Human Parvovirus
Authors: Francesca Bonvicini, Monica Musiani, Marialuisa Zerbini
VIROLOGY
The Parvoviridae family consists of small DNA viruses with an isometric morphology. The non enveloped viral particle is about 18 to 24 nm in diameter and the linear, single stranded DNA genome is typically about 5 kilobases. The Parvoviridae family includes two subfamilies, the Densovirinae which infects invertebrates and the Parvovirinae which infects vertebrates (127). Some viruses of the Parvovirinae subfamily have been recovered from human specimens and/or have been associated with clinical manifestations in humans: these viruses belong to the Dependovirus, Erythrovirus and Bocavirus genus. Moreover, human parvoviruses 4 and 5 have recently been isolated from humans but till today have not been classified.
The Dependovirus genus includes Adeno-associated viruses (AAV, six serotypes), which are commonly recovered from the human genital tract, notably in cells of the uterine cervix, but their role in both the pathogenicity and in the potential suppression of human papillomavirus-mediated oncogenesis is controversial and needs elucidations (131, 133).
Human parvoviruses 4 and 5 (Parv4 and Parv5), members of an unassigned genus, have been recently found in plasma of specific risk groups (injecting drug users and hemophiliacs) and in pooled-plasma or plasma-derived blood products (47, 60, 84, 116,120). The clinical significance of these new viruses requires further investigations (79). The Erythrovirus and Bocavirus genera include human parvovirus B19 (B19V, three genotypes) and human Bocavirus respectively. These two parvoviruses are the most important viruses of human interest associated with defined clinical manifestations.
EPIDEMIOLOGY
Human parvovirus B19V genotype 1 is a global and common infectious pathogen in humans. The infection can occur in any month of the year in sporadic or epidemic form. In temperate climates, however, infections usually occur in the spring with epidemic peaks every 2 to 4 years. The overall prevalence of IgG antibodies against B19V has been estimated ranging from 20.4% in children, 66.9% in adolescents to 88.5% in the elderly (80, 114). The prevalence of B19V DNA in asymptomatic blood donors has been estimated at between 0.002% and 0.38% (50, 51, 71), depending on the period of testing and the sensitivity of the assay used.
B19V genotype 2 and B19V genotype 3 are currently rare genotypes with a defined geographical distribution: B19V genotype 2 is found in Europe, the USA and other Western countries, while B19V genotype 3 is restricted to sub-Saharan Africa and South America.
Specific data on the seroprevalence of genotypes 2 and 3 are not available as the three genotypes, despite their differences in genome sequences, constitute a single serotype (41).
Studies based on the detection of life-long persistent DNA in solid tissues led to the discovery of a time-related circulation of genotypes. In particular, genotype 2 was shown to be frequent in tissues of subjects born before 1973 in Finland and Germany (102), before 1963 in Scotland (84) and before 1957 in Italy (11). Genotype 3, on the other hand, never attained wide circulation in Europe during the past ≥ 70 years (84). As a consequence, the detection of genotypes 2 and 3 in blood donors and manufacturing products from Europe can be considered a sporadic event (8, 57, 54, 116) Conversely, B19V genotype 3 was found to be prevalent in donations from Ghana (1.3%) (30). B19V transmission generally occurs by the respiratory route. Transmission takes place frequently among household and school contacts during outbreaks of B19V infection, with higher risks being associated with close contact between infected and susceptible individuals for a prolonged time.
B19V vertical transmission has been estimated at between 24% and 51% of cases of maternal infection during pregnancy (92, 101) and the risk of adverse fetal outcome between 3% and 12% (22).
Although B19V transmission by transfusion of single-donor derived blood components may be a relative rare event (34, 61), iatrogenic transmission by pooled factor VIII and IX, albumin, clotting factor concentrates and immunoglobulins has been frequently reported (5, 25, 70).
B19V transmission after hematopoietic stem cell and solid-organ transplantations has been described (40, 55). The prevalence of B19V DNA in donors of bone marrow progenitors or umbilical cord blood hematopoietic stem cells has been estimated at 3.3% and 2.9%, respectively (10). Recent studies have documented the presence of B19V DNA in the blood of 23%–31% of kidney transplant recipients, particularly those with anemia (31, 69). Transmission of B19V infection by the donor (95, 139) or else reactivation of B19V from a persistent state of infection (23, 49) have been proposed.
