Diabetic Foot Infections
Updated July, 2011
Authors: Paul B. Cornia, M.D., Benjamin A. Lipsky, M.D.
Foot infections are common, costly, potentially limb or even life-threatening complications of diabetes mellitus. Diabetic foot infection may be defined most simply as any acute or chronic inflammatory response to a microbial invasion in the infra-malleolar area in a person with diabetes. Because of the comorbidities associated with diabetes, these infections may begin as a seemingly minor problem but often progress, sometimes rapidly, if not managed appropriately. Proper treatment often requires appropriate wound care (usually including debridement) as well as antimicrobial therapy. Most often antibiotic therapy must be initiated empirically in persons with diabetic foot infection while awaiting the results of wound cultures. Because of the complexity of diabetic foot infection, an evidence-based, well coordinated, multi-disciplinary team approach improves outcomes (1, 7, 27).
EPIDEMIOLOGY
Most diabetic foot infections begin with a break in the protective cutaneous barrier, typically in the form of a neuropathic ulcer. The lifetime risk of foot ulceration in persons with diabetes is about 15-25% and about 60% of ulcers are clinically infected at presentation (4). A recent prospective study found that despite patient education and provider follow-up, 9% of diabetic patients developed a foot infection during a two year observation period (4); nearly all of these were precipitated by a foot wound. In developed countries, 85% of lower extremity amputations in diabetic patients are preceded by a foot ulcer. A complex, incompletely understood interplay of risk factors may conspire to cause foot ulceration and foot infection in persons with diabetes (Table 1 ).
PATHOGENESIS
Peripheral sensory neuropathy plays the central role in the development of foot ulcers, primarily by causing a loss of protective sensation. Importantly, most patients do not recognize the loss of protective sensation, underscoring the need for preventive efforts including serial surveillance screening and daily foot inspection. Skin ulceration usually results from unperceived repetitive sheer-type trauma due to altered weight bearing (foot deformities and excess plantar pressure), ill fitting shoes, or less commonly, skin-penetrating trauma. Peripheral motor neuropathy can cause abnormal foot biomechanics and lead to distorted foot anatomy. Dry, thickened, and cracked skin related to peripheral autonomic neuropathy increases the risk of skin breaks, offering a portal of entry for bacteria. Most foot ulcers develop on the plantar surface of the foot, especially at the metatarsal heads and to a lesser degree on the toes and calcaneum, the sites of highest pressure with standing and ambulation. Once a foot ulcer develops, several poorly characterized immunological and metabolic perturbations may impair healing and allow the infection to progress. Another risk factor for developing an infection, and for worsening its severity, is limb ischemia. Peripheral arterial disease typically affecting the major arteries between the knee and ankle, is twice as common in persons with diabetes as in non-diabetics (14). Diminished tissue perfusion inhibits wound healing and impairs delivery of antibiotics to infected tissue. Diabetes-related immune dysfunction, caused by impaired neutrophil and macrophage chemotaxis and phagocytosis, also predisposes to more frequent, rapidly spreading and severe infections (13).
Microbiology
Selecting an appropriate empiric antibiotic regimen requires knowing the usual etiologic organisms (Table 2). Numerous studies have assessed the microbiology of diabetic foot infection, but because of the heterogeneity of the patient populations and culture methods used the reported results vary substantially (24). Isolation of multiple species of bacteria from a wound specimen is common with many studies reporting a mean of 2-5 isolates per case (24). Isolation of bacteria from a wound specimen does not define pathogenicity and distinguishing pathogens from colonizers may be difficult. Nonetheless, in almost all cases collecting appropriately obtained specimens for culture is helpful. The results of these cultures may allow tailoring of the antibiotic regimen – either to a narrower spectrum or one targeted to specific bacteria not covered by the initial empiric regimen. Almost all studies, however, show that aerobic gram-positive cocci, particularly Staphylococcus aureus, are responsible for most acute infections, especially in patients who have not recently received antibiotic therapy. Methicillin-resistantStaphylococcus aureus(MRSA) is an increasingly frequent pathogen in diabetic foot infections. Recent studies have reported that an increasing frequency of MRSA isolates in diabetic foot infections. A recent review of studies conducted between 1993 and 2007 found a prevalence of MRSA in diabetic foot infection ranging from 5-30%; the majority reported rates of 10-20% (9). Coagulase-negative staphylococci are also frequently isolated, and may be mistakenly dismissed as contaminants. Because persons with diabetes are immunologically compromised, these are often true pathogens. Beta-hemolytic streptococci (usually group B) are also relatively frequent pathogens especially in patients with cellulitis. Enterococci are among the more common isolates in many studies, but their clinical significance is uncertain. Because cephalosporins are active against many common pathogens in diabetic foot infection, but not enterococci, treatment with this class predisposes to infection withEnterococcusspp.
Aerobic gram-negative bacilli, usually including Enterobacteriaceae (Escherichia coli, Proteus, Klebsiella, and Enterobacter) are also frequently isolated from diabetic foot infection, especially chronic or previously treated infections. Gram-negative rods are rarely the sole, or even predominant, pathogen. Many broad-spectrum antibiotic agents will cover Enterobacteriaceae, but notP Pseudomonas aeroginosa. Pseudomonas deserves specific mention, as it is a relatively frequent isolate and usually requires specifically targeted therapy. This water-borne organism often colonizes, and sometimes infects, wounds that have been soaked or subjected to hydrotherapy. It is also reported more frequently from countries with warmer climates (11, 17, 26); this may be a consequence of excessive sweating into shoes or wearing open or no footwear with exposure to the soil. Gram-negative organisms that elaborate extended spectrum beta-lactamases (ESBL) are also a growing problem in diabetic foot infection. As withPseudomonas, these generally require specifically targeted regimens.
Obligately anaerobic organisms may also cause diabetic foot infection. Recognizing these isolates requires obtaining proper (usually tissue) specimens and then quickly and appropriately processing them. The most frequent anaerobic isolates are peptococci and peptostreptococci, and less often Bacteroides species. These may be important pathogens, but almost exclusively occur as part of a polymicrobial infection with an ischemic or necrotic wound. The clinician must also be aware that the causative organism may change over time, especially if the patient’s infection fails to clinically respond to simple regimens.
(Printable Version of Causes of Diabetic Foot Infections)
Initial Assessment
Not all ulcers are infected. Since all wounds are colonized by microorganisms, infection must be diagnosed clinically rather than microbiologically. Various authoritative committees (Infectious Disease Society of America [IDSA], International Working Group on the Diabetic Foot [IWGDF], and American Diabetes Association) have defined infection in the diabetic foot as the presence of purulent secretions or at least two symptoms or signs of infection (erythema, warmth, tenderness, pain, or induration). Importantly, local and systemic inflammatory responses to infection may be diminished in those with peripheral neuropathy or arterial insufficiency – additional signs of infection that may be useful include purulent secretions, friable or discolored granulation tissue, undermining of the wound edges or a foul odor. In a patient with limb ischemia, infection may reach a limb-threatening state before the patient or clinician recognizes the problem. Eradicating infection in a wound will certainly facilitate healing, but it will usually take additional time for the wound to completely heal. As long as the wound remains, it is at continued risk of re-infection. Thus, curing infection is a separate, albeit related, issue to wound healing.
