L. Twelve hours later, the concentrations remained above 1 mg/L, suggesting that meropenem may be an alternative therapeutic option for Pseudomonas endophthalmitis (195).
Ear Infection
Perichondritis of the Ear
Removal of the earring, and debridement, along with appropriate anti-Pseudomonal antibiotic (i.e.oral fluoroquinolones) should be promptly initiated (122).
Otitis Externa
Simple otitis externa can be treated adequately with local therapy. Crusts, scales, and debris must be evacuated from the ear canal. Wicks are commonly used. Topical antibiotics active in vitro against P. aeruginosa such as colistin sulfate, neomycin sulfate, aminoglycosides, and ciprofloxacin are preferred. With increased use of topical ciprofloxacin treatments, ciprofloxacin-resistantP. aeruginosa is emerging (113). A double blind trial found no significant difference between treatment outcomes in patients given ofloxacin drops (0.3%) twice daily in comparison to Cortisporin (neomycin sulfate, polymyxin B sulfate, and hydrocortisone) otic drops four times daily. Ofloxacin otic solution (0.3%) used once daily for 7 days was highly effective; adherence to this regimen was 98%(198). Topical endogenous antiseptic N-chlorotaurine for acute otitis externa appeared to be more effective than topical Cortisporin (158).
Alternative treatment with topical povidone-iodine in chronic suppurative otitis media was as effective as topical ciprofloxacin, with the advantage of preventing drug resistance (114). A double-blind study found that antiseptic drops (aluminum acetate), which were inactive in vitro against P. aeruginosa, were as effective as topical gentamicin (40).
Malignant External Otitis
Malignant external otitis can be life-threatening, necessitating aggressive IV therapy. A retrospective study of 22 cases of malignant otitis secondary to Pseudomonas found a cure rate of 95% in patient treated with parenteral combination of anti-Pseudomonal penicillins with aminoglycosides as well as surgical debridement. Topical antibiotics, even fluoroquinolones, are ineffective.
Systemic fluoroquinolone antibiotics, especially ciprofloxacin, have emerged as the treatment of choice for malignant external otitis. However with widespread use of both oral and topical quinolones, the emergence of ciprofloxacin resistance in P. aeruginosa is becoming a major problem (183). When the P. aeruginosa is sensitive, oral ciprofloxacin therapy (750 mg twice daily) appears to be as effective as parenteral combination anti-Pseudomonal therapy (84, 184, 185). Duration should be 6 to 10 weeks, depending on the severity of infection.
The erythrocyte sedimentation rate (ESR) is useful in monitoring responsiveness to therapy. Hyperbaric oxygen has been used in anecdotal reports, but efficacy is uncertain(53). Surgery is rarely necessary except for local debridement or excision of accessible foci of infection such as polypectomy or removal of sequestrum (183).
Skin and Soft Tissue Infections
Hot Tub Folliculitis
Folliculitis is often a mild and self-limiting illness. In healthy persons, lesions resolve spontaneously and scarring is rare, necessitating no treatment. Topical 0.1% polymyxin B has been useful, or a short course (5-7 days) of oral ciprofloxacin. Treatment involves cleaning and debriding the infected skin and avoiding wetness. Therapy with dilute acetic acid can successfully suppress P. aeruginosa.
Puncture Wounds/Osteomyelitis
Antibiotic therapy with adjunctive surgical debridement is the current standard. Most cases of P. aeruginosa osteochondritis/osteomyelitis can be treated with single-antibiotic therapy consisting of a beta-lactam or fluoroquinolone (52,54). In children and adolescents with puncture wounds, incision and drainage, along with 2-3 weeks of anti-Pseudomonal therapy is adequate.
Immunosuppressed patients with associated P. aeruginosa bacteremia should receive combination therapy with an aminoglycoside. In the absence of vascular compromise or diabetes mellitus, a 4-week course of antibiotics may be adequate, dating from initiation of therapy or from the last major surgical debridement (112). In chronic osteomyelitis, in which prolonged therapy is needed, oral ciprofloxacin can play a major role as single-agent therapy. This should follow adequate debridement, hardware removal, and a course of 4 to 6 weeks of parenteral therapy (4, 11). The cure rate approached 70% in 23 patients with chronic pseudomonas osteomyelitis treated with oral ciprofloxocin; however, resistance to ciprofloxocin was noted to emerge in nearly 18% (77). The future usefulness of oral monotherapy will be limited by the extent of emergence of resistant organisms (4, 11).
Foot Infections
Treatment involves cleaning and debriding the infected skin and avoiding moisture. Therapy with diluted acetic acid can suppress P. aeruginosa.
Green Nail Syndrome
The patient should be advised to avoid excessive immersion in hot water, even when wearing protective gloves. After washing, the nails should be dried thoroughly, with the use of a hair dryer to keep the nail plate-nail bed space as dry as possible. Trimming of the nail, until the nail reattaches, should be repeated frequently for 4-6 weeks. Treatment of the underlying fungal infection is indicated. Other treatments include: Clorox diluted 1:4 two to three times a day to the space to suppress the growth of P. aeruginosa, applying acetic acid in 50% alcohol, 4% thymol in chloroform, or 15% sulphacetamide in 70 % ethyl alcohol; or ciprofloxacin (500 to 750 mg bid, orally) for 4-6 weeks has been found to be effective in treating this infection (93).
Ecythma Gangrenosum/Necrotizing Fasciitis
Combination anti-Pseudomonal antibiotic therapy is necessary. Leukocyte transfusions or colony stimulating factors are often used. Mortality is high, even with aggressive antibiotic therapy and surgical resection. Initial therapy should consist of parenteral anti-Pseudomonal therapy. Long-term anti-Pseudomonal therapy (for example, oral ciprofloxacin) may be necessary for cure.
Burns
Topical agents are frequently used to prevent infection of burn wounds, including silver sulfadiazine and mafenide (Sulfanylon). Outbreaks of sulfadiazine-resistant organisms have occurred in burn units with its heavy usage. When topical agents are used prophylactically, there may be a delay in P. aeruginosa colonization for more than 7 days. Mafemide has superior eschar penetration compared to silver sulfadiazine. Early and frequent debridement of necrotic tissue and excision of infected burn wounds is probably more important than topical therapy in preventing infection. Bacteriophages have been tried (see Adjunctive Therapy section).
Patients with overt infection should be treated aggressively with combination IV antibiotics: an aminoglycoside and a beta-lactam. Antibiotic susceptibility testing is critical for antibiotic choice, since nosocomial strains may be multiply resistant. Patients with significant burns have dramatic alterations in pharmacokinetics of most drugs. The risk in most patients may be under treatment rather than antibiotic toxicity (236). The applicability of once daily dosing of aminoglycosides in burns patients is unknown, but is possibly advantageous. Individualized pharmacokinetic dosing with monitoring of aminoglycoside serum concentrations is recommended.
ADJUNCTIVE THERAPY
Prompt removal of infected intravenous catheters or other hardware such as a ventriculoperitoneal shunt or ear piercing, should be performed, whenever possible. In addition incision and drainage of abscesses, as well as debridement of soft tissue should be performed. Debridement of the bony involvement in a puncture wound of the foot is necessary for resolution of the osteochondritis infection.
Bacteriophages have been advocated as a potential topical application of treatment for post-burn P. aeruginosa infection (5,202). A variety of phages are highly specific for P. aeruginosa (UNL-1, ACQ, UT1). A potential benefit of phage therapy is the lack of potential toxic effects, as well as diminished cost compared to systemic therapy. Limited clinical studies have been performed on this approach to therapy (1), with a clear need for further exploration of this therapy (207).
ENDPOINTS FOR MONITORING THERAPY
Bacteremia due to P. aeruginosa is associated with a high mortality rate compared to bacteremia due to other gram-negative bacilli and gram-positive organisms. Risk factors for mortality include severe sepsis, pneumonia, and a delay in starting effective antimicrobial therapy. The choice and timing of antibiotic therapy is particularly crucial. As an example, in one study of 410 episodes ofP. aeruginosa bacteremia, cure was substantially higher in patients receiving appropriate antibiotics than in those who did not (67% versus 14%), and a one- to two-day delay in administering appropriate antibiotics reduced the cure rate from 74% to 46%. The prognosis of P. aeruginosa bacteremia is closely related to the underlying condition of the host and the primary site of infection (106). In a patient with primary or secondary bacteremia, blood cultures should become negative. For urinary tract infection, urine culture should become negative. In a patient with P. aeruginosa meningitis, a repeat spinal tap after 48 to 72 hours may be helpful to document microbiologic clearance. The duration of therapy after an initial favorable clinical response is generally empiric. Bacteremia and urinary tract infections require at least 10 days of therapy. Meningitis should be treated for 21 days, and endocarditis for at least 42 days.
The goal for most therapy is a curative course of antibiotics for P. aeruginosa, with the exception of chronic infection such as occurs in cystic fibrosis. Demonstration of sterilization of cultures, resolution of pain, soft tissue swelling, and erythema are all clinical features to follow in patients with P. aeruginosa infections. In cystic fibrosis, a course of systemic antibiotics will reduce the bacterial burden of chronic infection with P. aeruginosa and use of other measurements to determine response are used (i.e. pulmonary function tests, appetite and weight gain, improved cough and diminished sputum production). Long-term suppressive therapy with inhaled TOBI and/or azithromycin should be considered for all cystic fibrosis patients with chronic infection by P. aeruginosa.
The endpoint for monitoring therapy of P. aeruginosa pneumonia has usually been documented as clinical, radiological and microbiologic clearance of signs of infection. Persistent endotracheal colonization frequently occurs despite clinical response (171). Currently, duration of therapy of 14-21 days is suggested for P. aeruginosa pneumonia. However, if clinical criteria were used (and ongoing colonization ignored), a duration of 8 days would appear reasonable (37).
VACCINES
Interest in a vaccine to prevent infection in susceptible hosts is tantalizing, especially in the care of patients with cystic fibrosis. At present, no vaccine is commercially available, but development of vaccines against type II secretion system proteins, as well as LPS, is ongoing. P. aeruginosa virulence associated and cellular factors have been suggested as antigens for immunotherapy (103).
INFECTION CONTROL MEASURES
In general P. aeruginosa bacteria are ubiquitous, i.e. being present commonly in the home, clinic and hospital environments. The bacterium is a difficult organism to eradicate from areas that become contaminated, such as operating rooms, hospital rooms, clinics, and medical equipment. In a hospital room occupied by a patient with a known infection from P. aeruginosa, within a matter of days, the same strain of the bacteria can be found in the sink drain and faucet, the shower stall, toilet and on surfaces in the hospital room. Bars of soap can become contaminated with P. aeruginosa, as can refillable containers of liquid soap.