CLINICAL MANIFESTATIONS
Human Parvovirus B19V infection can occur asymptomatically or can cause a broad spectrum of clinical manifestations whose outcome depends strongly on the host’s physical status. In immunocompetent subjects the major clinical manifestations of B19V infection are erythema infectiosum, arthropathy and transient aplastic crisis. Exceptional manifestations such as myocarditis (28,42, 52, 108), hepatitis (1, 56), encephalitis (7, 19, 66), chronic fatigue syndrome (59, 67) and autoimmune diseases (33, 36, 74) have also been associated with B19V infection.
In immunocompromised subjects, the clinical manifestation most frequently recognised is pure red cell aplasia. In pregnant women, B19V infection can result in non-immune fetal hydrops, spontaneous abortion and fetal death.
Erythema infectiosum
Erythema infectiosum, also known as fifth disease, is the prevalent B19V-associated clinical manifestation in children. Prodromal symptoms often go unnoticed but they may include low grade fever, headache, cough, myalgia, nausea and diarrhea. Normally, about 18 days after infection, a facial erythema of medium intensity appears on the cheeks but with a relative perioral pallor (hence the common name “slapped cheek”). Then, the maculopapular rash spreads involving the trunk and, bilaterally/symmetrically, arms and legs. Palms and soles are only rarely involved. Finally, the elements of maculopapular rash become clear in the centre giving the characteristic reticular or lacy pattern (4).
Erythema infectiosum may be transient or recurrent, and fluctuations in intensity can be linked to environmental factors such as exposure to sunlight and heat (96, 100).
Arthropathy
Joint involvement can occur as a consequence of B19V infection in the presence or absence of cutaneous rash. Arthropathy is uncommon in children (<10%) while it is prevalent in adults (~ 50%) and above all in middle-aged women (~80%). Joint symptoms typically present as acute and moderately severe symmetric polyarthritis, affecting above all the proximal interphalangeal joints of the hands and feet, and less frequently the wrists, elbows, knees and ankles (113, 137). The symptoms usually disappear after several weeks, but in some cases they can reoccur or persist for months or even years (46, 77, 90, 103, 125).
Transient Aplastic Crises
Transient aplastic crisis is an acute self-limited episode of red cell aplasia characterized by the temporary depression of the erythrocyte production in bone marrow and the absence of reticulocytes in the peripheral blood (110). Transient aplastic crises: may potentially occur in all patients with a shortened red-cell life span. Patients with transient aplastic crises: usually present with pallor, weakness, and lethargy, and are highly viremic, thereby posing a risk of transmission to others.
Examples of predisposing conditions are chronic hemolytic anemias and other disorders including thalassemia, hereditary spherocytosis, sickle-cell anemia, malaria and even iron deficiency. During transient aplastic crises:, in addition to a cessation of the erythroid production, other blood cell lineages can be affected with clinically significant thrombocytopenia, granulocytopenia or pancytopenia (22).
Pure Red Cell Aplasia
The clinical picture of B19V infection in the immunocompromised host differs significantly from immunocompetent subjects. Immunocompromised patients usually do not exhibit fever, rash or arthritis but the detection of the anemia is the first indication of B19V infection. Many immunocompromised patients are unable to produce neutralizing antibodies to clear the virus and this can lead to persistent infection, resulting in chronic anemia. Predisposing conditions include congenital or acquired immunodeficiency syndrome (AIDS), iatrogenic immunodeficiencies, solid-organ transplant recipients and lymphoproliferative disorders (39, 45, 138).
B19V Infection and Pregnancy
In pregnant women acute B19V infection can be entirely asymptomatic (30-50%) or associated with either arthropathies (30%) and/or rash (30-40% of cases).
The average proportion of women of child-bearing age susceptible to B19V infection and the risk of pregnant women acquiring infection during pregnancy have been estimated to be 26% and 0.61% in Belgium, 38% and 0.69% in England and Wales, 43.5% and 1.24% in Finland, 39.9% and 0.92% in Italy, and 36.8% and 1.58 % in Poland, respectively. The overall rate of transplacental transmission has been estimated at between 24 and 51% (92). B19V fetal infection may resolve spontaneously with delivery of an apparently normal infant, or lead to severe consequences such as non-immune fetal hydrops, spontaneous abortion and fetal death. The risk of adverse fetal outcome following B19V infection has been evaluated in the 3-12% range with the highest chance during the hepatic period of hematopoietic activity (between 11 and 23 weeks of gestation) (44, 101).