Because of the complex nature of diabetic foot infection and the potential for
rapid worsening (sometimes within hours), the clinician must assess the patient
promptly, methodically and repeatedly (Figure 1 and Figure 2, IDSA guidelines). Evaluate for systemic
evidence of infection (e.g., fever, chills, leukocytosis), examine the affected
limb (i.e., for foot deformities, altered biomechanics, neuropathy, and arterial
insufficiency) and finally the wound (size ![]() , depth, tissues involved
, depth, tissues involved ![]() , necrosis/gangrene
, necrosis/gangrene ![]() ,
foreign objects). There are several classification schemes for diabetic foot ulcers
,
foreign objects). There are several classification schemes for diabetic foot ulcers ![]() and the lack of consensus on wound definitions and infection classification
makes comparison of published studies difficult and is confusing to clinicians.
Most, however agree that the critical factors in evaluating a diabetic foot
wound are its depth and the limb’s vascular status. The recently published
guidelines from the IWGDF (15) and IDSA (5) (update in progress, publication
planned 2011) are similar (Table 3). The IDSA
scheme has been validated and predicts clinical outcome (6). Assessing the
component features should influence decisions regarding site of therapy
(inpatient vs. outpatient); the spectrum, route of administration and duration
of antibiotic therapy; the urgency of any necessary surgical intervention; and
likely, the outcome. Identifying causative pathogens using proper wound
culturing techniques guides antibiotic therapy, especially for chronic
infections and persons recently treated with antibiotics. A Gram-stained smear
of a wound specimen can provide real-time information on the likely causative
organisms. When selecting an initial antibiotic regimen, it is most helpful for
deciding whether or not to add coverage for gram-negative rods in a patient with
mild infection.
and the lack of consensus on wound definitions and infection classification
makes comparison of published studies difficult and is confusing to clinicians.
Most, however agree that the critical factors in evaluating a diabetic foot
wound are its depth and the limb’s vascular status. The recently published
guidelines from the IWGDF (15) and IDSA (5) (update in progress, publication
planned 2011) are similar (Table 3). The IDSA
scheme has been validated and predicts clinical outcome (6). Assessing the
component features should influence decisions regarding site of therapy
(inpatient vs. outpatient); the spectrum, route of administration and duration
of antibiotic therapy; the urgency of any necessary surgical intervention; and
likely, the outcome. Identifying causative pathogens using proper wound
culturing techniques guides antibiotic therapy, especially for chronic
infections and persons recently treated with antibiotics. A Gram-stained smear
of a wound specimen can provide real-time information on the likely causative
organisms. When selecting an initial antibiotic regimen, it is most helpful for
deciding whether or not to add coverage for gram-negative rods in a patient with
mild infection.
Determining the Severity of Infection
Systemic Evidence of Infection:
Systemic symptoms and signs of infection include fevers, chills, diaphoresis, anorexia, hemodynamic instability (tachycardia, hypotension), metabolic derangements (e.g., acidosis, dysglycemia, volume depletion, renal failure), leukocytosis and inflammatory markers. Surprisingly to many clinicians, these are uncommon in patients with a diabetic foot infection. When systemic signs or symptoms are present they generally signify severe infection with extensive tissue involvement or more virulent pathogens. But, elevated temperature, white blood cell count, or sedimentation rate are absent in up to 50% of severe diabetic foot infection (10).
Extent of Tissue Involvement:
A key factor in determining the outcome of a diabetic
foot infection is to assess the wound depth and which tissues are involved. This
requires first debriding ![]() any necrotic material or callus, then gently probing to any abscesses, sinus tracts, foreign bodies or bone or joint involvement
any necrotic material or callus, then gently probing to any abscesses, sinus tracts, foreign bodies or bone or joint involvement ![]() . Occasionally, defining the extent of infection
requires an imaging study (usually MRI) or surgical exploration. If there is any
concern for necrotizing deep space infection
. Occasionally, defining the extent of infection
requires an imaging study (usually MRI) or surgical exploration. If there is any
concern for necrotizing deep space infection ![]() ,
an experienced surgeon should evaluate the patient. Deeper and more extensive
infections may respond more slowly to appropriate antibiotic therapy. Palpating bone
,
an experienced surgeon should evaluate the patient. Deeper and more extensive
infections may respond more slowly to appropriate antibiotic therapy. Palpating bone ![]() in a diabetic foot ulcer using a steel probe (a positive “probe-to-bone” test)
is a simple and useful bedside test to aid in the diagnosis of osteomyelitis (6, 8). The positive predictive value approaches 90% when the pre-test probability
of osteomyelitis is high (12), but
is closer to 55% when the prevalence (usually ~20%) is lower (21). Visibly
exposed bone probably provides similar information as probing bone.
in a diabetic foot ulcer using a steel probe (a positive “probe-to-bone” test)
is a simple and useful bedside test to aid in the diagnosis of osteomyelitis (6, 8). The positive predictive value approaches 90% when the pre-test probability
of osteomyelitis is high (12), but
is closer to 55% when the prevalence (usually ~20%) is lower (21). Visibly
exposed bone probably provides similar information as probing bone.
Assessment of Peripheral Arterial Perfusion:
The presence of PAD is an independent risk
factor for developing a diabetic foot infection (20) and is present in up to 40%
of cases ![]() .
The presence of significant arterial insufficiency in an infected limb adversely
affects host immunological responses and wound healing and impairs delivery of
systemic antibiotics to the infected tissues. The absence of pedal pulses
suggests peripheral artery disease, but this method of assessment of arterial
perfusion is often not reliable, especially in persons with diabetes.
Determining the ratio of ankle to brachial artery systolic blood pressure
(ankle/brachial index [ABI]) is a simple, reliable, non-invasive, bedside
procedure to assess for peripheral artery disease (30) and should be performed
in most patients with diabetic foot infection especially if pedal pulses are
absent or diminished. An ABI <0.90 is abnormal, and <0.40 signifies severe
ischemia (Table 4), but arterial calcification of
vessels may falsely elevate the ankle/brachial index in patients with diabetes.
Assessing the transcutaneous partial pressure of oxygen (TcpO2) in the skin of
the foot is another non-invasive method of assessing peripheral arterial
perfusion. TcpO2 values of less than 30mmHg signify critical limb ischemia and
predict poor wound healing. If significant peripheral artery disease is
suspected based on history, physical examination, or non-invasive testing,
vascular surgery consultation is appropriate. Limb revascularization may be
necessary to cure infection and promote healing. Having access to an active
lower extremity revascularization program can decrease amputation rates and
increase the incidence of foot sparing surgeries that have a more favorable
long-term outcome.
.