Nosocomial spread of bacteria is frequently by hands, including P. aeruginosa. Bacterial hand counts are higher with rings; long fingernails and artificial fingernails are associated with higher gram-negative bacterial hand contamination. Education of hospital and all medical personnel on proper hand hygiene is vital for successful infection control of P. aeruginosa (22, 123). Those who enter the room of a patient colonized with a MDR organism should wear gloves and gowns and practice appropriate hand hygiene on leaving the patient’s room and removal of the protective apparel. However, patient to patient transmission of multiply resistant P. aeruginosaisolates does occur (132, 164). In one investigation, three P. aeruginosa strains accounted for nearly half of all MDR isolates in 5 different hospitals (132).
Molecular epidemiologic techniques (i.e. pulsed field gel electrophoresis) should be applied to investigate apparent outbreaks of infection. A search for a common environmental source should be undertaken. Contact isolation precaution measures should be used as a mode of control of spread of such organisms if clonality is confirmed and no environmental source is found. Such an approach requires the identification of asymptomatic carriers of the organism and then accommodation of such individuals in single rooms or cohorting with other colonized patients.
Restriction of use of anti-Pseudomonal antibiotics should also be considered to reduce selective pressure leading to mutations contributing to multidrug resistance. Cycling of antimicrobial agents used for empiric therapy has been attempted with some success in hospital intensive care units (6, 94, 95, 125, 154, 172, 222), while more recent studies showed that cycling of antimicrobial agents did not control the emergence of gram-negative antimicrobial resistant organisms.
Steam sterilization is the preferred method for preprocessing heat-stable medical devices. However manual cleaning to remove biological material is a necessary first step in reprocessing any medical device. Disinfection and sterilization protocols do not work effectively on visibly soiled surfaces. The practice of rinsing equipment in tap water after preprocessing may contaminate a device. P. aeruginosa may colonize the tap water, and has the ability to form biofilms on medical devices that may be difficult to remove (33). Patients with infections with P. aeruginosa should receive Standard Transmission Precautions in the clinic and hospital setting, with Contact Isolation for a patient with MDR- P. aeruginosa infection. Recommendations for good infection control practices for P. aeruginosa include the following:
- Perform proper hand hygiene
- Use hand soap from non-reusable containers; no bar soap
- Limit fingernail length, and artificial fingernails or nail extenders in medical/ hospital personnel
- Place all cystic fibrosis patients in a private room that does not share common facilities (i.e. bathroom or shower)
- Avoid direct contact between cystic fibrosis-patients in the hospital unless they are co-habitants at home
- Follow published recommendations for sterilization and disinfection of patient care equipment
- Use sterile or 0.2 micron filtered (not distilled or tap) water for rinsing disposable devices after they have been chemically disinfected. Dry this equipment after rinsing
- Use a one-step process and an EPA-registered hospital grade disinfectant/detergent designed for housekeeping environmental surfaces (22, 33, 193)
- Limit broad spectrum antibiotic usage.
REFERENCES
1. Abdul-Hassan H, El-Tahan E, Massoud B, Gomaa R. Bacteriopharge therapy of Pseudomonas burn wound sepsis. Ann Medit Burn Club 1990;3:262-264.
2. Adamo R, Sokol S, Soong G, Gomez MI, Prince A. Pseudomonas aeruginosa flagella activate airway epithelial cells through asialoGM1 and toll-like receptor 2 as well as toll-like receptor 5. Am J Respir Cell Mol Biol 2004;30(5):627-34. [PubMed]
3. Afessa B, Green B. Bacterial pneumonia in hospitalized patients with HIV infection: the Pulmonary Complications, ICU Support, and Prognostic Factors of Hospitalized Patients with HIV (PIP) Study. Chest 2000;117(4):1017-22. [PubMed]
4. Aguado J, Arjona R, Valle R, Moreno J. Prolonged oral ciprofloxacin treatment of recalcitrant and severe osteomyelitis [Abstract 980]. 30th Interscience Conference on Antimicrobial Agents and Chemotherapy, Atranta.
5. Ahmad SI. Treatment of post-burns bacterial infections by bacteriophages, specifically ubiquitous Pseudomonas spp. notoriously resistant to antibiotics. Med Hypotheses 2002;58(4):327-31. [PubMed]
6. Allegranzi B, Luzzati R, Luzzani A, Girardini F, Antozzi L, Raiteri R, et al. Impact of antibiotic changes in empirical therapy on antimicrobial resistance in intensive care unit-acquired infections. J Hosp Infect 2002;52(2):136-40. [PubMed]
7. Alvarez-Lerma F. Modification of empiric antibiotic treatment in patients with pneumonia acquired in the intensive care unit. ICU-Acquired Pneumonia Study Group. Intensive Care Med 1996;22(5):387-94. [PubMed]
8. Apple J, Hunt JL, Wait M, Purdue G. Delayed presentations of aortic valve endocarditis in patients with thermal injury. J Trauma 2002;52(2):406-9. [PubMed]
9. Arancibia F, Bauer TT, Ewig S, Mensa J, Gonzalez J, Niederman MS, et al. Community-acquired pneumonia due to gram-negative bacteria and pseudomonas aeruginosa: incidence, risk, and prognosis. Arch Intern Med 2002;162(16):1849-58. [PubMed]
10. Backhed F, Normark S, Schweda EK, Oscarson S, Richter-Dahlfors A. Structural requirements for TLR4-mediated LPS signalling: a biological role for LPS modifications. Microbes Infect 2003;5(12):1057-63. [PubMed]
11. Ball P. Emergent resistance to ciprofloxacin amongst Pseudomonas aeruginosa and Staphylococcus aureus: clinical significance and therapeutic approaches. J Antimicrob Chemother 1990;26 Suppl F:165-79. [PubMed]
12. Baltch A, Smith R. Pseudomonas aeruginosa: infections and treatment. New York, NY: Marcel Dekker; 1994.
13. Battikhi MN, Ammar SI. Otitis externa infection in Jordan. Clinical and microbiological features. Saudi Med J 2004;25(9):1199-203.[PubMed]
14. Bayer AS, Hirano L, Yih J. Development of beta-lactam resistance and increased quinolone MICs during therapy of experimental Pseudomonas aeruginosa endocarditis. Antimicrob Agents Chemother 1988;32(2):231-5. [PubMed]
15. Bayer AS, Peters J, Parr TR, Jr., Chan L, Hancock RE. Role of beta-lactamase in in vivo development of ceftazidime resistance in experimental Pseudomonas aeruginosa endocarditis. Antimicrob Agents Chemother 1987;31(2):253-8. [PubMed]
16. Beaucaire G, Nicolas MH, Martin C, Offenstadt G, Philippon A, Holzapfel L, et al. [Phare study. Comparative study of combined cefepime-amikacin versus ceftazidime combined with amikacin in the treatment of nosocomial pneumonias in ventilated patients. Multicenter group study]. Ann Fr Anesth Reanim 1999;18(2):186-95. [PubMed]
17. Bell SC, Senini SL, McCormack JG. Macrolides in cystic fibrosis. Chron Respir Dis 2005;2(2):85-98. [PubMed]
18. Bhat S, Fujitani S, Potoski BA, Capitano B, Linden PK, Shutt K, et al. Pseudomonas aeruginosa infections in the Intensive Care Unit: can the adequacy of empirical beta-lactam antibiotic therapy be improved? Int J Antimicrob Agents 2007;30(5):458-62. [PubMed]
19. Bjarnsholt T, Jensen PO, Burmolle M, Hentzer M, Haagensen JA, Hougen HP, et al. Pseudomonas aeruginosa tolerance to tobramycin, hydrogen peroxide and polymorphonuclear leukocytes is quorum-sensing dependent. Microbiology 2005;151(Pt 2):373-83. [PubMed]
20. Bodmann KF. Current guidelines for the treatment of severe pneumonia and sepsis. Chemotherapy 2005;51(5):227-33. [PubMed]
21. Bowe BE, Snyder JW, Eiferman RA. An in vitro study of the potency and stability of fortified ophthalmic antibiotic preparations. Am J Ophthalmol 1991;111(6):686-9. [PubMed]
22. Boyce JM, Pittet D. Guideline for Hand Hygiene in Health-Care Settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Society for Healthcare Epidemiology of America/Association for Professionals in Infection Control/Infectious Diseases Society of America. MMWR Recomm Rep 2002;51(RR-16):1-45, quiz CE1-4. [PubMed]
23. Boyle EM, Ainsworth JR, Levin AV, Campbell AN, Watkinson M. Ophthalmic Pseudomonas infection in infancy. Arch Dis Child Fetal Neonatal. 2001;85(2):F139-40. [PubMed]
24. Brun-Buisson C, Sollet JP, Schweich H, Briere S, Petit C. Treatment of ventilator-associated pneumonia with piperacillin-tazobactam/amikacin versus ceftazidime/amikacin: a multicenter, randomized controlled trial. VAP Study Group. Clin Infect Dis 1998;26(2):346-54. [PubMed]
25. Bui KQ, Banevicius MA, Nightingale CH, Quintiliani R, Nicolau DP. In vitro and in vivo influence of adjunct clarithromycin on the treatment of mucoid Pseudomonas aeruginosa. J Antimicrob Chemother 2000;45(1):57-62. [PubMed]
26. Burgess DS, Hall RG, Hardin TC. In vitro evaluation of the activity of two doses of Levofloxacin alone and in combination with other agents against Pseudomonas aeruginosa. Diagn Microbiol Infect Dis 2003;46(2):131-7. [PubMed]
27. Burgess DS, Hastings RW. Activity of piperacillin/tazobactam in combination with amikacin, ciprofloxacin, and trovafloxacin against Pseudomonas aeruginosa by time-kill. Diagn Microbiol Infect Dis 2000;38(1):37-41. [PubMed]
28. Burns JL, Emerson J, Stapp JR, Yim DL, Krzewinski J, Louden L, et al. Microbiology of sputum from patients at cystic fibrosis centers in the United States. Clin Infect Dis 1998;27(1):158-63. [PubMed]
29. Burns JL, Van Dalfsen JM, Shawar RM, Otto KL, Garber RL, Quan JM, et al. Effect of chronic intermittent administration of inhaled tobramycin on respiratory microbial flora in patients with cystic fibrosis. J Infect Dis 1999;179(5):1190-6. [PubMed]
30. Canadian CCTG. A randomized trial of diagnostic techniques for ventilator-associated pneumonia. N Engl J Med 2006;355(25):2619-30. [PubMed]
31. Capitano B, Nicolau DP, Potoski BA, Byers KE, Horowitz M, Venkataramanan R, et al. Meropenem administered as a prolonged infusion to treat serious gram-negative central nervous system infections. Pharmacotherapy 2004;24(6):803-7. [PubMed]
32. Carmeli Y, Troillet N, Eliopoulos GM, Samore MH. Emergence of antibiotic-resistant Pseudomonas aeruginosa: comparison of risks associated with different antipseudomonal agents. Antimicrob Agents Chemother 1999;43(6):1379-82. [PubMed]
33. Centers for Disease Control and Prevention. Infection Control Practices Advisory Committee. In: Chicago, IL; American Society for Healthcare; 2004.