Besides fetal anemia, thrombocytopenia secondary to B19V infection has also been reported (118). Rare cases of congenital anomalies have been described and include chronic anemia (26), meconium peritonitis (140), congenital heart disease (134), fetal hepatic calcifications (119) and bilateral opacification of the cornea (112). Malformations in aborted fetuses have been reported in a few isolated cases and include ocular anomalies (130), multiple structural defects (129) and hydrocephalus (64).
LABORATORY DIAGNOSIS
The laboratory diagnosis of B19V infection mainly relies on the detection of B19V genome in the clinical specimens (viral diagnosis) and/or on the determination of anti-B19V antibodies in serum samples (serological diagnosis) (Table 1).
Viral Diagnosis
Several approaches are available to detect B19V genome in clinical specimens. However, polymerase chain reaction assays (PCR) and in situ hybridisation techniques are the most commonly used because of their high sensitivity and specificity. Qualitative PCR assays are the methods routinely used in diagnostic laboratories (37, 97,98). The assays can be performed on several clinical specimens. Peripheral blood is the most easily available specimen for B19V detection; however, depending on the clinical features of the patients, the assays can be routinely carried out on bone marrow aspirates, cerebrospinal fluids, peritoneal fluids, cord blood and amniotic fluid samples, and several biopsy tissues.
Quantitative PCR assays are essential when the viremia or the clearance of B19V have to be monitored, such as in the course of persistent and recurrent infections, and when the efficacy of the therapy has to be evaluated. For this purpose in-house Real Time PCR assays (2, 12, 48, 82) and commercially available kits (Roche LightCycler Parvovirus B19 Quantification Kit-Roche Diagnostics; RealArt Parvo B19 LC PCR Kit-Artus GmbH) are used (Table 2); however these assays detect mainly genotype 1 (8, 9, 21, 35, 57).
In situ hybridization assays can give information not only on the presence and distribution of viral nucleic acids but they can also identify cellular and tissue morphology. In situ hybridization can be performed on different clinical specimens: it is mainly used for the diagnosis of B19V infections in hematological patients and in hydropic fetuses in bone marrow cells and fetal specimens, respectively. No kits are commercially available, however home-made procedures have been described (17, 20).
Serological Diagnosis
B19V antibodies are mainly detected by using enzyme immunoassays, immunofluorescence, and Western blot assays. Enzyme immunoassays is the method of choice in diagnostic laboratories dealing with large numbers of serum samples since the most frequently used commercially available tests are amenable to automation (29, 63). All these assays make use of B19V recombinant antigens that can be expressed in both eukaryotic and prokaryotic systems. B19V capsid proteins (VP1 and VP2) expressed in eukaryotic systems can self-assemble to form empty capsids which are morphologically and antigenically similar to native B19V and are thus particularly suited for the study of antibody response against conformational epitopes in enzyme immunoassay and immunofluorescence assays.
B19V capsid proteins expressed in prokaryotic systems have the advantage of being produced in large amounts but, losing the native conformation of B19V proteins, are generally suitable for the detection of antibodies directed to B19V linear epitopes, mainly in Western blot formats.
Enzyme immunoassays rely on the antibody reactivity with either or both B19V capsid proteins and employ either undenatured baculovirus-expressed (VP1 and/or VP2) or denaturated prokaryotically expressed VP1 protein as antigens (Table 2). The use of enzyme immunoassays based on undenatured antigens, is advisable for the serological diagnosis of B19V infection. In fact, these assays are sensitive and specific to detect antibodies to B19V conformational antigens which induce a strong and long lasting immune response in the course of infection (63, 83).
In the kits, IgM antibodies are mainly detected in capture-enzyme immunoassays format, while IgG antibodies are generally revealed using indirect enzyme-linked immunosorbent assay format with antigen coated to a solid phase.
Enzyme immunoassays have also been used to measure IgG avidity to discriminate primary from secondary infection and to confirm IgM positivity in specific cases. In fact, despite the availability of new and improved technologies, cross-reactivity between rubella, measles and B19V IgM antibodies has been described (128). B19V antibodies can also be detected by commercial immunofluorescent assays that employ glass slides coated with insect cells infected with a recombinant baculovirus expressing VP1 antigen (Table 2). For the selectively detection of B19V IgM, serum samples should be pretreated with a suitable adsorbent reagent. These assays are labor-intensive and cannot be automated.