The presence of significant arterial insufficiency in an infected limb adversely
affects host immunological responses and wound healing and impairs delivery of
systemic antibiotics to the infected tissues. The absence of pedal pulses
suggests peripheral artery disease, but this method of assessment of arterial
perfusion is often not reliable, especially in persons with diabetes.
Determining the ratio of ankle to brachial artery systolic blood pressure
(ankle/brachial index [ABI]) is a simple, reliable, non-invasive, bedside
procedure to assess for peripheral artery disease (30) and should be performed
in most patients with diabetic foot infection especially if pedal pulses are
absent or diminished. An ABI <0.90 is abnormal, and <0.40 signifies severe
ischemia (Table 4), but arterial calcification of
vessels may falsely elevate the ankle/brachial index in patients with diabetes.
Assessing the transcutaneous partial pressure of oxygen (TcpO2) in the skin of
the foot is another non-invasive method of assessing peripheral arterial
perfusion. TcpO2 values of less than 30mmHg signify critical limb ischemia and
predict poor wound healing. If significant peripheral artery disease is
suspected based on history, physical examination, or non-invasive testing,
vascular surgery consultation is appropriate. Limb revascularization may be
necessary to cure infection and promote healing. Having access to an active
lower extremity revascularization program can decrease amputation rates and
increase the incidence of foot sparing surgeries that have a more favorable
long-term outcome.
Laboratory Diagnosis
Obtaining Cultures in Diabetic Foot Infections
Properly obtained wound cultures (Table 5) are
useful for guiding antibiotic therapy in diabetic foot infections, particularly
in patients with chronic infections or who have recently been treated with
antibiotics. Culture specimens should be obtained after the wound has been
cleansed and debrided. A sample obtained by curettage, the aseptic scraping of
tissue at an ulcer base using a scalpel blade or dermal curette, more accurately
identifies pathogens than a wound swab ![]() .
Swabs are often contaminated with normal skin flora or colonizers (24) and are
less likely to grow anaerobic, and some fastidious aerobic organisms. Specimens
must be promptly transported to the laboratory, in an appropriate sterile
transport system, where they should be processed for aerobic and anaerobic
cultures and a Gram-stained smear. Other acceptable methods of culturing wounds
include aspiration of cellulitic tissue or purulent secretions, and tissue
biopsy obtained either at the bedside or at surgery. A bone biopsy, obtained
surgically or percutaneously
.
Swabs are often contaminated with normal skin flora or colonizers (24) and are
less likely to grow anaerobic, and some fastidious aerobic organisms. Specimens
must be promptly transported to the laboratory, in an appropriate sterile
transport system, where they should be processed for aerobic and anaerobic
cultures and a Gram-stained smear. Other acceptable methods of culturing wounds
include aspiration of cellulitic tissue or purulent secretions, and tissue
biopsy obtained either at the bedside or at surgery. A bone biopsy, obtained
surgically or percutaneously ![]() ,
processed for culture (and histological assessment, if possible) is the
criterion standard for diagnosing osteomyelitis. The
results of wound or sinus tract cultures do not accurately reflect those of bone
culture. Blood cultures are not
frequently positive in these infections but should be obtain in patients with
systemic symptoms and signs of infection. In the minority of cases with
bacteremia,S. aureusis the most frequently isolated pathogen.
,
processed for culture (and histological assessment, if possible) is the
criterion standard for diagnosing osteomyelitis. The
results of wound or sinus tract cultures do not accurately reflect those of bone
culture. Blood cultures are not
frequently positive in these infections but should be obtain in patients with
systemic symptoms and signs of infection. In the minority of cases with
bacteremia,S. aureusis the most frequently isolated pathogen.
Imaging Studies
Imaging studies may be useful in a patient with diabetic foot infection to
assess for any foreign material, soft tissue abscesses![]() ,
or bony abnormalities. Plain radiographs are usually the appropriate first study
but have limited diagnostic utility in assessing for osteomyelitis
,
or bony abnormalities. Plain radiographs are usually the appropriate first study
but have limited diagnostic utility in assessing for osteomyelitis ![]() . They lack sensitivity early in infection because abnormalities on plain film may
not be evident until 50% of the bone is resorbed which typically requires 2-3
weeks. They also lack specificity because neuroarthropathy (Charcot foot) may
have a similar radiographic appearance. If suspicion for osteomyelitis remains
despite an initial negative radiograph, repeating plain films in a few weeks can
either exclude the diagnosis (if still negative) or suggest that it has
developed (if there is cortical erosion, periosteal elevation or other
suggestive changes in one underlying the affected soft tissue).
. They lack sensitivity early in infection because abnormalities on plain film may
not be evident until 50% of the bone is resorbed which typically requires 2-3
weeks. They also lack specificity because neuroarthropathy (Charcot foot) may
have a similar radiographic appearance. If suspicion for osteomyelitis remains
despite an initial negative radiograph, repeating plain films in a few weeks can
either exclude the diagnosis (if still negative) or suggest that it has
developed (if there is cortical erosion, periosteal elevation or other
suggestive changes in one underlying the affected soft tissue).
Radionucleotide bone scans (using bisphosphonate-linked technetium or other
radionuclides) are more sensitive than plain radiographs for diagnosing
osteomyelitis, but uptake occurs with any type of inflammation ![]() ,
resulting in poor specificity (~50%). Labeled (e.g., with Indium111) white cell
or immunoglobulin scans have better specificity (~75%) than bone scans
,
resulting in poor specificity (~50%). Labeled (e.g., with Indium111) white cell
or immunoglobulin scans have better specificity (~75%) than bone scans ![]() .
Among imaging modalities, magnetic resonance imaging
.
Among imaging modalities, magnetic resonance imaging ![]() has the best overall sensitivity (>90%) and specificity (>80%) for detecting
osteomyelitis and higher resolution for soft tissue abnormalities. It is now
considered the imaging procedure of choice (17, 21), but it is still relatively
expensive and often not readily available.<
has the best overall sensitivity (>90%) and specificity (>80%) for detecting
osteomyelitis and higher resolution for soft tissue abnormalities. It is now
considered the imaging procedure of choice (17, 21), but it is still relatively
expensive and often not readily available.<
(Printable Version of Assessment of Diabetic Foot Infections)
MANAGEMENT
Antibiotic Therapy
Diabetic foot infection may be limb- or even life-threatening and may progress rapidly. Too narrow an empiric antibiotic regimen may therefore lead to a poor clinical outcome. Furthermore, diabetic foot infection are commonly polymicrobial. Thus, clinicians should generally opt for broad-spectrum empiric therapy while awaiting culture and sensitivity results. This is not, however, always necessary. Unnecessarily broad-spectrum therapy also has potential adverse consequences, such as increasing antibiotic resistance (a potential problem for both the individual patient and society as a whole), increased financial cost, and potentially increased risk of drug toxicity. Properly obtained specimens for microbial evaluation are crucial to optimally direct antibiotic therapy and to potentially allow the empiric antibiotic regimen to be narrowed or targeted.
