Urinary Tract Infections In Children
Authors: FME Wagenlehner, MD, PhD, KG Naber, MD, PhD
The urinary tract is a common source of infection in children and infants. It represents the most common bacterial infection in children less than 2 years of age. The outcome of a UTI is usually benign, but in early infancy it can progress to renal scarring, especially when associated with congenital anomalies of the urinary tract. Delayed sequelae related to renal scarring include hypertension, proteinuria, renal damage and even chronic renal failure, requiring dialysis treatment in a significant number of adults.
The risk of a UTI during the first decade of life is 1% in males and 3% in females. It has been suggested that 5% of schoolgirls and up to 0.5% of schoolboys undergo at least one episode of UTI during their school life. The incidence is different for children under 3 months of age, when it is more common in males. The incidence of asymptomatic bacteriuria is 0.7-3.4% in neonates, 0.7-1.3% in infants under 3 months of age and between 0.2% and 0.8% in preschool boys and girls, respectively. The incidence of symptomatic bacteriuria is 0.14% in neonates, with a further increase to 0.7% in boys and 2.8% in girls aged less than 6 months. The overall recurrence rate for the neonatal period has been reported to be 25%.
Etiology
The common pathogenic sources are Gram-negative, mainly enteric, organisms. Of these, Escherichia coli is responsible for 90% of episodes of UTIs. Gram-positive organisms (particularly enterococci and staphylocci) represent 5-7% of cases. Hospital-acquired infections show a wider pattern of aggressive organisms, such as Klebsiella spp., Serratia and Pseudomonas spp. Groups A and B streptococci are relatively common in the newborn. There is an increasing trend towards the isolation of Staphylococcus saprophyticus in UTIs in children, although the role of this organism is still debatable.
Pathogenesis and Risk Factors
Obstruction and dysfunction are among the most common causes of urinary infection. Phimosis predisposes to UTI. Enterobacteria derived from intestinal flora colonize the preputial sac, glandular surface and the distal urethra. Among these organisms are strains of E. coli expressing P. fimbriae which adhere to the inner layer of the preputial skin and to uroepithelial cells. A wide variety of congenital urinary tract abnormalities can cause UTIs through obstruction, e.g. urethral valves, pelvi-ureteric junction obstruction or non-obstructive urinary stasis (e.g. prune belly syndrome, vesicoureteral reflux). More mundane but significant causes of UTIs include labial adhesion and chronic constipation. Dysfunctional voiding in an otherwise normal child may result in infrequent bladder emptying aided by delaying manoeuvres, e.g. crossing legs, sitting on heels. Neuropathic bladder dysfunction (spina bifida, sphincter dyssynergia, etc) may lead to postvoid residual urine and secondary vesicoureteral reflux.
The link between renal damage and UTIs is controversial. The mechanism in obstructive nephropathy is self-evident, but more subtle changes occur where there is vesicoureteral reflux. Almost certainly the necessary components include vesicoureteral reflux, intrarenal reflux and a UTI. These must all work together in early childhood when the growing kidney is likely to be susceptible to parenchymal infection. Later on in childhood, the presence of bacteriuria seems irrelevant to the progression of existing scars or the very unusual formation of new scars. Another confounding factor is that many so-called scars are dysplastic renal tissue which developed in utero.
Signs and Symptoms
Symptoms are non-specific, and vary with the age of the child and the severity of the disease. Epididymoorchitis is extremely unusual. With scrotal pain and inflammation in a boy, testicular torsion has to be considered.
A UTI in neonates may be non-specific and with no localization. In small children, a UTI may present with gastrointestinal signs, such as vomiting and diarrhea. In the first weeks of life, 13.6% of patients with fever have a UTI. Rarely, septic shock will be the presentation. Signs of a UTI may be vague in small children, but later on, when they are older than 2 years, frequent voiding, dysuria and suprapubic, abdominal or lumbar pain may appear with or without fever.
Classification
Urinary tract infections may be classified either as a first episode or recurrent, or according to severity (simple or severe). Recurrent UTI may be subclassified into three groups:
• Unresolved infection: subtherapeutic level of antimicrobial, non-compliance with treatment, malabsorption, resistant pathogens.
• Bacterial persistence: may be due to a nidus for persistent infection in the urinary tract. Surgical correction or medical treatment for urinary dysfunction may be needed.