Western Blot assays are also commercially available in a strip-immunoassay format (Table 2). These assays usually employ a combination of various recombinant VP1/VP2 and NS fragments presenting linear epitopes, and a yeast-expressed VP2 antigen presenting conformational epitopes (43, 111). According to the kind of antibody pattern revealed, the assays can provide helpful information on the course of B19V infection.
PATHOGENESIS
The pathogenesis of B19V associated diseases may consist of both direct effect of the virus on target cells and indirect effect mainly due to B19V-specific immune response. The antibody response appears to mediate the rash illness and arthropathy. It was first demonstrated by volunteer studies in which the occurrence of the symptoms (rash and joint involvement) coincided with the appearance of IgG antibodies (3). The immune-complex deposition in skin, blood vessels and in joints is supposed to be the cause of these manifestations. B19-associated arthropathy can also be caused by an inflammatory response in synovial tissue supported by the phospholipase A2 activity of the capsid protein VP1 (78).
B19V shows a specific tropism for erythroid progenitor cells in the bone marrow, due to the presence of both globoside, which acts as a receptor for the virus (24), and of specific integrins (136) which are co-receptors necessary for viral internalization. In these cells, B19V replicates lytically causing a block in the erythropoiesis. In normal subjects, the transient cessation of red-cell production does not affect haemoglobin levels because of the long life span of erythrocytes. However, in subjects with a shortened red-cell life span or with an impaired immune response, B19V can lead to transient aplasia or chronic aplasia, respectively.
In bone marrow, B19V can infect abortively megakaryocytes (122) and B19V-induced thrombocytopenia has been observed (14) as a consequence of the cytotoxic effect of NS protein (32, 107).
The pathogenetic mechanism of fetal hydrops or fetal death mainly involves the infection of fetal erythroid precursors. Details on the mechanism of transplacental transmission of B19V and subsequent fetal infection have not been elucidated. However, infection of different cell types within the placental villi, such as trophoblasts and endothelial cells has been suspected (62, 109, 135). This event can mediate the transport of the virus through fetal membranes and, once in the fetal circulation, the virus may disseminate and infect susceptible cells such as erythroid precursors in the liver and bone marrow, myocytes and endothelial cells. B19V-induced fetal myocarditis may considerably contribute to cardiac failure and hydrops fetalis (91, 104, 121).
SUSCEPTIBILITY IN VITRO AND IN VIVO
Since animal models are not available, cultures of human bone marrow (105, 123), umbilical blood (124), peripheral blood (117) and some myeloblastoid cell lines have been used to reproduce the effects of the virus on natural target cells. Particularly, the erythroid progenitor cell lines KU812Ep6 and UT7/EpoS1 were used to study B19V infectivity (16, 88) and were found to be sensitive to both B19V genotypes 1 and 2; no differences in expression profiling at either mRNAs or protein level were detected (15). UT7/EpoS1 cell lines were used in infectivity assays to establish the neutralizing activity of B19V IgG in immunoglobulin preparations (89).
ANTIVIRAL THERAPY
Antiviral drugs are not available for the treatment of B19V infection but clinical symptoms and/or viral clearance can be controlled by blood transfusions, intravenous immunoglobulins (IVIGs) or non steroidal anti-inflammatory drugs (NSAIDs), depending on clinical presentation (Table 3).
Generally erythema infectiosum is self-limited and does not require specific treatment, while patients with B19V-induced arthralgia may need non steroidal anti-inflammatory drug treatment (22, 77). Recently, in patients with arthritis and persistent B19V infection success with IVIG has been reported (77, 103, 125). IVIG therapy neutralizes the virus and may increase the levels of both interleukin 2 and 4, which protect against chronic symptoms. Moreover IVIG can regulate the production of TNF-α by B19V infected immune cells in the infiltrated synovial tissue (68, 75, 87, 94). IVIG therapy has been shown to be effective against clinical syndromes associated with persistent B19V infection, such as chronic fatigue syndrome (68) and encephalitis (7, 19).
ADJUNCTIVE THERAPY
In cases of transient aplastic crisis, the patient may require blood transfusions to prevent congestive heart failure. If promptly treated, the prognosis is good with restored erythropoiesis within 1-10 days (53).