34. Centers for Disease Control and Prevention. Pseudomonas aeruginosa infections associated with transrectal ultrasound-guided prostate biopsies--Georgia, 2005. MMWR Morb Mortal Wkly Rep 2006;55(28):776-7. [PubMed]
35. Centers for Disease Control and Prevention. Pseudomonas dermatitis/folliculitis associated with pools and hot tubs--Colorado and Maine, 1999-2000. MMWR Morb Mortal Wkly Rep 2000;49(48):1087-91. [PubMed]
36. Chang WN, Lu CH, Huang CR, Chuang YC. Mixed infection in adult bacterial meningitis. Infection 2000;28(1):8-12. [PubMed]
37. Chastre J, Wolff M, Fagon JY, Chevret S, Thomas F, Wermert D, et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. Jama 2003;290(19):2588-98. [PubMed]
38. Cheng BC, Chang WN, Lu CH, Chen JB, Chang CS, Lee CH, et al. Bacterial meningitis in hemodialyzed patients. J Nephrol 2004;17(2):236-41. [PubMed]
39. Chmelik V, Gutvirth J. Meropenem treatment of post-traumatic meningitis due to Pseudomonas aeruginosa. J Antimicrob Chemother 1993;32(6):922-3. [PubMed]
40. Clayton MI, Osborne JE, Rutherford D, Rivron RP. A double-blind, randomized, prospective trial of a topical antiseptic versus a topical antibiotic in the treatment of otorrhoea. Clin Otolaryngol Allied Sci 1990;15(1):7-10. [PubMed]
41. CLSI. Clinical and Laboratory Standards Institute. Seventh ed: Approved Standard; 2006.
42. Cometta A, Baumgartner JD, Lew D, Zimmerli W, Pittet D, Chopart P, et al. Prospective randomized comparison of imipenem monotherapy with imipenem plus netilmicin for treatment of severe infections in nonneutropenic patients. Antimicrob Agents Chemother 1994;38(6):1309-13. [PubMed]
43. Committee on Infectious Diseases. The use of systemic fluoroquinolones. Pediatrics 2006;118(3):1287-92.
44. Conway SP, Etherington C, Munday J, Goldman MH, Strong JJ, Wootton M. Safety and tolerability of bolus intravenous colistin in acute respiratory exacerbations in adults with cystic fibrosis. Ann Pharmacother 2000;34(11):1238-42. [PubMed]
45. Corpus KA, Weber KB, Zimmerman CR. Intrathecal amikacin for the treatment of pseudomonal meningitis. Ann Pharmacother 2004;38(6):992-5. [PubMed]
46. Craig W, Ebert S. Antimicrobial therapy in Pseudomonas aeruginosa infections. New York, NY: Marcel Dekker; 1994.
47. Crnich CJ, Gordon B, Andes D. Hot tub-associated necrotizing pneumonia due to Pseudomonas aeruginosa. Clin Infect Dis 2003;36(3):e55-7. [PubMed]
48. Crouch Brewer S, Wunderink RG, Jones CB, Leeper KV, Jr. Ventilator-associated pneumonia due to Pseudomonas aeruginosa. Chest 1996;109(4):1019-29. [PubMed]
49. Cruciani M, Malena M, Amalfitano G, Monti P, Bonomi L. Molecular epidemiology in a cluster of cases of postoperative Pseudomonas aeruginosa endophthalmitis. Clin Infect Dis 1998;26(2):330-3. [PubMed]
50. Cystic Fibrosis Foundation. Cystic Fibrosis Foundation. Seventh ed. Bethesda, MD: Approved Standard; 1994.
51. Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry 2006 Annual Data Report. Seventh ed. Bethesda, MD: Approved Standard; 2007.
52. Dan M, Siegman-Igra Y, Pitlik S, Raz R. Oral ciprofloxacin treatment of Pseudomonas aeruginosa osteomyelitis. Antimicrob Agents Chemother 1990;34(5):849-52. [PubMed]
53. Davis JC, Gates GA, Lerner C, Davis MG, Jr., Mader JT, Dinesman A. Adjuvant hyperbaric oxygen in malignant external otitis. Arch Otolaryngol Head Neck Surg 1992;118(1):89-93. [PubMed]
54. Dellamonica P, Bernard E, Etesse H, Garraffo R, Drugeon HB. Evaluation of pefloxacin, ofloxacin and ciprofloxacin in the treatment of thirty-nine cases of chronic osteomyelitis. Eur J Clin Microbiol Infect Dis 1989;8(12):1024-30. [PubMed]
55. Demko CA, Byard PJ, Davis PB. Gender differences in cystic fibrosis: Pseudomonas aeruginosa infection. J Clin Epidemiol 1995;48(8):1041-9. [PubMed]
56. Drobnic ME, Sune P, Montoro JB, Ferrer A, Orriols R. Inhaled tobramycin in non-cystic fibrosis patients with bronchiectasis and chronic bronchial infection with Pseudomonas aeruginosa. Ann Pharmacother 2005;39(1):39-44. [PubMed]
57. El-Solh AA, Sikka P, Ramadan F, Davies J. Etiology of severe pneumonia in the very elderly. Am J Respir Crit Care Med 2001;163(3 Pt 1):645-51. [PubMed]
58. El Amari EB, Chamot E, Auckenthaler R, Pechere JC, Van Delden C. Influence of previous exposure to antibiotic therapy on the susceptibility pattern of Pseudomonas aeruginosa bacteremic isolates. Clin Infect Dis 2001;33(11):1859-64. [PubMed]
59. Engleberg NC dV, Dermondy TS. Fourth ed: Lippincott Williams & Wilkins; 2007.
60. Erdem I, Kaynar-Tascioglu J, Kaya B, Goktas P. The comparison of in the vitro effect of imipenem or meropenem combined with ciprofloxacin or levofloxacin against multidrug-resistant Pseudomonas aeruginosa strains. Int J Antimicrob Agents 2002;20(5):384-6.[PubMed]
61. Erdem I, Kucukercan M, Ceran N. In vitro activity of combination therapy with cefepime, piperacillin-tazobactam, or meropenem with ciprofloxacin against multidrug-resistant Pseudomonas aeruginosa strains. Chemotherapy 2003;49(6):294-7. [PubMed]
62. Evans ME, Feola DJ, Rapp RP. Polymyxin B sulfate and colistin: old antibiotics for emerging multiresistant gram-negative bacteria. Ann Pharmacother 1999;33(9):960-7. [PubMed]
63. Fagon JY, Chastre J, Domart Y, Trouillet JL, Gibert C. Mortality due to ventilator-associated pneumonia or colonization with Pseudomonas or Acinetobacter species: assessment by quantitative culture of samples obtained by a protected specimen brush. Clin Infect Dis 1996;23(3):538-42. [PubMed]
64. Fagon JY, Chastre J, Rouby JJ. Is bronchoalveolar lavage with quantitative cultures a useful tool for diagnosing ventilator-associated pneumonia? Crit Care 2007;11(2):123. [PubMed]
65. Fagon JY, Chastre J, Wolff M, Gervais C, Parer-Aubas S, Stephan F, et al. Invasive and noninvasive strategies for management of suspected ventilator-associated pneumonia. A randomized trial. Ann Intern Med 2000;132(8):621-30. [PubMed]
66. Falagas ME, Kasiakou SK. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin Infect Dis 2005;40(9):1333-41. [PubMed]
67. Fantin B, Farinotti R, Thabaut A, Carbon C. Conditions for the emergence of resistance to cefpirome and ceftazidime in experimental endocarditis due to Pseudomonas aeruginosa. J Antimicrob Chemother 1994;33(3):563-9. [PubMed]
68. Favero MS, Carson LA, Bond WW, Petersen NJ. Pseudomonas aeruginosa: growth in distilled water from hospitals. Science 1971;173(999):836-8. [PubMed]
69. Feng X, Zhao G, Cui L, Guan X, Wang H. [Interaction between biofilm formed by Pseudomonas aeruginosa and antibacterial agents]. Wei Sheng Wu Xue Bao 2000;40(2):210-3. [PubMed]
70. Fink MP, Snydman DR, Niederman MS, Leeper KV, Jr., Johnson RH, Heard SO, et al. Treatment of severe pneumonia in hospitalized patients: results of a multicenter, randomized, double-blind trial comparing intravenous ciprofloxacin with imipenem-cilastatin. The Severe Pneumonia Study Group. Antimicrob Agents Chemother 1994;38(3):547-57. [PubMed]
71. Fiscella RG, Nguyen TK, Cwik MJ, Phillpotts BA, Friedlander SM, Alter DC, et al. Aqueous and vitreous penetration of levofloxacin after oral administration. Ophthalmology 1999;106(12):2286-90. [PubMed]
72. FitzSimmons S. Meeting of the International B. cepacia. Vigtoria, BC, Canada: 1997; 1977.
72a. Flanagan JL, Brodie EL, Weng L, Lynch SV, Garcia O, Brown R, Hugenholtz P, DeSantis TZ, Andersen GL, Wiener-Kronish JP, Bristow J. Loss of bacterial diversity during antibiotic treatment of intubated patients colonized with Pseudomonas aeruginosa. J Clin Microbiol 2007;45(6):1954-1962.
73. Flores G, Stavola JJ, Noel GJ. Bacteremia due to Pseudomonas aeruginosa in children with AIDS. Clin Infect Dis 1993;16(5):706-8.[PubMed]
73a. Fujitani S, Sun HY, Yu VL, Weingarten JA. Pneumonia due to Pseudomonas aeruginosa: part I: epidemiology, clinical diagnosis, and source. Chest 2011;139(4):909-919.[PubMed]
74. Fong IW, Tomkins KB. Penetration of ceftazidime into the cerebrospinal fluid of patients with and without evidence of meningeal inflammation. Antimicrob Agents Chemother 1984;26(1):115-6. [PubMed]
75. Fung-Tomc J, Minassian B, Kolek B, Washo T, Huczko E, Bonner D. In vitro antibacterial spectrum of a new broad-spectrum 8-methoxy fluoroquinolone, gatifloxacin. J Antimicrob Chemother 2000;45(4):437-46. [PubMed]
76. Fung-Tomc JC, Minassian B, Kolek B, Huczko E, Aleksunes L, Stickle T, et al. Antibacterial spectrum of a novel des-fluoro(6) quinolone, BMS-284756. Antimicrob Agents Chemother 2000;44(12):3351-6. [PubMed]
77. Galanakis N, Giamarellou H, Moussas T, Dounis E. Chronic osteomyelitis caused by multi-resistant Gram-negative bacteria: evaluation of treatment with newer quinolones after prolonged follow-up. J Antimicrob Chemother 1997;39(2):241-6. [PubMed]
78. Gales AC, Reis AO, Jones RN. Contemporary assessment of antimicrobial susceptibility testing methods for polymyxin B and colistin: review of available interpretative criteria and quality control guidelines. J Clin Microbiol 2001;39(1):183-90. [PubMed]
79. Garg P, Sharma S, Rao GN. Ciprofloxacin-resistant Pseudomonas keratitis. Ophthalmology 1999;106(7):1319-23. [PubMed]
80. Gavin PJ, Suseno MT, Cook FV, Peterson LR, Thomson RB, Jr. Left-sided endocarditis caused by Pseudomonas aeruginosa: successful treatment with meropenem and tobramycin. Diagn Microbiol Infect Dis 2003;47(2):427-30. [PubMed]