• Reinfection: each episode is a new infection acquired from periurethral, perineal or rectal flora. From the clinical point of view, severe and simple forms of UTIs should be differentiated because to some extent the severity of symptoms dictates the degree of urgency with which investigation and treatment are to be undertaken.
Severe UTI
Severe UTI is related to the presence of fever of > 39ºC, the feeling of being ill, persistent vomiting, and moderate or severe dehydration.
Simple UTI
A child with a simple UTI may have only mild pyrexia, but is able to take fluids and oral medication. The child is only slightly or not dehydrated and has a good expected level of compliance. When a low level of compliance is expected, such a child should be managed as one with a severe UTI.
Diagnosis
Physical Examination
It is mandatory to look for phimosis, labial adhesion, signs of pyelonephritis, epididymo-orchitis, and stigmata of spina bifida, e.g. hairy patch on the sacral skin. The absence of fever does not exclude the presence of an infective process.
Laboratory Tests
The definitive diagnosis of infection in children requires a positive urine culture. Urine must be obtained under bacteriologically reliable conditions when undertaking a urine specimen culture. A positive urine culture is defined as the presence of more than 100,000 cfu/mL of one pathogen. The urine specimen may be difficult to obtain in a child less than 4 years old and different methods such as suprapubic bladder aspiration, bladder catheterization, plastic bag collection are advised since there is a high risk of contamination (Table 1).
Imaging of the Urinary Tract
A ‘gold standard’ imaging technique has to be cost-effective, painless, safe, with minimal or nil radiation, and an ability to detect any significant structural anomaly. Current techniques do not fulfil all such requirements.
Ultrasonography
Ultrasonography (US) has become very useful in children because of its safety, speed and high accuracy in identifying the anatomy and size of the renal parenchyma and collecting system. It is subjective and therefore operator-dependent, and gives no information on renal function. However, scars can be identified, although not as well as with technetium-99m dimercaptosuccinic acid (Tc-99m DMSA) scanning. This technique has been shown to be very sensitive and excretory urography must be reserved only for when images need to be morphologically clarified.
Radionuclide Studies
Tc-99m DMSA is a radiopharmaceutical that is bound to the basement membrane of proximal renal tubular cells; half of the dose remains in the renal cortex after 6 hours. This technique is helpful in determining functional renal mass and ensures an accurate diagnosis of cortical scarring by showing areas of hypoactivity indicating lack of function. A UTI interferes with the uptake of this radiotracer by the proximal renal tubular cells, and may show areas of focal defect in the renal parenchyma. A star-shaped defect in the renal parenchyma may indicate an acute episode of pyelonephritis. A focal defect in the renal cortex usually indicates a chronic lesion or a ‘renal scar’.
A focal scarring or a smooth uniform loss of renal substance as demonstrated by Tc-99m DMSA has generally been regarded as being associated with vesicoureteral reflux (reflux nephropathy). The use of Tc-99m DMSA scans can be helpful in the early diagnosis of acute pyelonephritis. About 50-85% of children will show positive findings in the first week. Minimal parenchymal defects, when characterized by a slight area of hypoactivity, can resolve with antimicrobial therapy. However, defects lasting longer than 5 months are considered to be renal scarring. Tc-99m DMSA scans are considered more sensitive than excretory urography and ultrasonography in the detection of renal scars.
Cystourethrography
1. Conventional Voiding Cystourethrography: Voiding cystourethrography is the most widely used radiological exploration for the study of the lower urinary tract and especially of vesicoureteral reflux. It is considered mandatory in the evaluation of UTIs in children less than 1 year of age. Its main drawbacks are the risk of infection, the need for retrogrades filling of the bladder and the possible deleterious effect of radiation on children. In recent years, tailored low-dose fluoroscopic voiding cystourethrography has been used for the evaluation of vesicoureteral reflux in girls in order to minimize radiological exposure. Voiding cystourethrography is mandatory in the assessment of febrile childhood UTI, even in the presence of normal ultrasonography. Up to 23% of these patients may reveal vesicoureteral reflux.
2. Radionuclide Cystography (Indirect):This investigation is performed by prolonging the period of scanning after the injection of Tc-99m diethylene triamine pentaacetate (DTPA) or mercaptoacetyltriglycine (MAG-3) as part of a dynamic renography. It represents an attractive alternative to conventional cystography, especially when following patients with reflux, because of its lower dose of radiation. Disadvantages are a poor image resolution and difficulty in detecting lower urinary tract abnormalities.