Deciding upon an empiric antibiotic regimen for diabetic foot infection is challenging, but understanding some basic principles can guide clinicians (22). An important place to start is to realize that about half of diabetic foot wounds have no clinical evidence of infection (20). Some believe that the presence of a high density of organisms, usually defined as >105 per gram of tissue, represents “critical colonization” that may require antibiotic therapy (5), but the few published trials of antibiotic therapy for uninfected lesions do not support this practice. Thus, clinically uninfected wounds generally require neither a culture nor antibiotic therapy.
For clinically infected lesions, mild, and most moderate, infections (Table 3) can be treated with relatively narrow spectrum agents predominantly directed at staphylococci and streptococci. An exception to this would be a patient who has recently been treated with an antibiotic. This may predispose to infection with more unusual and antibiotic resistant organisms. More severe infections generally mandate a broader-spectrum regimen. All empiric regimens should cover aerobic gram-positive cocci, especially Staphylococcus aureus, since they are the most frequently isolated organism from a diabetic foot infection (acute and chronic; mild, moderate and severe).
The decision as to whether or not to provide empiric coverage for MRSA is based on the presence of known risk factors and the local prevalence MRSA. Previous history of MRSA infection or colonization may be the best predictor. Other risk factors include recent long-term antibiotic use, recent hospitalization, prolonged duration of the wound, and presence of osteomyelitis, but these have not been consistently reported in all studies.
Aerobic gram-negative bacilli are also commonly isolated as part of a
polymicrobial infection ![]() ,
especially in a chronic wound in a patient who has received antibiotic therapy.
Because Pseudomonas aeruginosa is a hydrophilic organism, hydrotherapy (e.g., soaking the affected foot in
water or debridement with water lavage) is the main risk factor. Even when
isolated in diabetic foot infection, Pseudomonas is usually part of a mixed
infection and rarely the predominant pathogen and patients often improve without
anti-pseudomonal antibiotic therapy. Selecting a regimen aimed at this
frequently antibiotic resistant organism should thus be reserved for patients
with one of the noted risk factors or who have green-blue colored wound drainage
(Table 2) and a more severe infection
,
especially in a chronic wound in a patient who has received antibiotic therapy.
Because Pseudomonas aeruginosa is a hydrophilic organism, hydrotherapy (e.g., soaking the affected foot in
water or debridement with water lavage) is the main risk factor. Even when
isolated in diabetic foot infection, Pseudomonas is usually part of a mixed
infection and rarely the predominant pathogen and patients often improve without
anti-pseudomonal antibiotic therapy. Selecting a regimen aimed at this
frequently antibiotic resistant organism should thus be reserved for patients
with one of the noted risk factors or who have green-blue colored wound drainage
(Table 2) and a more severe infection ![]() .
As previously mentioned, recent reports from developing countries with a
tropical or arid climate have shown more frequent isolation of aerobic
gram-negative bacilli, including pseudomonas, rather than gram-positive cocci.
Clinicians in such regions may consider broader spectrum (i.e., covering both
aerobic gram-negative bacilli and gram-positive cocci) empirical therapy.
.
As previously mentioned, recent reports from developing countries with a
tropical or arid climate have shown more frequent isolation of aerobic
gram-negative bacilli, including pseudomonas, rather than gram-positive cocci.
Clinicians in such regions may consider broader spectrum (i.e., covering both
aerobic gram-negative bacilli and gram-positive cocci) empirical therapy.
Obligate anaerobic bacteria are isolated from a substantial minority of cases, but almost always as part of a polymicrobial infection and usually associated with a chronic wound. Anaerobes are more often isolated from necrotic or gangrenous wounds with limb ischemia. A foul, feculent odor (the so called “fetid foot”) is also a clue to infection with anaerobes. Since oxygen is a potent anti-anaerobic agent, adequate treatment may require only debridement of necrotic tissue and exposing the remaining organisms to air. An empiric antibiotic regimen directed at anaerobic organisms may be appropriate when clinical suspicion is high and the infection is moderate to severe. The anaerobes in diabetic foot infection are mainly gram-positives (peptococci or peptostreptococci) rather than Bacteroides spp. Especially with anaerobic infections, antibiotic therapy is no substitute for adequate debridement and drainage.
One final consideration is fungal infection. While tinea infections of the webspaces between the toes and onychomycosis are frequent in diabetic patients, pathogenic fungal infections are not. Occasionally, however, a patient who has received multiple antibiotics, who has severe hyperglycemia, or who is receiving corticosteroid therapy will develop an invasive soft tissue fungal infection.
Surprisingly few studies have been performed to assess the efficacy of various antibiotics to treat diabetic foot infection. Furthermore, the available studies are not well standardized, making comparisons difficult. In virtually all comparative studies the regimens produced equivalent results. Thus, while several agents have been used successfully to treat diabetic foot infection, no specific antibiotic regimens have emerged as being preferred for a particular type of infection. Based on a review of available studies, the IDSA formulated suggested antibiotic regimens for the treatment of soft-tissue diabetic foot infection, as shown in Table 6. The general approach is to select as narrow spectrum, safe, and convenient a regimen as possible (IDSA guidelines). In about two-thirds of patients this will need to be empiric. When culture (or Gram-stained smear) results are available, the clinician should of course use this information. After 2-3 days, the clinician should reassess the patient and the infection and consider altering the definitive regimen based on the culture and sensitivity results and the patient’s clinical response to the selected regimen.
Mild infections ![]() may be treated with relatively narrow spectrum oral therapy
directed at these aerobic gram-positive cocci, such as penicillinase-resistant
penicillins (e.g., dicloxacillin), first-generation cephalosporins (e.g., cephalexin), or clindamycin. Some mildly infected wounds can be treated with
topical antimicrobial therapy, either antiseptics (e.g., silver or iodine based)
or antibiotic (e.g., mupirocin or bacitracin) but there are currently few
published studies to support this approach. New topical products are now
undergoing testing and may emerge as useful treatments for selected cases.
may be treated with relatively narrow spectrum oral therapy
directed at these aerobic gram-positive cocci, such as penicillinase-resistant
penicillins (e.g., dicloxacillin), first-generation cephalosporins (e.g., cephalexin), or clindamycin. Some mildly infected wounds can be treated with
topical antimicrobial therapy, either antiseptics (e.g., silver or iodine based)
or antibiotic (e.g., mupirocin or bacitracin) but there are currently few
published studies to support this approach. New topical products are now
undergoing testing and may emerge as useful treatments for selected cases.