The management of B19V induced fetal hydrops mainly depends on both the severity of anemia and the gestational age. If anemia, assessed by middle cerebral artery peak systolic velocity measurements (85), is severe, intrauterine blood transfusion can be indicated (44) and usually one intrauterine transfusion is sufficient for fetal recovery (99). Since fetal thrombocytopenia may also occur, an increased risk of hemorrhage may complicate cordocentesis and transfusion. For this reason it is advised that platelets should be available for transfusion during procedures. Otherwise, if anemia is mild, the “wait and see” policy is advisable in order to avoid an ineffective invasive therapy. The expectant management involves regular assessment of the fetal conditions by ultrasound examination and MCV-PSV measurements. Spontaneous resolution of hydrops has been documented (13, 65). IVIG therapy has also been described successfully in the treatment of fetal hydrops (86, 115).
Immunocompromised patients suffering from pure red cell aplasia are treated successfully with IVIG therapy. They usually respond with recovery of erythropoiesis following 1 or 2 administrations. Repeated courses of IVIG are required if the condition of immunosuppression is long-lasting (76, 93, 126). Solid-organ transplant recipients that developed B19V infection and suffered from pure red cell aplasia have been successfully treated by IVIG (40).
Commercially available IVIGs may contain different amounts of specific B19V IgG because each batch is made from different blood donor populations. However, large screening studies in the US and Europe have estimated the minimum B19V IgG titre in IVIG preparations at 10 IU/ml (73, 89, 103). The neutralizing activity of IVIG was analysed by in vitro infectivity assays and was found to be 4 log B19V at only 4 IU/ml of B19V IgG (89).
ENDPOINTS FOR MONITORING THERAPY
Quantitative Real Time PCR assays can be used to monitor B19V viral load in the course of persistent and recurrent infections and to assess the efficacy of the therapy in clearing the virus.
The quantitative evaluation of B19V DNA should be considered in cases of persistent anemia in high-risk patients such as in renal transplant recipients, during the period of major immunosuppresion in order to assess the therapy (12,31). In immunocompromised patients suffering from persistent B19V infection, the monitoring of B19 viral load could improve the usage of IVIG doses during IVIG therapy (126).
VACCINES
No vaccines for B19V are currently commercially available. A recombinant B19V vaccine (MEDI-491; MedImmune) composed of the VP1 (~25%) and VP2 (~75%) capsid proteins and formulated with MF59C.1 adjuvant (Chiron) has been reported in a randomized, double blind, phase I study. The vaccine was found to be safe, well tolerated and highly immunogenic eliciting a strong humoral response (6). The vaccine would be primarily intended for risk groups of severe B19V infection. For example it could be used to prevent transient aplastic crisis in patients with a shortened red-cell life span and pure red cell aplasia in immunocompromised hosts. Whether seronegative women of childbearing age should be included is a matter of controversy. The risk of fatal outcome following maternal primary infection may be too low to warrant a generalized vaccination program.
PREVENTION OR INFECTION CONTROL MEASURES
Control measures for B19V infection need to balance the low risk of clinical relevant infections with the potential severe adverse outcome for particular groups, notably the fetus, immunocompromised and hematological patients. The issue of infection control exclusively arises in relation to these three groups.
Since B19V transmission may occur through respiratory secretions, blood-derived products and donated organs, screening of blood components before transfusion and testing organ donors prior to transplantation may be considered as preventive strategies. Measures to prevent nosocomial spread of B19V and to minimize health care worker exposure should be taken when highly viremic patients are hospitalized with transient aplastic crisis. Regarding iatrogenic transmission of B19V infection, nucleic acid amplification technique (NAT) to detect B19V DNA has been initiated voluntarily by some companies as an in-process control in the screening of pooled source plasma or mini-pools (18, 25). In Europe, the European Pharmacopoeia monograph “Human Anti-D immunoglobulin” (58) stipulated that plasma pools used in the production of anti-D immunoglobulin and plasma treated for virus inactivation should contain less than 104 International Units (IU)/ml of B19V DNA. In the US, a similar proposed limit has been applied to manufacturing pools destined for all plasma derivatives. This safety level was based on the results of a post-marketing surveillance study for solvent-detergent treated plasma: individuals receiving plasma with viral titres of approximately 108 geq/ml became DNA positive and developed anti-B19V antibodies, while recipients of low-titre contaminated plasma (<104 geq/ml) did not produce antibodies and did not show any evidence of B19V transmission (132). Hence, highly viremic blood donations have to be discharged before the pooling process, and the level of B19V DNA must be verified after the manufacturer’s processes (57, 71).