Cystosonography
Contrast material-enhanced voiding ultrasonography has been introduced for the diagnoses of vesicoureteral reflux without irradiation. Further studies are necessary to determine the role of this new imaging modality in UTI.
Additional Imaging
Excretory urography remains a valuable tool in the evaluation of the urinary tract in children, but its use in UTIs is debatable unless preliminary imaging has demonstrated abnormalities requiring further investigation. The major disadvantages in infants are the risks of side effects from exposure to contrast media and radiation. However, the role of excretory urography is declining with the increasing technical superiority of CT and MRI. However, the indications for their use is still limited in UTI.
Urodynamic Evaluation
When voiding dysfunction is suspected, e.g. incontinence, residual urine, increased bladder wall thickness, urodynamic evaluation with uroflowmetry, (video) cystometry, including pressure flow studies, and electromyography should be considered.
Schedule of Investigation
Screening of infants for asymptomatic bacteriuria is unlikely to prevent pyelonephritic scar formation, as these usually develop very early in infancy. Only a minority of children with a UTI have an underlying urological disorder, but when present such a disorder can cause considerable morbidity. Thus, after a maximum of two UTI episodes in a girl and one episode in a boy, investigations should be undertaken (Figure 1), but not in the case of asymptomatic bacteriuria. The need for DTPA/MAG-3 (Radioisoptope renography) scanning is determined by the ultrasound findings, particularly if there is suspicion of an obstructive lesion.
Treatment
Treatment has four main goals:
1. elimination of symptoms and eradication of bacteriuria in the acute episode
2. prevention of renal scarring
3. prevention of a recurrent UTI
4. correction of associated urological lesions.
Severe UTI's
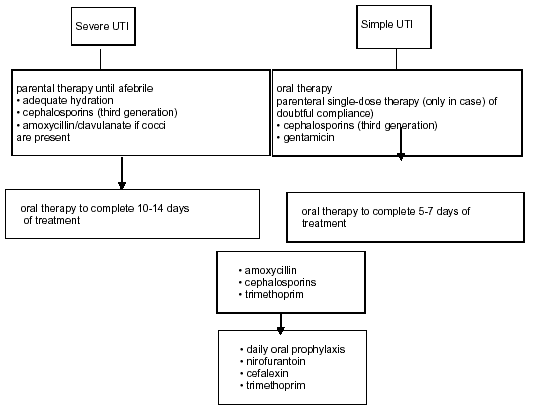
A severe UTI requires adequate parenteral fluid replacement and appropriate antimicrobial treatment, preferably with cephalosporins (third generation). If a Gram-positive UTI is suspected by Gram stain, it is useful to administer aminoglycosides in combination with ampicillin or amoxicillin/clavulanate. Antimicrobial treatment has to be initiated on an empirical basis, but should be adjusted according to culture results as soon as possible. In patients with an allergy to cephalosporins, aztreonam or gentamicin may be used. When aminoglycosides are necessary, serum levels should be monitored for dose adjustment. Chloramphenicol, sulfonamides, tetracyclines, rifampicin, amphotericin B and quinolones should be avoided. The use of ceftriaxone must also be avoided due to its undesired side effect of jaundice. A wide variety of antimicrobials can be used in older children, with the exception of tetracyclines (because of teeth staining). Fluorinated quinolones may produce cartilage toxicity, but if necessary may be used as second-line therapy in the treatment of serious infections, since musculoskeletal adverse events are of moderate intensity and transient. For a safety period of 24-36 hours, parenteral therapy should be administered. When the child becomes afebrile and is able to take fluids, he/she may be given an oral agent to complete the 10-14 days of treatment, which may be continued on an outpatient basis. This provides some advantages, such as less psychological impact on the child and more comfort for the whole family. It is also less expensive, well tolerated and eventually prevents opportunistic infections. The preferred oral antimicrobials are: trimethoprim (TMP), co-trimoxazole (TMP plus sulphamethoxazole), an oral cephalosporin, or amoxicillin/clavulanate. However, the indication for TMP is declining in areas with increasing resistance. In children less than 3 years of age, who have difficulty taking oral medications, parenteral treatment for 7-10 days seems advisable, with similar results to those with oral treatment. If there are significant abnormalities in the urinary tract (e.g. vesicoureteral reflux, obstruction), appropriate urological intervention should be considered. If renal scarring is detected, the patient will need careful follow-up by a paediatrician in anticipation of sequelae such as hypertension, renal function impairment and recurrent UTI.