Moderate ![]() to severe infections
to severe infections ![]() often necessitate empirical regimens with activity against commonly isolated
gram-negative bacilli, MRSA (if there are risk factors as previously discussed)
and perhaps Enterococcus species. Enterococci are relatively frequent isolates, especially in patients
previously treated with cephalosporins, but they are often colonizers and rarely
primary pathogens. Broadening empirical antibiotic coverage based on previously
mentioned clinical findings (Table 1
) is often
appropriate. As a general rule, we err on the side of overly broad initial
coverage, put our effort into obtaining high quality specimens for culture,
ensuring proper wound care and thinking more about the definitive antibiotic
regimen to complete the course when culture results are available. We think most
errors in treatment consist of failing to alter (and curtail) antibiotic therapy
rather than in making a poor initial antibiotic choice.
often necessitate empirical regimens with activity against commonly isolated
gram-negative bacilli, MRSA (if there are risk factors as previously discussed)
and perhaps Enterococcus species. Enterococci are relatively frequent isolates, especially in patients
previously treated with cephalosporins, but they are often colonizers and rarely
primary pathogens. Broadening empirical antibiotic coverage based on previously
mentioned clinical findings (Table 1
) is often
appropriate. As a general rule, we err on the side of overly broad initial
coverage, put our effort into obtaining high quality specimens for culture,
ensuring proper wound care and thinking more about the definitive antibiotic
regimen to complete the course when culture results are available. We think most
errors in treatment consist of failing to alter (and curtail) antibiotic therapy
rather than in making a poor initial antibiotic choice.
Additional considerations include the site of treatment and route of drug administration. Intravenous antibiotic therapy and hospitalization are not only costly but also potentially expose patients to iatrogenic complications and nosocomial infection. The severity of infection is usually the most important determinant of the need for hospitalization. Other factors may also influence the decision, such as the clinician’s desire to closely monitor the wound and response to treatment, a need for expedited diagnostic testing or consultant evaluation, and adverse social circumstances (e.g., inability of the patient to adequately care for the wound, off-load the foot, or uncertainty regarding compliance with antibiotics or follow-up care). Most patients with mild to moderate infections can be successfully treated as outpatients with oral antibiotic therapy ( 23). Many oral antibiotics have high bioavailability allowing therapeutic serum levels. An antibiotic’s pharmacokinetic and pharmacodynamic properties determine its serum, and therefore tissue, levels. When parenteral therapy is needed, it can often be offered by outpatient intravenous therapy with a once daily agent when this service is available. The arterial supply to the foot must also be adequate to ensure delivery of therapeutic antibiotic levels to the infected tissue.
After
wound culture and antibiotic susceptibility results become available, the
clinician should consider narrowing the antibiotic coverage to the isolated
pathogen(s). Clinical assessment of the patient and wound, including the
response to the empirical regimen, are paramount in tailoring the antibiotic
regimen. If the wound is clinically responding, it is reasonable to continue an
empirical regimen, even if it does not have activity against all the isolated
organisms. Conversely, if a wound is not improving, broaden antibiotic coverage
to cover all isolated organisms, even those whose pathogenicity is uncertain.
The optimal duration of antibiotic therapy has not been studied, but we
recommend one to two weeks for mild to moderate soft tissue infections and at
least two weeks (occasionally longer) for deeper or more complex soft tissue
infections (Table 7). It is not usually necessary
to continue antibiotic therapy for soft tissue infections beyond the point when
all clinical signs if infection have resolved ![]() .
.
Surgical Intervention
Surgical
procedures designed to control infection and preserve as much the affected limb
as possible are frequently required in the treatment of diabetic foot infection
(29). Early, thorough debridement of infected and necrotic tissue helps define
the extent of infection and may reduce the likelihood of future amputation.
Severe infections may be immediately limb-threatening and require urgent
surgical consultation. Unexplained, persistent foot pain, particularly in a
patient known to have advanced neuropathy (i.e., a previously insensate foot) or
distortion of the superficial anatomy (e.g., bulging of the plantar surface ![]() or dorsal induration
or dorsal induration ![]() in a patient with a plantar ulcer) may indicate deep space infection that
warrants prompt evaluation by a surgeon. When amputation is necessary, the
surgeon should make every effort to perform a minor (below the ankle) rather
than a major amputation to preserve functionality. If severe peripheral arterial
disease is present, a vascular surgeon should be consulted promptly. Non-urgent
amputation should rarely be done without a vascular surgery consultation. Early
revascularization (i.e., within days) is generally preferred to delaying the
procedure for a prolonged course of potentially ineffective antibiotic therapy.
Endovascular or bypass procedures can be limb salvaging in some patients with a
diabetic foot infection.
in a patient with a plantar ulcer) may indicate deep space infection that
warrants prompt evaluation by a surgeon. When amputation is necessary, the
surgeon should make every effort to perform a minor (below the ankle) rather
than a major amputation to preserve functionality. If severe peripheral arterial
disease is present, a vascular surgeon should be consulted promptly. Non-urgent
amputation should rarely be done without a vascular surgery consultation. Early
revascularization (i.e., within days) is generally preferred to delaying the
procedure for a prolonged course of potentially ineffective antibiotic therapy.
Endovascular or bypass procedures can be limb salvaging in some patients with a
diabetic foot infection.
Wound Care
After the initial assessment and treatment, the patient requires ongoing wound care. Regular follow-up evaluations and repeated debridement of any residual dead tissue can help promote continued healing. Many dressings and wound-care products are marketed but none are favored based on evidence. A moist dressing that allows daily wound inspection is optimal. The roles of adjunctive treatments such as hyperbaric oxygen and granulocyte colony stimulating factors have not yet been adequately defined. Meta-analyses of these treatments suggest that an as yet undefined subset of patients may benefit from these treatments. Considering their cost they should rarely be used as initial therapy but rather reserved for patients who are not responding to what should otherwise be appropriate treatment.
Many clinicians and patients do not recognize the importance of off-loading pressure from the affected foot ( 2, 4). A variety of footwear devices are designed to redistribute pressure off the affected part of the foot. The total contact cast has been shown to be the best device, but the clinician and patient cannot easily view the infected wound in this device. In some patients healing sandals, half shoes, and removable cast walkers are adequate. Complete offloading of the affected limb may also be achieved with crutches, a walker or a wheelchair; however, these devices may increase the risk of ulceration of the contralateral limb and confinement to a wheelchair may not be practical and leads to deconditioning. With the exception of the total contact cast (which the patient cannot remove), patient compliance is often suboptimal. Select a dressing and off-loading device with the fact in mind that infected wounds must generally be examined and dressed daily.