81. Gerald B, Rhamphal R. Pseudomonas aeruginosa. Sixth ed. Philadelphia, PA: Elsevier; 1994.
82. Giamarellos-Bourboulis EJ, Adamis T, Laoutaris G, Sabracos L, Koussoulas V, Mouktaroudi M, et al. Immunomodulatory clarithromycin treatment of experimental sepsis and acute pyelonephritis caused by multidrug-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother 2004;48(1):93-9. [PubMed]
83. Giamarellos-Bourboulis EJ, Sambatakou H, Galani I, Giamarellou H. In vitro interaction of colistin and rifampin on multidrug-resistant Pseudomonas aeruginosa. J Chemother 2003;15(3):235-8. [PubMed]
84. Giamarellou H. Malignant otitis externa: the therapeutic evolution of a lethal infection. J Antimicrob Chemother 1992;30(6):745-51.[PubMed]
85. Godfrey AJ, Bryan LE, Rabin HR. beta-Lactam-resistant Pseudomonas aeruginosa with modified penicillin-binding proteins emerging during cystic fibrosis treatment. Antimicrob Agents Chemother 1981;19(5):705-11. [PubMed]
86. Gordin FM, Rusnak MG, Sande MA. Evaluation of combination chemotherapy in a lightly anesthetized animal model of Pseudomonas pneumonia. Antimicrob Agents Chemother 1987;31(3):398-403. [PubMed]
87. Gordon A, Isaacs D. Late onset neonatal Gram-negative bacillary infection in Australia and New Zealand: 1992-2002. Pediatr Infect Dis J 2006;25(1):25-9. [PubMed]
88. Gordon KA, Pfaller MA, Jones RN. BMS284756 (formerly T-3811, a des-fluoroquinolone) potency and spectrum tested against over 10,000 bacterial bloodstream infection isolates from the SENTRY antimicrobial surveillance programme (2000). J Antimicrob Chemother 2002;49(5):851-5. [PubMed]
89. Gouello JP, Asfar P, Brenet O, Kouatchet A, Berthelot G, Alquier P. Nosocomial endocarditis in the intensive care unit: an analysis of 22 cases. Crit Care Med 2000;28(2):377-82. [PubMed]
90. Gradelski E, Valera L, Bonner D, Fung-Tomc J. Synergistic activities of gatifloxacin in combination with other antimicrobial agents against Pseudomonas aeruginosa and related species. Antimicrob Agents Chemother 2001;45(11):3220-2. [PubMed]
91. Grandis JR, Curtin HD, Yu VL. Necrotizing (malignant) external otitis: prospective comparison of CT and MR imaging in diagnosis and follow-up. Radiology 1995;196(2):499-504. [PubMed]
92. Green S, Nathwani D, Gourlay Y, McMenamin J, Goldberg D, Kennedy D. Nebulized colistin (polymyxin E) for AIDS-associated Pseudomonas aeruginosa pneumonia. Int J STD AIDS 1992;3:130-1. [PubMed]
93. Greenberg JH. Green fingernails: a possible pathway of nosocomial pseudomonas infection. Mil Med 1975;140(5):356-7. [PubMed]
94. Gruson D, Hilbert G, Vargas F, Valentino R, Bebear C, Allery A, et al. Rotation and restricted use of antibiotics in a medical intensive care unit. Impact on the incidence of ventilator-associated pneumonia caused by antibiotic-resistant gram-negative bacteria. Am J Respir Crit Care Med 2000;162(3 Pt 1):837-43. [PubMed]
95. Gruson D, Hilbert G, Vargas F, Valentino R, Bui N, Pereyre S, et al. Strategy of antibiotic rotation: long-term effect on incidence and susceptibilities of Gram-negative bacilli responsible for ventilator-associated pneumonia. Crit Care Med 2003;31(7):1908-14. [PubMed]
96. Gunderson BW, Ibrahim KH, Hovde LB, Fromm TL, Reed MD, Rotschafer JC. Synergistic activity of colistin and ceftazidime against multiantibiotic-resistant Pseudomonas aeruginosa in an in vitro pharmacodynamic model. Antimicrob Agents Chemother 2003;47(3):905-9. [PubMed]
97. Gupta K, Sahm DF, Mayfield D, Stamm WE. Antimicrobial resistance among uropathogens that cause community-acquired urinary tract infections in women: a nationwide analysis. Clin Infect Dis 2001;33(1):89-94. [PubMed]
98. Hajjar AM, Ernst RK, Tsai JH, Wilson CB, Miller SI. Human Toll-like receptor 4 recognizes host-specific LPS modifications. Nat Immunol 2002;3(4):354-9. [PubMed]
99. Harris AD, Perencevich E, Roghmann MC, Morris G, Kaye KS, Johnson JA. Risk factors for piperacillin-tazobactam-resistant Pseudomonas aeruginosa among hospitalized patients. Antimicrob Agents Chemother 2002;46(3):854-8. [PubMed]
100. Hatchette TF, Gupta R, Marrie TJ. Pseudomonas aeruginosa community-acquired pneumonia in previously healthy adults: case report and review of the literature. Clin Infect Dis 2000;31(6):1349-56. [PubMed]
101. Hilf M, Yu VL, Sharp J, Zuravleff JJ, Korvick JA, Muder RR. Antibiotic therapy for Pseudomonas aeruginosa bacteremia: outcome correlations in a prospective study of 200 patients. Am J Med 1989;87(5):540-6. [PubMed]
102. Hoffken G, Niederman MS. Nosocomial pneumonia: the importance of a de-escalating strategy for antibiotic treatment of pneumonia in the ICU. Chest 2002;122(6):2183-96. [PubMed]
103. Holder IA. Pseudomonas immunotherapy: a historical overview. Vaccine 2004;22(7):831-9. [PubMed]
104. Hooper D, Wolfson J. Mechanisms of bacterial resistance to quinolones. 2nd ed. Washigton: American Society for Microbiology; 1993.
105. Horii T, Muramatsu H, Morita M, Maekawa M. Characterization of Pseudomonas aeruginosa isolates from patients with urinary tract infections during antibiotic therapy. Microb Drug Resist 2003;9(2):223-9. [PubMed]
106. Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 2000;118(1):146-55. [PubMed]
107. Isaacs D, Slack MP, Wilkinson AR, Westwood AW. Successful treatment of Pseudomonas ventriculitis with ciprofloxacin. J Antimicrob Chemother 1986;17(4):535-8. [PubMed]
108. Ismail H, Hellier WP, Batty V. Use of magnetic resonance imaging as the primary imaging modality in the diagnosis and follow-up of malignant external otitis. J Laryngol Otol 2004;118(7):576-9. [PubMed]
109. Jaccard C, Troillet N, Harbarth S, Zanetti G, Aymon D, Schneider R, et al. Prospective randomized comparison of imipenem-cilastatin and piperacillin-tazobactam in nosocomial pneumonia or peritonitis. Antimicrob Agents Chemother 1998;42(11):2966-72.[PubMed]
110. Jackson G. Pseudomonas aeruginosa infection and treatment. New York, NY: Marcel Dekker; 1994.
111. Jackson GG, Riff LJ. Pseudomonas bacteremia: pharmacologic and other bases for failure of treatment with gentamicin. J Infect Dis 1971;124 Suppl:S185-91. [PubMed]
112. Jacobs RF, McCarthy RE, Elser JM. Pseudomonas osteochondritis complicating puncture wounds of the foot in children: a 10-year evaluation. J Infect Dis 1989;160(4):657-61. [PubMed]
113. Jang CH, Park SY. Emergence of ciprofloxacin-resistant pseudomonas in chronic suppurative otitis media. Clin Otolaryngol Allied Sci 2004;29(4):321-3. [PubMed]
114. Jaya C, Job A, Mathai E, Antonisamy B. Evaluation of topical povidone-iodine in chronic suppurative otitis media. Arch Otolaryngol Head Neck Surg 2003;129(10):1098-100. [PubMed]
115. Jimenez-Lucho VE, Saravolatz LD, Medeiros AA, Pohlod D. Failure of therapy in pseudomonas endocarditis: selection of resistant mutants. J Infect Dis 1986;154(1):64-8. [PubMed]
116. Johnson DE, Thompson B. Efficacy of single-agent therapy with azlocillin, ticarcillin, and amikacin and beta-lactam/amikacin combinations for treatment of Pseudomonas aeruginosa bacteremia in granulocytopenic rats. Am J Med 1986;80(5C):53-8. [PubMed]
117. Jones RN, Beach ML, Pfaller MA. Spectrum and activity of three contemporary fluoroquinolones tested against Pseudomonas aeruginosa isolates from urinary tract infections in the SENTRY Antimicrobial Surveillance Program (Europe and the Americas; 2000): more alike than different! Diagn Microbiol Infect Dis 2001;41(3):161-3. [PubMed]
118. Kandemir O, Oztuna V, Milcan A, Bayramoglu A, Celik HH, Bayarslan C, et al. Clarithromycin destroys biofilms and enhances bactericidal agents in the treatment of Pseudomonas aeruginosa osteomyelitis. Clin Orthop Relat Res 2005(430):171-5. [PubMed]
119. Kang CI, Kim SH, Kim HB, Park SW, Choe YJ, Oh MD, et al. Pseudomonas aeruginosa bacteremia: risk factors for mortality and influence of delayed receipt of effective antimicrobial therapy on clinical outcome. Clin Infect Dis 2003;37(6):745-51. [PubMed]
120. Kapusnik JE, Hackbarth CJ, Chambers HF, Carpenter T, Sande MA. Single, large, daily dosing versus intermittent dosing of tobramycin for treating experimental pseudomonas pneumonia. J Infect Dis 1988;158(1):7-12. [PubMed]
121. Karlowsky JA, Jones ME, Thornsberry C, Evangelista AT, Yee YC, Sahm DF. Stable antimicrobial susceptibility rates for clinical isolates of Pseudomonas aeruginosa from the 2001-2003 tracking resistance in the United States today surveillance studies. Clin Infect Dis 2005;40 Suppl 2:S89-98. [PubMed]
122. Keene WE, Markum AC, Samadpour M. Outbreak of Pseudomonas aeruginosa infections caused by commercial piercing of upper ear cartilage. Jama 2004;291(8):981-5. [PubMed]
123. Kennedy AM, Elward AM, Fraser VJ. Survey of knowledge, beliefs, and practices of neonatal intensive care unit healthcare workers regarding nosocomial infections, central venous catheter care, and hand hygiene. Infect Control Hosp Epidemiol 2004;25(9):747-52. [PubMed]