An overview of the treatment of febrile UTIs in children is given in Figure 2 and the dosing of antimicrobial agents is outlined in Table 2.
Simple UTI's
A simple UTI is considered to be a low-risk infection in children. Oral empirical treatment with TMP, an oral cephalosporin or amoxicillin/clavulanate is recommended, according to the local resistance pattern. The duration of treatment in uncomplicated UTIs treated orally should be 5-7 days. A single parenteral dose may be used in cases of doubtful compliance and with a normal urinary tract. If the response is poor or complications develop, the child must be admitted to hospital for parenteral treatment.
Prophylaxis
If there is an increased risk of pyelonephritis, e.g. vesicoureteral reflux, and recurrent UTI, low-dose antibiotic prophylaxis is recommended. It may also be used after an acute episode of UTI until the diagnostic work-up is completed. The most effective antimicrobial agents are: Nitrofurantoin, TMP, cephalexin and cefaclor.
References
1. Bloomfield P, Hodson EM, Craig JC. Antibiotics for acute pyelonephritis in children. Cochrane Database of Syst Rev 2005;(1):CD003772. [PubMed]
2. Britton KE. Renal radionuclide studies. In: Whitfield HN, Hendry WF, Kirby RS, Duckett JW, eds. Textbook of genitourinary surgery. Oxford: Blackwell Science, 1998, pp. 76-103. [PubMed]
3. Haycock GB. A practical approach to evaluating urinary tract infection in children. Pediatr Nephrol 1991;5:401-402. [PubMed]
4. Hoberman A, Chao HP, Keller DM, Hickey R, Davis HW, Ellis D. Prevalence of urinary tract infection in febrile infants. J Pediatr 1993;123:17-23. [PubMed]
5. Jacobson SH, Eklof O, Eriksson CG, Lins LE, Tidgren B, Winberg J. Development of uraemia and hypertension after pyelonephritis in childhood: 27 year follow up. BMJ 1989;299:703-706. [PubMed]
6. Jodal U. The natural history of bacteriuria in childhood. Infect Dis Clin North Am 1987;1:713-729. [PubMed]
7. Kass EJ, Fink-Bennett D, Cacciarelli AA, Balon H, Pavlock S. The sensitivity of renal scintigraphy and sonography in detecting nonobstructive acute pyelonephritis. J Urol 1992;148:606-608. [PubMed]
8. Lin DS, Huang SH, Lin CC, Tung YC, Huang TT, Chiu NC, Koa HA, Hung HY, Hsu CH, Hsieh WS, Yang DI, Huang FY. Urinary tract infection in febrile infants younger than eight weeks of Age. Paediatrics 2000;105:E20. [PubMed]
9. Mucci B, Maguire B. Does routine ultrasound have a role in the investigation of children with urinary tract infection? Clin Radiol 1994;49:324-325. [PubMed]
10. Naber KG, Bishop MC, Bjerklund-Johansen TE, Botto H, Cek M, Grabe M, Lobel B, Palou J, Tenke P. EAU guidelines on the management of urinary and male genital tract infections. EAU Working Group on Urinary and Male Genital Tract Infections. European Association of Urology (EAU) Guidelines Office. 2006 edition. 1-126. [PubMed]
11. Pickworth FE, Carlin JB, Ditchfield MR, de Campo MP, Cook DJ, Nolan T, Powell HR, Sloane R, Grimwood K. Sonographic measurement of renal enlargement in children with acute pyelonephritis and time needed for resolution: implications for renal growth assessment. Am J Roentgenol 1995;165:405-408. [PubMed]
12. Rosenberg AR, Rossleigh MA, Brydon MP, Bass SJ, Leighton DM, Farnsworth RH. Evaluation of acute urinary tract infection in children by dimercaptosuccinic acid scintigraphy: a prospective study. J Urol 1992;148:1746-1749.[PubMed]
13. Schulamn SL. Voiding dysfunction in children. Urol Clin North Am 2004;31:481-490, ix. [PubMed]