Osteomyelitis
Osteomyelitis frequently complicates diabetic foot infection but its evaluation and management remain controversial (16). Except for direct inoculation by a puncture wound, bone infection spreads from soft tissue to cortex to marrow. Thus, nearly all cases in the setting of a diabetic foot infection are chronic osteomyelitis. Traditionally, surgical resection of infected and necrotic bone has been viewed as the best approach to curing these infections. Recent retrospective reports have demonstrated clinical success in ~65-80% of selected cases using prolonged (3-6 months) antibiotic (usually fluoroquinolone) therapy with little or no surgery. Prospective trials are needed to define in which clinical circumstances osteomyelitis may be appropriately treated without surgery. Using the results of bone culture is the preferred means to guide antibiotic therapy, especially if it is to be prolonged. When all the necrotic bone has been resected, 4-6 weeks of antibiotic therapy for the residual infected bone is usually sufficient. If nonsurgical treatment is selected, initial parenteral therapy (for perhaps 1-2 weeks) is recommended. Oral antibiotic(s) with good bioavailability may then be used to complete a course of therapy. If all infected and necrotic bone has been resected, a brief course of antibiotic therapy (1-2 weeks) for any residual soft tissue infection is sufficient (3). While much discussed, the data on the accuracy of measuring and importance of achieving adequate bone levels of antibiotics to treat osteomyelitis are weak. Some studies support the use of clindamycin; clinical experience suggests that beta-lactams and selected fluoroquinolones work well and some recent case series support using linezolid (for the shortest necessary duration and with careful monitoring for hematological and neurological toxicity).
PREVENTION
Having a diabetic foot infection increases the risk of future recurrence – emphasize this to the patient at this teachable moment to underscore the importance of preventive strategies. Identifying patients with peripheral neuropathy, foot deformity or peripheral artery disase, and educating them about the risk of foot ulcer and diabetic foot infection is a simple, but critical and often overlooked, way to prevent diabetic foot infection (28). Primary and secondary prevention measures include optimizing metabolic (glycemic, lipid) control, wearing appropriate footwear, avoiding mechanical or thermal foot trauma, and performing a daily foot inspection. Fungal infections of the foot are more common in people with diabetes. Patients with tinea pedis should be treated with a topical anti-fungal, while those with severe onychomycosis may benefit from oral anti-fungal therapy. Persons with major foot complications should be referred to a specialist (e.g., podiatric, orthopedic or vascular surgeons).
CONCLUSIONS
Diabetic foot infection is a common, complex complication of diabetes. An early and thorough clinical evaluation, with attention to the presence of systemic symptoms and signs infection, the extent of local tissue involvement, and an assessment of peripheral arterial perfusion is essential. Obtaining proper culture specimens (e.g., biopsy or curettage of the cleansed, debrided ulcer base or aspiration of purulent secretions) will help guide antibiotic selection. Most patients should have plain radiographs of the foot. If osteomyelitis is suspected, this is best diagnosed by MRI and bone biopsy for microbiological and histological evaluation. Initial antibiotic therapy is usually empirical. In the absence of culture results, this should be directed at the most commonly encountered pathogens, i.e., aerobic gram-positive cocci. Certain clinical clues can suggest the presence of gram-negative rods or anaerobes. The spectrum of pathogens targeted by the empirical regimen, as well as the route of administration, duration of therapy, and need for hospitalization are primarily determined by the severity of infection. The antibiotic regimen should be re-assessed when culture and sensitivity tests become available, but any changes should be guided by the patient’s clinical response to the empirical agents. Deep space and extensive infections typically require surgical interventions. Consider early revascularization for a severely ischemic limb. Amputation may be required, but much of the foot can often be spared. Since infection often recurs, patients must be educated to examine their feet routinely and counseled to seek prompt medical attention at the first appearance of symptoms or signs of infection.
(Printable Version of Management of Diabetic Foot Infections)
REFERENCES
1. Armstrong DG, Harless LB. Outcomes of preventative care in a diabetic foot clinic. J Foot Ankle Surg. 1998; 37: 460-6. [PubMed]
2. Armstrong DG, Lavery LA, Nixon BP, Boulton AM. It’s not what you put on, but what you take off: techniques for debriding and off-loading the diabetic foot wound. Clin Infect Dis. 2004; 39 Suppl 2: S92-9. [PubMed]
3. Berendt AR, Lipsky BA, Jeffcoate WJ, et al. Progress report on the diagnosis and treatment of diabetic foot osteomyelitis. International Working Group on the Diabetic Foot, interactive DVD available at www.idf.org/bookshop.
4. Boulton AJ. Pressure and the diabetic foot: clinical science and off-loading techniques. Am J Surg 2004; 187: 17S-24S. [PubMed]
5. Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev. 2001; 14: 244-69. [PubMed]
6. Butalia S, Palda VA, Sargeant RJ, Detsky AS, Mourad O. Does this patient with diabetes have osteomyelitis of the lower extremity? JAMA. 2008; 299: 806-13. [PubMed]
7. Crane M, Werber B. Critical pathway approach to diabetic pedal infections in a multidisciplinary setting. J Foot Ankle Surg. 1999; 38: 30-3. [PubMed]
8. Dinh MY, Abad CL, Safdar N. Diagnostic accuracy of the physical examination and imaging tests for osteomyelitis underlying diabetic foot ulcers: meta-analysis. Clin Infect Dis. 2008; 47: 519-27. [PubMed]
9. Eleftheriadou I, Tentolouris N, Argiana V, Jude E, Boulton AJ. Methicillin-resistant Staphylococcus aureus in diabetic foot infections. Drugs 2010; 70(14): 1785-97. [PubMed]
10. Eneroth M, Apelqvist J, Stenstrom A. Clinical characteristics and outcome in 223 diabetic patients with deep foot infections. Foot Ankle Int 1997; 18: 716-22. [PubMed]
11. Gadepalli R, Dhawan B, Sreenivas V, Kapil A, Ammini AC, Chaudhry R. A clinico-microbiological study of diabetic foot ulcers in an Indian tertiary care. Diabetes Care. 2006; 29: 1727-32. [PubMed]
12. Grayson ML, Gibbons GW, Balogh K, Levin E, Karchmer AW. Probing to bone in infected pedal ulcers. A clinical sign of underlying osteomyelitis in diabetic patients. JAMA 1995; 273: 721-3. [PubMed]
13. Geerlings SE, Hoepelman AI. Immune dysfunction in patients with diabetes mellitus. FEMS Immuneol Med Microbiol. 1999; 26: 259-65. [PubMed]
14. Gregg EW, Solie P, Paulose-Ram R, et al. Prevalence of lower-extremity disease in the US adult population ≥40 years or age with and without diabetes: 1999-2000 National Health and Nutritional Examination Survey. Diabetes Care. 2004; 27: 1591-97. [PubMed]
15. International Working Group on the Diabetic Foot. International consensus on the diabetic foot [CD-ROM]. Brussels: International Diabetes Foundation, May 2003. < [PubMed]
16. Jeffcoate WJ, Lipsky BA. Controversies in diagnosing and managing osteomyelitis of the foot in diabetes. Clin Infect Dis. 2004;39: S115-22. [PubMed]
17. Kandemir O, Akbay E, Sahin E, Milcan A, Gen R. Risk factors for infection of the diabetic foot with multi-drug resistant organisms. J Infect; 2007;54(5): 439-45. [PubMed]
18. Kapoor A, Page S, LaValley M, Gale DR, Felson DT. Magnetic resonance imaging for diagnosing foot osteomyelitis: a meta-analysis. Arch Intern Med. 2007; 167: 125-32. [PubMed]
19. Lavery LA, Armstrong DG, Murdoch DP, Peters EJ, Lipsky BA. Validation of the Infectious Disease Society of America’s diabetic foot classification system. Clin Infect Dis. 2007; 44: 562-5. [PubMed]
20. Lavery LA, Armstrong DG, Wunderlich RP, Mohler MJ, Wendel CS, Lipsky BA. Risk factors for foot infection in patients with diabetes mellitus. Diabetes Care. 2006; 29: 1288-93. [PubMed]
21. Lavery LA, Armstrong DG, Peters EJ, Lipsky BA. Probe-to-bone test for diagnosing diabetic foot osteomyelitis: reliable or relic? Diabetes care. 2007; 30: 270-4. [PubMed]
2 2. Lipsky BA. Empirical therapy for diabetic foot infections: are there clinical clues to guide antibiotic selection? Clin Microbiol Infect. 2007; 13: 351-3. [PubMed]
23. Lipsky BA, Pecoraro RE, Larson SA, Hanley ME, Ahroni JH. Outpatient management of uncomplicated lower-extremity infections in diabetic patients. Arch Intern Med. 1990; 150: 790-7. [PubMed]
24. Lipsky BA, Pecoraro RE, Wheat LJ. The diabetic foot: soft tissue and bone infection. Infect Dis North Am. 1990; 4: 409-32. [PubMed]
25. Lipsky BA, Berendt AR, Deery HG, et al. Diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2004;39: 885-910. [PubMed]
26. Martínez-Gómez DA, Ramírez-Almagro C, Campillo-Soto A, Morales-Cuenca G, Pagán-Ortiz J, Aguayo-Albasini JL. [Diabetic foot infections. Prevalence and antibiotic sensitivity of the causative microorganisms]. Enferm Infecc Microbiol Clin. 2009 Jun;27(6): 317-21.