124. Knauf HP, Silvany R, Southern PM, Jr., Risser RC, Wilson SE. Susceptibility of corneal and conjunctival pathogens to ciprofloxacin. Cornea 1996;15(1):66-71. [PubMed]
125. Kollef MH, Vlasnik J, Sharpless L, Pasque C, Murphy D, Fraser V. Scheduled change of antibiotic classes: a strategy to decrease the incidence of ventilator-associated pneumonia. Am J Respir Crit Care Med 1997;156(4 Pt 1):1040-8. [PubMed]
126. Kollef MH, Ward S. The influence of mini-BAL cultures on patient outcomes: implications for the antibiotic management of ventilator-associated pneumonia. Chest 1998;113(2):412-20. [PubMed]
127. Komshian SV, Tablan OC, Palutke W, Reyes MP. Characteristics of left-sided endocarditis due to Pseudomonas aeruginosa in the Detroit Medical Center. Rev Infect Dis 1990;12(4):693-702. [PubMed]
128. Kowalski RP, Pandya AN, Karenchak LM, Romanowski EG, Husted RC, Ritterband DC, et al. An in vitro resistance study of levofloxacin, ciprofloxacin, and ofloxacin using keratitis isolates of Staphylococcus aureus and Pseudomonas aeruginosa. Ophthalmology 2001;108(10):1826-9. [PubMed]
129. Kusne S, Eibling DE, Yu VL, Fitz D, Johnson JT, Kahl LE, et al. Gangrenous cellulitis associated with gram-negative bacilli in pancytopenic patients: dilemma with respect to effective therapy. Am J Med 1988;85(4):490-4. [PubMed]
130. Laguno M, Miro O, Font C, de la Sierra A. Pacemaker-related endocarditis. Report of 7 cases and review of the literature.Cardiology 1998;90(4):244-8. [PubMed]
131. Lamont IL, Beare PA, Ochsner U, Vasil AI, Vasil ML. Siderophore-mediated signaling regulates virulence factor production in Pseudomonasaeruginosa. Proc Natl Acad Sci U S A 2002;99(10):7072-7. [PubMed]
132. Landman D, Quale JM, Mayorga D, Adedeji A, Vangala K, Ravishankar J, et al. Citywide clonal outbreak of multiresistant Acinetobacter baumannii and Pseudomonas aeruginosa in Brooklyn, NY: the preantibiotic era has returned. Arch Intern Med 2002;162(13):1515-20. [PubMed]
133. Leibovici L, Paul M, Poznanski O, Drucker M, Samra Z, Konigsberger H, et al. Monotherapy versus beta-lactam-aminoglycoside combination treatment for gram-negative bacteremia: a prospective, observational study. Antimicrob Agents Chemother 1997;41(5):1127-33. [PubMed]
134. Letendre ED, Mantha R, Turgeon PL. Selection of resistance by piperacillin during Pseudomonas aeruginosa endocarditis. J Antimicrob Chemother 1988;22(4):557-62. [PubMed]
135. Levin AS, Barone AA, Penco J, Santos MV, Marinho IS, Arruda EA, et al. Intravenous colistin as therapy for nosocomial infections caused by multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Clin Infect Dis 1999;28(5):1008-11. [PubMed]
136. Li J, Turnidge J, Milne R, Nation RL, Coulthard K. In vitro pharmacodynamic properties of colistin and colistin methanesulfonate against Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Antimicrob Agents Chemother 2001;45(3):781-5. [PubMed]
137. Linden PK, Kusne S, Coley K, Fontes P, Kramer DJ, Paterson D. Use of parenteral colistin for the treatment of serious infection due to antimicrobial-resistant Pseudomonas aeruginosa. Clin Infect Dis 2003;37(11):e154-60. [PubMed]
138. Lipman J, Allworth A, Wallis SC. Cerebrospinal fluid penetration of high doses of intravenous ciprofloxacin in meningitis. Clin Infect Dis 2000;31(5):1131-3. [PubMed]
139. Livermore DM. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clin Infect Dis 2002;34(5):634-40. [PubMed]
140. Lodise TP, Jr., Lomaestro B, Drusano GL. Piperacillin-tazobactam for Pseudomonas aeruginosa infection: clinical implications of an extended-infusion dosing strategy. Clin Infect Dis 2007;44(3):357-63. [PubMed]
141. Lodise TP, Jr., Patel N, Kwa A, Graves J, Furuno JP, Graffunder E, et al. Predictors of 30-day mortality among patients with Pseudomonas aeruginosa bloodstream infections: impact of delayed appropriate antibiotic selection. Antimicrob Agents Chemother 2007;51(10):3510-5. [PubMed]
142. Lomholt JA, Kilian M. Ciprofloxacin susceptibility of Pseudomonas aeruginosa isolates from keratitis. Br J Ophthalmol 2003;87(10):1238-40. [PubMed]
143. Lorente L, Lorenzo L, Martin MM, Jimenez A, Mora ML. Meropenem by continuous versus intermittent infusion in ventilator-associated pneumonia due to gram-negative bacilli. Ann Pharmacother 2006;40(2):219-23. [PubMed]
144. Lu CH, Chang WN, Chuang YC, Chang HW. Gram-negative bacillary meningitis in adult post-neurosurgical patients. Surg Neurol 1999;52(5):438-43; discussion 443-4. [PubMed]
145. Lu CH, Chang WN, Chuang YC, Chang HW. The prognostic factors of adult gram-negative bacillary meningitis. J Hosp Infect 1998;40(1):27-34. [PubMed]
146. Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007;44 Suppl 2:S27-72. [PubMed]
147. Mendelson MH, Gurtman A, Szabo S, Neibart E, Meyers BR, Policar M, et al. Pseudomonas aeruginosa bacteremia in patients with AIDS. Clin Infect Dis 1994;18(6):886-95. [PubMed]
148. Menon J, Rennie IG. Endogenous Pseudomonas endophthalmitis in an immunocompetent patient: a case for early diagnosis and treatment. Eye 2000;14 (Pt 2):253-4. [PubMed]
149. Meyer JM, Neely A, Stintzi A, Georges C, Holder IA. Pyoverdin is essential for virulence of Pseudomonas aeruginosa. Infect Immun 1996;64(2):518-23. [PubMed]
150. Micek ST, Lloyd AE, Ritchie DJ, Reichley RM, Fraser VJ, Kollef MH. Pseudomonas aeruginosa bloodstream infection: importance of appropriate initial antimicrobial treatment. Antimicrob Agents Chemother 2005;49(4):1306-11. [PubMed]
151. Michalopoulos AS, Tsiodras S, Rellos K, Mentzelopoulos S, Falagas ME. Colistin treatment in patients with ICU-acquired infections caused by multiresistant Gram-negative bacteria: the renaissance of an old antibiotic. Clin Microbiol Infect 2005;11(2):115-21. [PubMed]
152. Millar MR, Bransby-Zachary MA, Tompkins DS, Hawkey PM, Myles Gibson R. Ciprofloxacin for Pseudomonas aeruginosa meningitis. Lancet 1986;1(8493):1325. [PubMed]
153. Mizuta M, Linkin DR, Nachamkin I, Fishman NO, Weiner MG, Sheridan A, et al. Identification of optimal combinations for empirical dual antimicrobial therapy of Pseudomonas aeruginosa infection: potential role of a Combination Antibiogram. Infect Control Hosp Epidemiol 2006;27(4):413-5. [PubMed]
154. Moss WJ, Beers MC, Johnson E, Nichols DG, Perl TM, Dick JD, et al. Pilot study of antibiotic cycling in a pediatric intensive care unit. Crit Care Med 2002;30(8):1877-82. [PubMed]
155. Musher DM. Pseudomonas pneumonia in smokers. Clin Infect Dis 2001;33(3):415. [PubMed]
156. Nau R, Prange HW, Martell J, Sharifi S, Kolenda H, Bircher J. Penetration of ciprofloxacin into the cerebrospinal fluid of patients with uninflamed meninges. J Antimicrob Chemother 1990;25(6):965-73. [PubMed]
157. Navon-Venezia S, Ben-Ami R, Carmeli Y. Update on Pseudomonas aeruginosa and Acinetobacter baumannii infections in the healthcare setting. Curr Opin Infect Dis 2005;18(4):306-13. [PubMed]
158. Neher A, Nagl M, Appenroth E, Gstottner M, Wischatta M, Reisigl F, et al. Acute otitis externa: efficacy and tolerability of N-chlorotaurine, a novel endogenous antiseptic agent. Laryngoscope 2004;114(5):850-4. [PubMed]
159. Nicolau DP, Banevicius MA, Nightingale CH, Quintiliani R. Beneficial effect of adjunctive azithromycin in treatment of mucoid Pseudomonas aeruginosa pneumonia in the murine model. Antimicrob Agents Chemother 1999;43(12):3033-5. [PubMed]
160. Norrby SR. Carbapenems in serious infections: a risk-benefit assessment. Drug Saf 2000;22(3):191-4. [PubMed]
161. Nseir S, Di Pompeo C, Pronnier P, Beague S, Onimus T, Saulnier F, et al. Nosocomial tracheobronchitis in mechanically ventilated patients: incidence, aetiology and outcome. Eur Respir J 2002;20(6):1483-9. [PubMed]
162. Obritsch MD, Fish DN, MacLaren R, Jung R. Nosocomial infections due to multidrug-resistant Pseudomonas aeruginosa: epidemiology and treatment options. Pharmacotherapy 2005;25(10):1353-64. [PubMed]
163. Patel JB, Giles RE. Meropenem: evidence of lack of proconvulsive tendency in mice. J Antimicrob Chemother 1989;24 Suppl A:307-9. [PubMed]
164. Paterson D, Cornaglia G, Mazzariol A, Lamonaca V, Gruttadauria S, Marino I. A true return to the pre-antibiotic era? Endovascular infections as sequelae of bacteremia with Pseudomonas aeruginosa producing VIM-1, resistant to all carbapenems, cephalosporins, penicillins, quinolones and aminoglycosides. In: Program and abstracts of the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy; Chicago: Abstract K-1183; 2001:129-158.
165. Paul M, Silbiger I, Grozinsky S, Soares-Weiser K, Leibovici L. Beta lactam antibiotic monotherapy versus beta lactam-aminoglycoside antibiotic combination therapy for sepsis. Cochrane Database Syst Rev 2006(1):CD003344.