14. Stutley JE, Gordon I. Vesico-ureteric reflux in the damaged non-scarred kidney. Pediatr Nephrol 1992;6:25-29. [PubMed]
15. To T, Agha M, Dick PT, Feldman W. Cohort study on circumcision of newborn boys and subsequent risk of urinary-tract infection. Lancet 1998;352:1813-1816. [PubMed]
16. Westwood ME, Whiting PF, Cooper J, Watt IS, Kleijnen J. Further investigation of confirmed urinary tract infection (UTI) in children under five years: a systematic review. BMC Pediatr 2005;5:2. [PubMed]
17. Yeung CK, Godley ML, Dhillon HR, Gordon I, Duffy PG, Ransley PG. The characteristics of primary vesico-ureteric reflux in male and female infants with pre-natal hydronephrosis. Br J Urol 1997;80:319- 327. [PubMed]
Table 1. Criteria of UTI in Children
| Urine specimen from suprapubic bladder puncture | Urine specimen from bladder catheterization | Urine specimen from midstream void |
|---|---|---|
| Any number of cfu/mL (at least 10 identical colonies) | ≥ 1,000-50,000 cfu/mL | ≥ 104 cfu/mL with symptoms |
| ≥ 105 cfu/mL without symptoms |
Table 2. Dosing of Antimicrobial Agents in Children Aged 3 Months to 12 Years*
| Antimicrobial agent | Application | Age | Total dosage per day | Doses per day |
|---|---|---|---|---|
| Ampicillin | Intravenous | 3-12 months | 100-300 mg/kg BW | 3 |
| Intravenous | 1-12 years | 60-150 (-300) mg/kg BW | 3 | |
| Amoxycillin | Oral | 3 months to 12 years | 50-100 mg/kg BW | 2-3 |
| Amoxycillin/clavulanate | Intravenous | 3 months to 12 years | 60-100 mg/kg BW | 3 |
| Oral | 3 months to 12 years | 37.5-75 mg/kg BW | 2-3 | |
| Cephalexin Treatment | Oral | 3 months to 12 years | 50-100 mg/kg BW | 3 |
| Prophylaxis Cefaclor | ||||
| • Treatment | Oral | 1-12 years | 10 mg/kg BW | 1-2 |
| Oral | 3 months to 12 years | 50-100 mg/kg BW | 3 | |
| • Prophylaxis | Oral | 1-12 years | 10 mg/kg BW | 1-2 |
| Cefixime | Oral | 3 months to 12 years | 8-12 mg/kg BW | 1-2 |
| Cetriaxone | Intravenous | 3 months to 12 years | 50-100 mg/kg BW | 1 |
| Aztreonam | Intravenous | 3 months to 12 years | (50)-100 mg/kg BW | 3 |
| Gentamicin | Intravenous | 3-12 months | 5-7.5 mg/kg BW | 1-3 |
| Intravenous | 1-2 years | 5 mg/kg BW | 1-3 | |
| Trimethoprim | ||||
| • Treatment | Oral | 1-12 years | 6 mg/kg BW | 2 |
| • Prophylaxis | Oral | 1-12 years | 1-2 mg/kg BW | 1 |
| Nitrofurantoin | ||||
| • Treatment | Oral | 1-12 years | 3-5 mg/kg BW | 2 |
| • Prophylaxis | Oral | 1-12 years | 1mg/kg BW | 1-2 |
BW = body weight.
Figure 1. Schedule of Investigation of A UTI in A Child
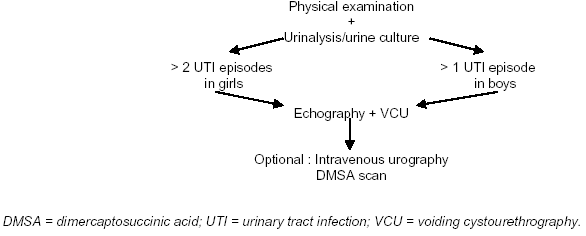
Figure 2. Treatment of Febrile UTIs in Children
Salo J, et al. Cranberry Juice for the Prevention of Recurrences of Urinary Tract Infections in children: A Randomized Placebo-Controlled Trial. J Clinical Infectious Diseases. 2012;54(3): 340-6.
Etoubleau C, Reveret M, et al. Moving from bag to catheter for urine collection in non-toilet-trained children suspected of having urinary tract infection: a paired comparison of urine cultures. J Pediatr. 2009 Jun;154:803-6.
Montini G, et al. Prophylaxis After First Febrile Urinary Tract Infection in Children? A Multicenter, Randomized, Controlled, Noninferiority Trial. Pediatrics 2008;122:1064-1071.
Shaikh N et al. Does this child have a urinary tract infection? JAMA. 2007 Dec 26;298(24):2895-904.
GUIDED MEDLINE SEARCH FOR
López-Medrano F, María Aguado J. Urinary Tract Infections in Transplant Recipients