The discovery that B19V was more genetically diverse than originally thought has led to a review of testing procedures. Currently, the Official Control Authority Batch Release (OCABR) guidelines require the detection of B19V genotype 1 and recommend the detection of A6 strains (genotype 2) and V9 strains (genotype 3) (106). Since commercially available kits and in-house assays mainly detect genotype 1, efforts should be made to improve B19V genotype detectability (72).
The main problem with B19V is the proteic structure of the capsid which is slightly sensitive to treatments usually applied to plasma products such as solvent-detergent and heat treatments. Therefore the screening of pooled source plasma and of final blood products to verify B19V contamination is fundamental to enhance the safety margins of plasma products. It seems advisable to give B19-safe materials at least to vulnerable recipients such as pregnant women, immunocompromized and hematological patients. Transplant recipients are also at risk of developing pure red cell aplasia due to B19V infection after transplantation (40); however surveillance strategies are not performed.
REFERENCES
1. Abe K, Kiuchi T, Tanaka K, Edamoto Y, Aiba N, Sata T. Characterization of erythrovirus B19 genomes isolated in liver tissues from patients with fulminant hepatitis and biliary atresia who underwent liver transplantation. Int J Med Sci 2007;4:105-109. [PubMed]
2. Aberham C, Pendl C, Gross P, Zerlauth G, Gessner M. A quantitative, internally controlled real-time PCR Assay for the detection of parvovirus B19 DNA. J Virol Methods 2001;92:183-191. [PubMed]
3. Anderson MJ, Higgins PG, Davis LR, Willman JS, Jones SE, Kidd I., Pattison JR, Tyrrell DA. Experimental parvoviral infection in humans. J Infect. Dis 1985;152:257–265. [PubMed]
4. Anderson MJ, Jones SE, Fisher-Hoch SP, Lewis E, Hall SM, Bartlett CLR, Cohen BJ, Mortimer PP, Pereira MS. Human parvovirus, the cause of erythema infectiosum (fifth disease)? Lancet 1985;1378. [PubMed]
5. Azzi A, Morfini M, Mannucci PM. The transfusion-associated transmission of parvovirus B19. Transfus Med Rev 1999;13:194-204.[PubMed]
6. Ballou WR, Reed JL, Noble W, Young NS, Koenig S. Safety and immunogenicity of a recombinant parvovirus B19 vaccine formulated with MF59C.1. J Infect Dis 2003;187: 675–8. [PubMed]
7. Barah F, Vallely PJ, Chiswick ML, Cleator GM, Kerr JR. Association of human parvovirus B19 infection with acute meningoencephalitis. Lancet 2001;358:729730. [PubMed]
8. Baylis SA, Shah N, Minor PD. Evaluation of different assays for the detection of parvovirus B19 DNA in human plasma. J Virol Methods 2004;121:7-16. [PubMed]
9. Baylis SA. Standardisation of nucleic acid amplification technique (NAT)-based assay for different genotypes of parvovirus B19: a meeting summary. Vox Sanguinis 2008;94:74-80. [PubMed]
10. Behzad-Behbahani A, Pouransari R, Tabei SZ, Rahiminejad MS, Robati M, Yaghobi R, Nourani H, Ramzi MM, Farhadi-Andarabi A, Mojiri A, Rahsaz M, Banihashemi M, Zare N. Risk of viral transmission via bone marrow progenitor cells versus umbilical cord blood hematopoietic stem cells in bone marrow transplantation. Transplant Proc 2005;37:3211-3212. [PubMed]
11. Bergallo M, Costa C, Sidoti F, Novelli M, Ponti R, Castagnoli C, Merlino C, Bernengo MG, Cavallo R. Variants of parvovirus B19: bioinformatical evaluation of nested PCR assays. Intervirology 2008;51:75-80. [PubMed]
12. Bergallo M, Merlino C, Daniele R, Costa C, Ponzi AN, Cavallo R. Quantitative Competitive-PCR assay to measure human parvovirus B19 DNA load in serum samples. Melecular Biotechnology 2006;32:23-29. [PubMed]