166. Peacock JE, Herrington DA, Wade JC, Lazarus HM, Reed MD, Sinclair JW, et al. Ciprofloxacin plus piperacillin compared with tobramycin plus piperacillin as empirical therapy in febrile neutropenic patients. A randomized, double-blind trial. Ann Intern Med 2002;137(2):77-87. [PubMed]
167. Pendland SL, Messick CR, Jung R. In vitro synergy testing of levofloxacin, ofloxacin, and ciprofloxacin in combination with aztreonam, ceftazidime, or piperacillin against Pseudomonas aeruginosa. Diagn Microbiol Infect Dis 2002;42(1):75-8. [PubMed]
168. Pennington JE, Johnson CE, Platt R. Third-generation cephalosporins in the treatment of pneumonia due to Pseudomonas aeruginosa in guinea pigs. J Infect Dis 1982;146(4):567. [PubMed]
169. Poole K. Bacterial multidrug resistance--emphasis on efflux mechanisms and Pseudomonas aeruginosa. J Antimicrob Chemother 1994;34(4):453-6. [PubMed]
170. Quinn JP, Studemeister AE, DiVincenzo CA, Lerner SA. Resistance to imipenem in Pseudomonas aeruginosa: clinical experience and biochemical mechanisms. Rev Infect Dis 1988;10(4):892-8. [PubMed]
171. Ramsey BW, Pepe MS, Quan JM, Otto KL, Montgomery AB, Williams-Warren J, et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. Cystic Fibrosis Inhaled Tobramycin Study Group. N Engl J Med 1999;340(1):23-30.[PubMed]
172. Raymond DP, Pelletier SJ, Crabtree TD, Gleason TG, Hamm LL, Pruett TL, et al. Impact of a rotating empiric antibiotic schedule on infectious mortality in an intensive care unit. Crit Care Med 2001;29(6):1101-8. [PubMed]
173. Reedy JS, Wood KE. Endogenous Pseudomonas aeruginosa endophthalmitis: a case report and literature review. Intensive Care Med 2000;26(9):1386-9. [PubMed]
174. Regelmann WE, Elliott GR, Warwick WJ, Clawson CC. Reduction of sputum Pseudomonas aeruginosa density by antibiotics improves lung function in cystic fibrosis more than do bronchodilators and chest physiotherapy alone. Am Rev Respir Dis 1990;141(4 Pt 1):914-21. [PubMed]
175. Rello J, Bodi M, Mariscal D, Navarro M, Diaz E, Gallego M, et al. Microbiological testing and outcome of patients with severe community-acquired pneumonia. Chest 2003;123(1):174-80. [PubMed]
176. Rello J, Gallego M, Mariscal D, Sonora R, Valles J. The value of routine microbial investigation in ventilator-associated pneumonia. Am J Respir Crit Care Med 1997;156(1):196-200. [PubMed]
177. Rello J, Lorente C, Diaz E, Bodi M, Boque C, Sandiumenge A, et al. Incidence, etiology, and outcome of nosocomial pneumonia in ICU patients requiring percutaneous tracheotomy for mechanical ventilation. Chest 2003;124(6):2239-43. [PubMed]
178. Reyes MP, Lerner AM. Current problems in the treatment of infective endocarditis due to Pseudomonas aeruginosa. Rev Infect Dis 1983;5(2):314-21. [PubMed]
179. Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in medical intensive care units in the United States. National Nosocomial Infections Surveillance System. Crit Care Med 1999;27(5):887-92. [PubMed]
180. Rodgers GL, Mortensen J, Fisher MC, Lo A, Cresswell A, Long SS. Predictors of infectious complications after burn injuries in children. Pediatr Infect Dis J 2000;19(10):990-5. [PubMed]
181. Roger T, David J, Glauser MP, Calandra T. MIF regulates innate immune responses through modulation of Toll-like receptor 4. Nature 2001;414(6866):920-4. [PubMed]
182. Rousseau JM, Soullie B, Villevielle T, Koeck JL. Efficiency of cefepime in postoperative meningitis attributable to Enterobacter aerogenes. J Trauma 2001;50(5):971. [PubMed]
183. Rubin Grandis J, Branstetter BFt, Yu VL. The changing face of malignant (necrotising) external otitis: clinical, radiological, and anatomic correlations. Lancet Infect Dis 2004;4(1):34-9. [PubMed]
184. Rubin J, Stoehr G, Yu VL, Muder RR, Matador A, Kamerer DB. Efficacy of oral ciprofloxacin plus rifampin for treatment of malignant external otitis. Arch Otolaryngol Head Neck Surg 1989;115(9):1063-9. [PubMed]
185. Rubin J, Yu VL. Malignant external otitis: insights into pathogenesis, clinical manifestations, diagnosis, and therapy. Am J Med 1988;85(3):391-8. [PubMed]
186. Rusnak MG, Drake TA, Hackbarth CJ, Sande MA. Single versus combination antibiotic therapy for pneumonia due to Pseudomonas aeruginosa in neutropenic guinea pigs. J Infect Dis 1984;149(6):980-5. [PubMed]
187. Rynn C, Wootton M, Bowker KE, Alan Holt H, Reeves DS. In vitro assessment of colistin's antipseudomonal antimicrobial interactions with other antibiotics. Clin Microbiol Infect 1999;5(1):32-36. [PubMed]
188. Sabath LD. Biochemical and physiologic basis for susceptibility and resistance of Pseudomonas aeruginosa to antimicrobial agents. Rev Infect Dis 1984;6 Suppl 3:S643-56. [PubMed]
189. Sadikot RT, Blackwell TS, Christman JW, Prince AS. Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med 2005;171(11):1209-23. [PubMed]
190. Safdar N, Handelsman J, Maki DG. Does combination antimicrobial therapy reduce mortality in Gram-negative bacteraemia? A meta-analysis. Lancet Infect Dis 2004;4(8):519-27. [PubMed]
191. Saiman L, Burns JL, Whittier S, Krzewinski J, Marshall SA, Jones RN. Evaluation of reference dilution test methods for antimicrobial susceptibility testing of Pseudomonas aeruginosa strains isolated from patients with cystic fibrosis. J Clin Microbiol 1999;37(9):2987-91. [PubMed]
192. Saiman L, Marshall BC, Mayer-Hamblett N, Burns JL, Quittner AL, Cibene DA, et al. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. Jama 2003;290(13):1749-56. [PubMed]
193. Saiman L, Siegel J. Infection control recommendations for patients with cystic fibrosis: microbiology, important pathogens, and infection control practices to prevent patient-to-patient transmission. Infect Control Hosp Epidemiol 2003;24(5 Suppl):S6-52. [PubMed]
194. Schaefer F, Bruttin O, Zografos L, Guex-Crosier Y. Bacterial keratitis: a prospective clinical and microbiological study. Br J Ophthalmol 2001;85(7):842-7. [PubMed]
195. Schauersberger J, Amon M, Wedrich A, Nepp J, El Menyawi I, Derbolav A, et al. Penetration and decay of meropenem into the human aqueous humor and vitreous. J Ocul Pharmacol Ther 1999;15(5):439-45. [PubMed]
196. Scheinberg P, Shore E. A pilot study of the safety and efficacy of tobramycin solution for inhalation in patients with severe bronchiectasis. Chest 2005;127(4):1420-6. [PubMed]
197. Schonwald S, Beus I, Lisic M, Car V, Gmajnicki B. Ciprofloxacin in the treatment of gram-negative bacillary meningitis. Am J Med 1989;87(5A):248S-249S. [PubMed]
198. Schwartz RH. Once-daily ofloxacin otic solution versus neomycin sulfate/polymyxin B sulfate/hydrocortisone otic suspension four times a day: a multicenter, randomized, evaluator-blinded trial to compare the efficacy, safety, and pain relief in pediatric patients with otitis externa. Curr Med Res Opin 2006;22(9):1725-36. [PubMed]
199. Senda K, Arakawa Y, Nakashima K, Ito H, Ichiyama S, Shimokata K, et al. Multifocal outbreaks of metallo-beta-lactamase-producing Pseudomonas aeruginosa resistant to broad-spectrum beta-lactams, including carbapenems. Antimicrob Agents Chemother 1996;40(2):349-53. [PubMed]
200. Shepp DH, Tang IT, Ramundo MB, Kaplan MK. Serious Pseudomonas aeruginosa infection in AIDS. J Acquir Immune Defic Syndr 1994;7(8):823-31. [PubMed]
201. Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med 2000;162(2 Pt 1):505-11. [PubMed]
202. Soothill JS. Bacteriophage prevents destruction of skin grafts by Pseudomonas aeruginosa. Burns 1994;20(3):209-11. [PubMed]
203. Stein A, Raoult D. Colistin: an antimicrobial for the 21st century? Clin Infect Dis 2002;35(7):901-2. [PubMed]
204. Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 2002;110(2 Pt 1):285-91. [PubMed]
205. Stoll BJ, Hansen NI, Higgins RD, Fanaroff AA, Duara S, Goldberg R, et al. Very low birth weight preterm infants with early onset neonatal sepsis: the predominance of gram-negative infections continues in the National Institute of Child Health and Human Development Neonatal Research Network, 2002-2003. Pediatr Infect Dis J 2005;24(7):635-9. [PubMed]
206. Stover DE, Mangino D. Macrolides: a treatment alternative for bronchiolitis obliterans organizing pneumonia? Chest 2005;128(5):3611-7. [PubMed]
207. Sulakvelidze A, Alavidze Z, Morris JG, Jr. Bacteriophage therapy. Antimicrob Agents Chemother 2001;45(3):649-59. [PubMed]
207a. Sun HY, Fujitani S, Quintiliani R, Yu VL. Pneumonia Due to Pseudomonas aeruginosa: Part II: Antimicrobial Resistance, Pharmacodynamic Concepts, and Antibiotic Therapy. Chest 2011;139(5):1172-85. [PubMed]