27. Prompers L, Huijberts M, Apelqvist J, et al. Delivery of care to diabetic patients with foot ulcers in daily practice: results from the Eurodiale Study, a prospective cohort study. Diabet Med 2008;25: 700-7.
28. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005; 293: 217-28. [PubMed]
29. van Baal JG. Surgical treatment of the infected diabetic foot. Clin Infect Dis. 2004; 39 Suppl 2: S123-8. [PubMed]
30. Wilson JF, Laine C, Goldmann D. In the clinic. Peripheral arterial disease. Ann Intern Med. 2007; 146: ITC 3-1 – ITC 3-16. [ PubMed]
Table 1. Risk Factors for Foot Ulceration and Infection
| Risk factor |
Mechanism leading to ulceration, impaired wound healing or infection |
|---|---|
Peripheral sensory neuropathy |
Loss of protective sensation (e.g., repetitive shear-type stress leading to ulceration) |
Peripheral motor neuropathy |
Abnormal foot anatomy and biomechanics resulting in excess pressure |
Peripheral autonomic neuropathy |
Impaired sweating leading to dry, cracked skin |
Arterial insufficiency |
Diminished delivery of nutrient, oxygen, neutrophils, etc. leading to impaired wound healing and clearance of infection |
Hyperglycemia |
Immune system (e.g., neutrophil) dysfunction and excess collagen cross-linking |
Patient disability or non-adherence |
Reduced vision (unable to inspect feet), prior amputation, lack of regular follow-up with medical care, poor hygiene, inappropriate footwear |
Table 2. Pathogens Associated with Diabetic Foot Infection Syndromes
| Diabetic foot infection syndrome |
Pathogen |
|---|---|
Cellulitis without ulceration |
Beta-hemolytic streptococci (especially group B) and Staphylococcus aureus |
Ulcer or wound, recently developed and no prior antibiotic treatment |
S. aureus and beta-hemolytic streptococci |
Ulcer or wound, chronic or recent antibiotic treatment |
Usually polymicrobial – S. aureus and beta-hemolytic streptococci plus Enterobacteriaceae. Enterococci if previous cephalosporin therapy. |
Ulcer or wound, prior hydrotherapy or green-blue colored drainage |
Pseudomonas aeroginosa (often in combination with other organisms) |
Extensive necrosis or gangrene, ischemic limb, feculent odor (“fetid foot”) |
Polymicrobial – mixed aerobic gram-positive cocci (including enterococci), Enterobacteriaceae, nonfermentative gram-negative rods, and obligate anaerobes |
Healthcare-associated |
MRSA; ESBL-producing gram-negative rods |
MRSA = Methicillin-resistant Staphylococcus aureus
ESBL = Extended spectrum beta-lactamase
Table 3. Clinical Classification of Diabetic Foot Infections
| Infection* severity |
Clinical manifestations of infection |
|---|---|
Uninfected |
Wound lacking purulence or any manifestations of inflammation |
Mild |
Infection localized to the skin and subcutaneous tissue (cellulitis/erythema extends ≤2 cm around an ulcer) without evidence of systemic illness |
Moderate |
More extensive local infection (i.e., local spread ≥2cm beyond an ulcer, lymphangitic streaking, abscess, gangrene, or involvement of deep soft tissue, muscle, fascia, tendon, joint or bone) without evidence systemic illness or severe metabolic derangements |
Severe |
Infection with systemic toxicity or severe metabolic derangements |
* Infection defined as the presence of purulent secretions (pus) or ≥2 signs or symptoms of inflammation (erythema, warmth, tenderness, induration, pain)
Table 4. Interpretation of Ankle-Brachial Index Results
| Ankle-brachial index (ABI)* |
Interpretation |
|---|---|
>1.30 |
Poorly compressible vessels, arterial calcification |
0.90-1.30 |
Normal |
0.60-0.89 |
Mild obstruction |
0.40-0.59 |
Moderate obstruction |
<0.40 |
Severe obstruction |
* Obtained by measuring the systolic blood pressure in the ankle divided by that in the brachial artery
Table 5. Recommendations for Collection of Specimens for Culture from Diabetic Foot Wounds
Do |
Cleanse and debride wound before obtaining specimen(s) for culture |
Obtain tissue specimen for culture by scraping with a sterile scalpel or dermal curette (curettage) or biopsy from the base of a debrided ulcer |
Aspirate any purulent secretions using sterile needle/syringe |
Promptly send specimens for culture in sterile container or appropriate transport media for aerobic and anaerobic culture |
Do Not |
Culture clinically uninfected lesions, unless for epidemiological studies |
Obtain specimen for culture without first cleansing or debriding the wound |
Obtain specimen for culture by swabbing the wound or wound drainage |
Table 6. Suggested Antibiotic Regimens for the Treatment of Soft-Tissue Diabetic Foot Infections
| Severity of infection |
Route of administration |
Recommended agents (choose on or more)* |
Alternative agents* |
|---|---|---|---|
Mild/moderate |
Oral |
(500 mg q.i.d.) OR (250 mg q.i.d.) OR (300 mg t.i.d.) OR (875/125 mg b.i.d.) |
(750 mg q.d.) ± Clindamycin (300 mg t.i.d.) OR (2 double-strength b.i.d.) |
Moderate/severe |
Intravenous until stable, then transition to an oral equivalent (or tailor based on culture results) |
(3.0 gm q.i.d.) OR Clindamycin (450 mg q.i.d.) + ciprofloxacin (750 mg b.i.d.) |
(3.3 gm q.i.d.) OR Clindamycin (600 mg q.i.d.) + ceftazidime (2 gm t.i.d.) OR (1 gm q.d.) |
Life-threatening |
Prolonged intravenous |
Imipenem/cilastin (500 mg q.i.d.) OR Clindamycin (900 mg q.i.d.) + tobramycin (5.1 mg/kg/d) + ampicillin (50 mg/kg q.i.d.) |
Vancomycin (15 mg/kg b.i.d.) + aztreonam (2.0 gm t.i.d.) + metronidazole (7.5 mg/kg q.i.d.) |
*Based on published evidence of efficacy for complicated skin infection or diabetic foot infections. Choice should be based on any available culture results, clinical factors (allergies, co-morbidities, etc.), local antibiotic susceptibility data, availability and cost.