208. Tablan OC, Reyes MP, Rintelmann WF, Lerner AM. Renal and auditory toxicity of high-dose, prolonged therapy with gentamicin and tobramycin in pseudomonas endocarditis. J Infect Dis 1984;149(2):257-63. [PubMed]
209. Takase H, Nitanai H, Hoshino K, Otani T. Impact of siderophore production on Pseudomonas aeruginosa infections in immunosuppressed mice. Infect Immun 2000;68(4):1834-9. [PubMed]
210. Tascini C, Ferranti S, Messina F, Menichetti F. In vitro and in vivo synergistic activity of colistin, rifampin, and amikacin against a multiresistant Pseudomonas aeruginosa isolate. Clin Microbiol Infect 2000;6(12):690-1. [PubMed]
211. Tascini C, Gemignani G, Ferranti S, Tagliaferri E, Leonildi A, Lucarini A, et al. Microbiological activity and clinical efficacy of a colistin and rifampin combination in multidrug-resistant Pseudomonas aeruginosa infections. J Chemother 2004;16(3):282-7. [PubMed]
212. Toleman MA, Rolston K, Jones RN, Walsh TR. blaVIM-7, an evolutionarily distinct metallo-beta-lactamase gene in a Pseudomonas aeruginosa isolate from the United States. Antimicrob Agents Chemother 2004;48(1):329-32. [PubMed]
213. Traill ZC, Miller RF, Ali N, Shaw PJ. Pseudomonas aeruginosa bronchopulmonary infection in patients with advanced human immunodeficiency virus disease. Br J Radiol 1996;69(828):1099-103. [PubMed]
214. Trias J, Dufresne J, Levesque RC, Nikaido H. Decreased outer membrane permeability in imipenem-resistant mutants of Pseudomonas aeruginosa. Antimicrob Agents Chemother 1989;33(8):1202-6. [PubMed]
215. Trouillet JL, Vuagnat A, Combes A, Kassis N, Chastre J, Gibert C. Pseudomonas aeruginosa ventilator-associated pneumonia: comparison of episodes due to piperacillin-resistant versus piperacillin-susceptible organisms. Clin Infect Dis 2002;34(8):1047-54.[PubMed]
216. Tsai AC, Tseng MC, Chang SW, Hu FR. Clinical evaluation of ciprofloxacin ophthalmic solution in the treatment of refractory bacterial keratitis. J Formos Med Assoc 1995;94(12):760-4. [PubMed]
217. Tunkel AR, Hartman BJ, Kaplan SL, Kaufman BA, Roos KL, Scheld WM, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis 2004;39(9):1267-84. [PubMed]
218. Vasil ML, Ochsner UA. The response of Pseudomonas aeruginosa to iron: genetics, biochemistry and virulence. Mol Microbiol 1999;34(3):399-413. [PubMed]
219. Vidal F, Mensa J, Almela M, Martinez JA, Marco F, Casals C, et al. Epidemiology and outcome of Pseudomonas aeruginosa bacteremia, with special emphasis on the influence of antibiotic treatment. Analysis of 189 episodes. Arch Intern Med 1996;156(18):2121-6. [PubMed]
220. Vidal F, Mensa J, Martinez JA, Almela M, Marco F, Gatell JM, et al. Pseudomonas aeruginosa bacteremia in patients infected with human immunodeficiency virus type 1. Eur J Clin Microbiol Infect Dis 1999;18(7):473-7. [PubMed]
221. Vidaur L, Sirgo G, Rodriguez AH, Rello J. Clinical approach to the patient with suspected ventilator-associated pneumonia. Respir Care 2005;50(7):965-74; discussion 974. [PubMed]
222. Warren DK, Hill HA, Merz LR, Kollef MH, Hayden MK, Fraser VJ, et al. Cycling empirical antimicrobial agents to prevent emergence of antimicrobial-resistant Gram-negative bacteria among intensive care unit patients. Crit Care Med 2004;32(12):2450-6.[PubMed]
223. Weinbren MJ, Forgeson G, Helenglass G, Jameson B, Powles R. Unusual presentation of pseudomonas infection. Bmj 1988;297(6655):1034-5. [PubMed]
224. Weller TM, Andrews JM, Jevons G, Wise R. The in vitro activity of BMS-284756, a new des-fluorinated quinolone. J Antimicrob Chemother 2002;49(1):177-84. [PubMed]
. 225. Weyers CM, Leeper KV. Nonresolving pneumonia. Clin Chest Med 2005;26(1):143-58. [PubMed]
226. Wieland M, Lederman MM, Kline-King C, Keys TF, Lerner PI, Bass SN, et al. Left-sided endocarditis due to Pseudomonas aeruginosa. A report of 10 cases and review of the literature. Medicine (Baltimore) 1986;65(3):180-9. [PubMed]
227. Williams HB, Breidenbach WC, Callaghan WB, Richards GK, Prentis JJ. Are burn wound biopsies obsolete? A comparative study of bacterial quantitation in burn patients using the absorbent disc and biopsy techniques. Ann Plast Surg 1984;13(5):388-95. [PubMed]
228. Wilson LA, Schlitzer RL, Ahearn DG. Pseudomonas corneal ulcers associated with soft contact-lens wear. Am J Ophthalmol 1981;92(4):546-54. [PubMed]
229. Winston DJ, Barnes RC, Ho WG, Young LS, Champlin RE, Gale RP. Moxalactam plus piperacillin versus moxalactam plus amikacin in febrile granulocytopenic patients. Am J Med 1984;77(3):442-50. [PubMed]
230. Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004;39(3):309-17. [PubMed]
231. Wong-Beringer A, Beringer P, Lovett MA. Successful treatment of multidrug-resistant Pseudomonas aeruginosa meningitis with high-dose ciprofloxacin. Clin Infect Dis 1997;25(4):936-7. [PubMed]
232. Wright ML, Romano MJ. Ventilator-associated pneumonia in children. Semin Pediatr Infect Dis 2006;17(2):58-64. [PubMed]
233. Yasuda H, Ajiki Y, Koga T, Kawada H, Yokota T. Interaction between biofilms formed by Pseudomonas aeruginosa and clarithromycin. Antimicrob Agents Chemother 1993;37(9):1749-55. [PubMed]
234. Yokoyama K, Doi Y, Yamane K, Kurokawa H, Shibata N, Shibayama K, et al. Acquisition of 16S rRNA methylase gene in Pseudomonas aeruginosa. Lancet 2003;362(9399):1888-93. [PubMed]
235. Yu VL, Singh N. Excessive antimicrobial usage causes measurable harm to patients with suspected ventilator-associated pneumonia. Intensive Care Med 2004;30(5):735-8. [PubMed]
235a. Yu VL. Guidelines for hospital-acquired pneumonia and health-care-associated pneumonia: a vulnerability, a pitfall, and a fatal flaw. Lancet Infect Dis 2011;11(3):248-252. [PubMed]
236. Zaske DE, Chin T, Kohls PR, Solem LD, Strate RG. Initial dosage regimens of gentamicin in patients with burns. J Burn Care Rehabil 1991;12(1):46-50.[PubMed]
237. Zloty P, Belin M. Ocular infections caused by Pseudomonas aeruginosa. New York, NY: Marcel Dekker; 1994.
238. Zhuo H, Yang K, Lynch SV, Dotson RH, Glidden DV, Singh G, Webb WR, Elicker BM, Garcia O, Brown R, Sawa Y, Misset B, Wiener-Kronish JP. Increased mortality of ventilated patients with endotracheal Pseudomonas aeruginosa without clinical signs of infection. Crit Care Med 2008;36 (9):2495-503.
Table 1. Minimum Inhibitory Concentrations of P. aeruginosa for Selected Antibiotics
| MIC(mg/L) | |||
|---|---|---|---|
| Antibiotic | MIC50 | MIC90 | Range |
Extended spectrum Penicillins Piperacillin Ticarcillin Carbenicillin |
4 32 64 |
64 128 256 |
1 - >128 1 - >128 8 - 512 |
Extended spectrum Penicillins plus Beta-lactamase inhibitors |
|||
Piperacillin-Tazobactam1 Ticarcillin-Clavulanate1 |
4 32 |
64 128 |
1 - >128 1 - >128 |
Cephalosporins Third Generations Ceftazidime Cefoperazone Cefotaxime Ceftriaxone Ceftizoxime Cefpodoxime |
2 8 16 16 32 >128 |
8 32 64 64 128 >128 |
0.5-128 0.25 - >128 0.25 - >64 0.25 - >64 2 - >128 >128 |
Fourth Generation Cefepime Cefpirome |
4 16 |
16 32 |
1 - 32 4 - >128 |
Other beta-lactams Meropenem Imipenem Ertapenem Aztreonam |
1 2 8 8 |
2 8 >16 16 |
0.06 - 16 0.5 - 256 0.12 - >16 0.5 - 256 |
Quinolones Ciprofloxacin Levofloxacin Ofloxacin Gatifloxacin Moxifloxacin Garenofloxacin Norfloxacin |
0.25 0.5 1 1 2 2 2 |
16 32 32 32 32 >32 32 |
0.32 - 32 0.12 - 32 0.25 - 32 0.5 - 32 0.5 - 32 0.25 - >32 0.2 - 32 |
Aminoglycosides Tobramycin Gentamicin Amikacin Netilmicin |
2 4 8 8 |
4 16 16 32 |
0.25 - >512 2 - >512 1 - 128 1 - 64 |
Other antibiotics Tigecycline |
8 |
>8 |
4 - >8 |
Adapted from (46, 75, 76, 88, 224)
Table 2: Drug of Choice [Total Daily Dose (Dosing Interval in Hours)]
| Class | Antibiotic | Route | Adult Dose | Pediatric Dose * | Alternative Dosing |
|---|---|---|---|---|---|
Aminoglycoside |
amikacin |
IV |
7.5 mg/kg IV (12) |
15-30 mg/kg IV (8) |
15 mg/kg IV (24) |
Aminoglycoside |
colistin |
IT/inhaled |
Toxic for IV Use |
Toxic for IV Use |
N/A |
Aminoglycoside |
gentamicin |
IV |
2 mg/kg load, then 5 mg/kg IV (8) |
7.5 mg/kg IV (8) |
5-7 mg/kg IV (24) |
Aminoglycoside |
tobramycin |
IV/inhaled |
2 mg/kg load, then 5 mg/kg IV (8) |
7.5-10.5 mg/kg IV (8) |
5-12 mg/kg IV (24) |
Carbapenem |
imipenem/cilastatin |
IV/IM |
1-4 gm IV (6) |
60-100 mg/kg IV (6) |
N/A |
Carbapenem |
meropenem |
IV |
1.5-6 gm IV (8) |
60-120 mg/kg IV (8) |
125-250mg/hour IV |
Cephalosporin: 3rd generation |
ceftazidime |
IV/IM |
3-6 gm IV (8-12) |
90-150mg/kg IV (8) |
N/A |
Cephalosporin: 4th generation |
cefepime |
IV |
2-6 gm IV (8) |
100-150mg/kg IV (8) |