Table 7. Suggested Route, Setting, and Duration of Antibiotic Therapy by Clinical Syndrome
| Site and severity of infection |
Route of antibiotic administration |
Usual setting for therapy |
Duration of therapy* |
|---|---|---|---|
Soft tissue only |
|||
Mild |
Oral |
Outpatient |
1-2 weeks, may extend up to 4 weeks if infection slow to resolve |
Moderate |
Oral (or initial parenteral) |
Outpatient or inpatient |
2-4 weeks |
Severe |
Initial parenteral, switch to oral when clinically stable |
Initial inpatient, discharge to outpatient when clinically stable |
2-4 weeks |
Bone or joint** |
|||
Post-amputation, no residual infected tissue |
Parenteral or oral |
Outpatient, unless patient ill or requires parenteral therapy (and no available OPAT) |
2-5 days |
Post-amputation, residual infected soft tissue but not infected bone |
Parenteral or oral |
2-4 weeks |
|
Post-amputation, residual infected but viable bone |
Initial parenteral, then consider switch to oral |
4-6 weeks |
|
Post-amputation, residual non-viable bone or no surgery |
Initial parenteral, then consider switch to oral |
>3 months |
OPAT = Outpatient antibiotic therapy
* Treat until resolution of all (or most) of the original symptoms and signs of infection
** With or without concurrent soft tissue infection
Figure 1. Approach to treating a diabetic patient with a foot wound
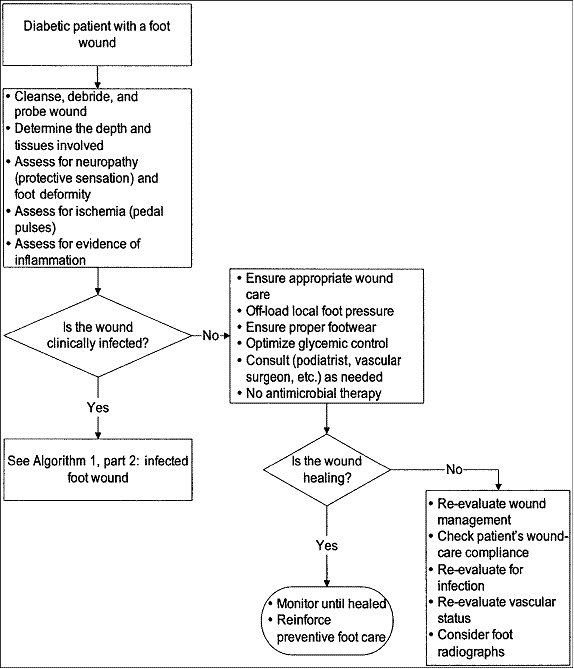
Figure 2. Approach to treating a diabetic patient with a foot infection.
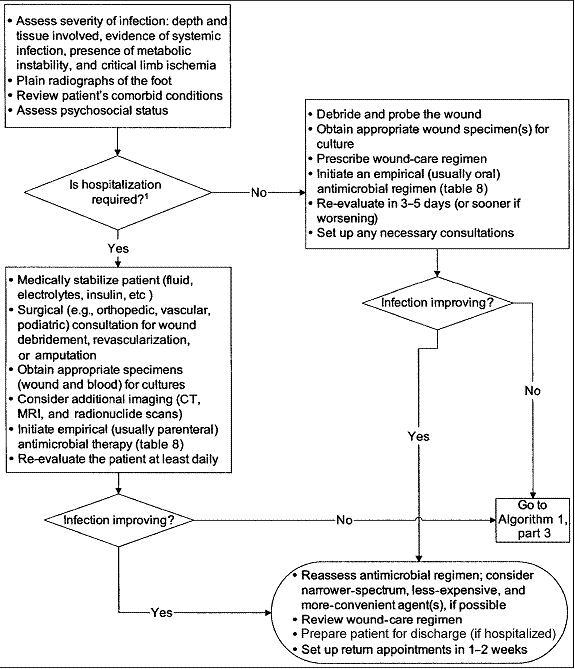
Consider hospitalization if any of the following criteria are present: systemic toxicity (e.g., fever and leukocytosis); metabolic instability (e.g., severe hypoglycemia or acidosis); rapidly progressive or deep tissue infection, substantial necrosis or gangrene, or presence of critical ischemia; requirement of urgent diagnostic or therapeutic interventions; and inability to care for self or inadequate home support.
What's New
Senneville E. et al. Needle Puncture and Transcutaneous Bone Biopsy Cultures are Inconsistent in Patients with Diabetes and Suspected Osteomyelitis of the Foot. Clin Infect Dis. 2009 Apr 1;48(7): 888-93.
Dinh MT, Abad CL, et al. Diagnostic Accuracy of the Physical Examination and Imaging Tests for Osteomyelitis Underlying Diabetic Foot Ulcers: Meta-Analysis. Clin Infect Dis. 2008 Aug 15;47: 519-27.
Eric Senneville, et al. Outcome of Diabetic Foot Osteomyelitis Treated Nonsurgically. Diabetes Care 2008;31: 637-642.
Vardakas KZ, et al. Factors associated with treatment failure in patients with diabetic foot infections: An analysis of data from randomized controlled trials. Diabetes Res Clin Pract 2008 Feb 19 [Epub ahead of print].
GUIDED MEDLINE SEARCH FOR:
REVIEW ARTICLES
Lipsky BA, et al. Topical Versus Systemic Antimicrobial Therapy for Treating Mildly Infected Diabetic Foot Ulcers: A Randomized, Controlled, Double-blinded, Multicenter Trial of Pexiganan Cream.Clin Infect Dis. 2008 Dec 15;47(12): 1537-45.
Lipsky BA. 2012 Infectious Disease Society of America. Clinical Practice Guideline for the Diagnosis and Treatment of Diabetic Foot Infections. Clin Infect Dis 2012;54:132-173.