Load 15 mg/kg then 100 mg/kg IV infused over 24 hours |
Fluoroquinolone |
ciprofloxacin |
PO/IV |
500-1500 mg po (12); 400-800 mg IV (8-12) |
30 mg/kg PO/IV (12) |
N/A |
Fluoroquinolone |
levofloxacin |
PO/ IV |
500-750 mg PO IV (24) |
10 mg/kg PO/ IV (24) |
N/A |
Monobactam |
aztreonam |
IV |
3-8 gm IV (6-8) |
90-200mg/kg (8) |
N/A |
Penicillin: antipseudomonal |
ticarcillin |
IV |
4-24 gm (4-6) |
300- 600 mg/kg IV (8) |
N/A |
Penicillin: antipseudomonal |
piperacillin |
IV |
8-24 gm IV (4-6)
|
200-600 mg/kg IV (8) |
3.375 g infused over 4 hours (8) |
| Polymyxin | colistin | IT (use In for inhaled ?)/inhaled | 150-300 mg IN (12) | 80-300 mg IN (12) | N/A |
| colistin | IV | 2.5-5 mg/kg IV (8) MAX 160 mg/dose | 2.5-5 mg/kg IV (8)-MAX 160 mg/dose | IM (extremely painful) | |
| colistin | IT | 3.2-10 mg IT (24) | Not sure there is data to recommend | N/A |
*Dose in neonates may need to be adjusted Note: Doses in renal failure may need to be adjusted
N/A=Not Available; IM= Intramuscular; IV= Intravenous; PO=Oral; gm=gram; mg= milligram; kg=kilogram
Table 3: Special Infections [Total Daily Dose (Dosing Interval in Hours)]
| Infection | Antibiotic | Adult Dose | Pediatric Dose* | Aminogylcoside? | Duration |
|---|---|---|---|---|---|
Cystic Fibrosis: mild exacerbation/ sinusitis |
ciprofloxacin |
1500 mg po (12) |
30 mg/kg po (12) |
No |
14-21 days |
Cystic Fibrosis: fails oral therapy |
**cefepime |
6 gm IV (12) |
150 mg/kg IV (12) |
tobramycin or amikacin |
10-21 days |
|
ceftazidime |
6 gm IV (8) |
150 mg/kg IV (8) |
tobramycin or amikacin |
10-21 days |
|
**meropenem |
6 gm IV (8) |
320 mg/kg IV (8) |
tobramycin or amikacin |
10-21 days |
|
**piperacillin or piperacillin/ tazobactam |
24gm IV (4) |
600 mg/kg IV (6) |
tobramycin or amikacin |
10-21 days |
|
ticarcillin or ticarcillin-clavulenate |
24 gm IV (4-6)
|
600 mg/kg IV (6-8)
|
tobramycin or amikacin |
10-21 days |
Infective Endocarditis |
cefepime |
6 gm IV (8) |
150 mg/kg IV (8) |
Yes |
42 days |
|
ceftazidime |
6 gm IV (8) |
150 mg/kg IV (8) |
Yes |
42 days |
|
piperacillin |
24 gm IV (4-6) |
600 mg/kg IV (6) |
Yes |
42 days |
|
ticarcillin |
20-24 gm IV (4-6) |
600 mg/kg IV (6-8) |
Yes |
42 days |
Meningitis |
cefepime |
6gm IV (8) |
150 mg/kg IV (8) |
+/- gentamicin |
21 days |
|
ceftazidime |
6gm IV (8) |
150 mg/kg (8) |
+/- gentamicin |
21 days |
|
ciprofloxacin |
800-1200 mg IV (8-12) |
30 mg/kg IV (12) |
+/- gentamicin |
21 days |
|
meropenem |
6gm IV (8) |
120 mg/kg IV (8) |
+/- gentamicin |
21 days |
Urinary Tract Infection |
ciprofloxacin |
500 mg po (12) |
30 mg/kg po (12) |
No (unless urosepsis) |
7-10 days as out patient |
** Consider continuous infusion (see Table 1 for dosing)
Note: Doses in renal failure may need to be adjustedPO=Oral; gm=gram; mg= milligram; kg=kilogram
Table 4: Doses of IV Aminogylcosides in Specific Infections [Total Daily Dose (Dosing Interval in Hours)]
| Infection | Aminoglycoside | Adult Dose | Pediatric Dose * | Peak (P), Trough (T) Recommended in mcg/ ml |
|---|---|---|---|---|
Cystic Fibrosis |
amikacin |
15-30 mg/kg (8-12) |
30 mg/kg (8) |
P 20-30, T 5-10 |
|
tobramycin |
3.3 mg/kg (8) or 10-12 mg/ kg (24) |
3.3 mg/kg (8) or 10-12 mg/ kg (24) |
P10-12 & T 1-2 for q 8hrs; T <1 once daily dosing & P 10 times MIC |
Endocarditis |
tobramycin |
2 mg/ kg load then 5-8 mg/kg (8) or 8-10 mg/kg (24) |
7.5 mg/kg (8) or 7-10 mg/kg (24) |
P10-12 & T 1-2 for q 8hrs; T <1 once daily dosing & P 10 times MIC |
|
gentamicin |
2 mg/kg load then 5-8 mg/kg (8) or 7 mg/kg (24) |
7.5 mg/kg (8) or 7 mg/kg (24) |
P 20-30, T 5-10 for q 8hr; T<1 once daily dosing |
Meningitis |
amikacin |
15 mg/kg (8) |
15-22.5 mg/kg (8) |
P 20-30, T 5-10 |
|
gentamicin |
5 mg/kg (8) |
7.5 mg/kg (8) |
P 6-10, T <2 |
|
tobramycin |
5 mg/kg (8) |
7.5 mg/kg (8) |
P 8-10, T <2 |
Sepsis |
amikacin |
7.5 mg/kg (12) or 15 mg/kg (24) |
15-30 mg/kg (8) |
P 15-30, T 5-10 for q8; P 56-64, T <1 once daily dosing |
|
gentamicin |
2 mg/ kg load then 5 mg/kg (8) or 7 mg/kg (24) |
7.5 mg/kg (8) or 7 mg/kg (24) |
P 20-30, T 5-10 for q 8hr; T<1 once daily dosing |
|
tobramycin |
2 mg/ kg load then 5 mg/kg (8) or 7 mg/kg (24) |
7.5 mg/kg (8) or 7-10 mg/kg (24) |
P 6-10, T <2 for q8h; T <1 once daily dosing |
Note: Doses in renal failure may need to be adjusted
gm=gram; mg= milligram; kg=kilogram; MIC= minimum inhibatory concentration
Table 5: Recommended Dosages of Antimicrobial Agents Given by the Intraventricular Route
| Antimicrobial Agent | Daily Intraventricular Dose, mg | Comments |
|---|---|---|
amikacin |
5-50 |
Usual daily dose is 30 mg |
colistin |
10 |
|
gentamicin |
1-8 |
Usual daily dose is 1-2 mg for infants and children; 4-8 mg for adults. |
tobramycin |
5-20 |
|
mg= milligram
Figure 1: Approach to Antipseudomonal Antibiotic Therapy for ICU Pneumonia: Empiric Therapy
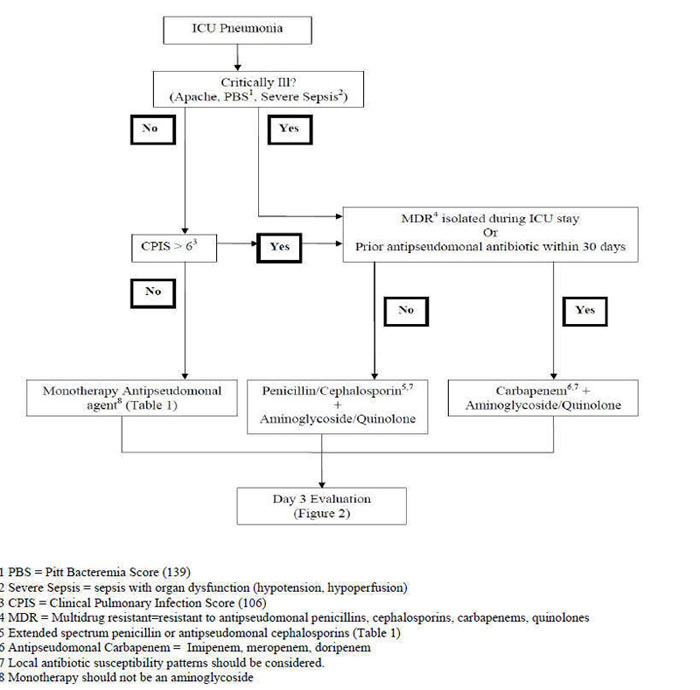
Figure 2: Day 3: Evaluation for Antipseudomonal Antibiotic Therapy in ICU Pneumonia

What's New
King P, Lomovskaya O, et al. In vitro Pharmacodynamics of Levofloxacin and Other Aerosolized Antibiotics Under Multiple Conditions Relevant to Chronic Pulmonary Infection in Cystic Fibrosis. Antimicrob Agents Chemother. 2010;54:143-8.
Dohar, JE et al. Differences in Bacteriologic Treatment Failures in Acute Otitis Externa Between Ciprofloxacin/Dexamethasone and Neomycin/Polymyxin B/Hydrocortisone: Results of a Combined Analysis. Curr Med Res Opin. 2009;25(2):287-91.
Goldstein EJ, Citron DM, et al. Introduction of Ertapenem into a Hospital Formulary: Effect on Antimicrobial Usage and Improved in vitro Susceptibility of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2009;53:5122-6.
Schechner V, et al. Gram-negative Bacteremia upon Hospital Admission: When Should Pseudomonas aeruginosa be Suspected? Clin Infect Dis. 2009;48:580-6.
Tam VH et al. Outcomes of Bacteremia due to Pseudomonas aeruginosa with Reduced Susceptibility to Piperacillin-Tazobactam: Implications on the Appropriateness of the Resistance Breakpoint. Clin Infect Dis. 2008;46:862-7.
Martinez-Solano L et al. Chronic Pseudomonas aeruginosa Infection in Chronic Obstructive Pulmonary Disease. Clin Infect Dis. 2008;47(12):1526-33.
Kusne S, Eibling DE, et al. Gangrenous Cellulitis Associated with Gram-Negative Bacilli in Pancytopenic Patients. Am J Med. 1988;85:490-4.
GUIDED MEDLINE SEARCH FOR
Review articles
Vaiman M, et al. Ecthyma gangrenosum and ecthyma-like lesions: review article. Eur J Clin Microbiol Infect Dis 2015;34:633-639.
Park SY, et al. Impact of adequate empirical combination therapy on mortality from bacteremic Pseudomonas aeurginosapneumonia. BMC Infect Dis 2012;12:308-313.
Sun HY, et al. Pneumonia Due to Pseudomonas aeruginosa: Part II: Antimicrobial Resistance, Pharmacodynamic Concepts, and Antibiotic Therapy. Chest 2011;139:1172-1185.
Fujitani S, et al. Pneumonia Due to Pseudomonas aeruginosa: Part I: Epidemiology, Clinical Diagnosis, and Source. Chest 2011;139:909-919.
Moffett KS. Pseudomonas aeruginosa in Patients with Cystic Fibrosis. 2010
Leid, JG. Bacterial Biofilms Resist Key Host Defenses. Microbe/Volume 4, Number 2, 2009.
Tabibian JH, et al. Uropathogens and Host Characteristics. J Clin Microbiol 2008;46:3980-3986.
O'Toole GA. How Pseudomonas aeruginosa regulates surface behaviors. Microbe 2008;3:65-71.
Poole K. Bacterial Multidrug Efflux Pumps Serve Other Functions. Microbe 2008;3:179-185.
Raad I, Hanna H, and Maki D. Intravascular Catheter-related Infections: Advances in Diagnosis, Prevention and Management. The LANCET Infectious Diseases 2007;7:645-657.
Hoiby N. P. aeruginosa in Cystic Fibrosis Patients Resists Host Defenses, Antibiotics. Microbe 2006;12:571-577.
Grandis JR, Branstetter BF, Yu VL. The changing face of malignant (necrotising) external otitis: clinical, radiological, and anatomic correlations. Lancet Infect Dis 2004;4:34-39.
Safdar N, Handelsman J, Maki DG. Does combination antimicrobial therapy reduce mortality in Gram-negative bacteraemia? A meta-analysis. Lancet Infect Dis. 2004;4(8): 519-27.
Efflux Pumps as a Mechanism of Antimicrobial Resistance.
Javey G, Zuravleff J. Keratitis.
Golnaz J, Zuravleff J. Retinitis and Endophthalmitis.
GUIDED MEDLINE SEARCH FOR RECENT REVIEWS
Table of Contents
- Microbiology
- Epidemiology
- Clinical Manifestations
- Laboratory Diagnosis
- Pathogenesis
- Susceptibility in Vitro and in Vivo
- Antimicrobial Therapy
- Special Situations
- Adjunctive Therapy
- Endpoints for Monitoring Therapy
- Vaccines
- Infection Control Measures