13. Bhal PS, Davies NJ, Westmoreland D, Jones A. Spontaneous resolution of non-immune hydrops fetalis secondary to transplacental parvovirus B19 infection. Ultrasound Obstet Gynecol 1996;7:55-57. [PubMed]
14. Bhattacharyya J, Kumar R, Tyagi S, Kishore J, Mahapatra M, Choudhry VP. Human parvovirus B19-induced acquired pure amegakaryocytic thrombocytopenia. British Journal of Haematology 2004;128:128-129. [PubMed]
15. Blümel J, Eis-Hübinger AM, Stühler A, Bönsch C, Gessner M, Löwer J. Characterization of Parvovirus B19 genotype 2 in KU812Ep6 cells. J Virol 2005;79:14197-14206. [PubMed]
16. Bonvicini F, Filippone C, Delbarba S, Manaresi E, Zerbini M, Musiani M, Gallinella G. Parvovirus B19 genome as a single, two state replicative and transcriptional unit. Virology 2006;347: 447-454. [PubMed]
17. Bonvicini F, Filippone C, Manaresi E, Gentilomi GA, Zerbini M, Musiani M, Gallinella G. Peptide Nucleic Acid-Based in situ hybridization assay for the detection of parvovirus B19 nucleic acids. Clinical Chemistry 2006;52:973-978.[PubMed]
18. Bonvicini F, Gallinella G, Gentilomi GA, Ambretti S, Musiani M, Zerbini M. Prevention of iatrogenic transmission of B19 infection: different approaches to detect, remove or inactivate virus contamination. Clinical Laboratory 2006;52:263-268. [PubMed]
19. Bonvicini F, Marinacci G, Pajno MC, Gallinella G, Musiani M, Zerbini M. Meningoencephalitis with persistent parvovirus B19 infection in an apparently healthy woman. Clin Infect Dis 2008;47:385-387. [PubMed]
20. Bonvicini F, Mirasoli M, Gallinella G, Zerbini M, Musiani M, Roda A. PNA-based probe for quantitative chemiluminescent in situ hybridisation imaging of cellular parvovirus B19 replication kinetics. The Analyst 2007;132:519-523. [PubMed]
21. Braham S, Gandhi J, Beard S, Cohen B. Evaluation of the Roche LightCycler parvovirus B19 quantification kit for the diagnosis of parvovirus B19 infections. J Clin Virol 2004;31:5-10.[PubMed]
22. Broliden K, Tolfvenstam T, Norbeck O. Clinical aspects of parvovirus B19 infection. J Intern Med 2006;260:285–304. [PubMed]
23. Broliden K. Parvovirus B19 infection in pediatric solid-organ and bone marrow transplantation. Pediatr Transplant 2001;5:320–330. [PubMed]
24. Brown KE, Anderson SM, Young NS. Erythrocyte P antigen: cellular receptor for B19 parvovirus. Science 1993;262:114–117.[PubMed]
25. Brown KE, Young NS, Alving BM, Barbosa LH. Parvovirus B19: implications for transfusion medicine. Summary of a workshop. Transfusion 2001;41:130-135. [PubMed]
26. Brown KE. Congenital anaemia after transplacental B19 parvovirus infection. Lancet 1994;343:895–896. [PubMed]
27. Brown KE. Haematological consequences of parvovirus B19 infection. Bailliere’s Clinical Haematology 2000;13:245-259.[PubMed]
28. Bultmann BD, Klingel K, Sotlar K, Bock CT, Baba HA, Sauter M, Kandolf R. Fatal parvovirus B19-associated myocarditis clinically mimicking ischemic heart disease: an endothelial cell-mediated disease. Hum Pathol 2003;34:92-95. [PubMed]
29. Butchko AR, Jordan JA. Comparison of three commercially available serologic assays used to detect human parvovirus B19-specific immunoglobulin M (IgM) and IgG antibodies in sera of pregnant women. J Clin Microbiol 2004;42:3191-3195. [PubMed]
30. Candotti D, Etiz N, Parsyan A, Allain JP. Identification and characterization of persistent human erythrovirus infection in blood donor samples. J Virol 2004;78:12169-12178. [PubMed]
31. Cavallo R, Merlino C, Re D, Bollero C, Bergallo M, Lembo D, Musso T, Leonardi G, Segoloni GP, Ponzi AN. B19 virus infection in renal transplant recipients. J Clin Virol 2003;26:361-368. [PubMed]
32. Chisaka H, Morita E, Yaegashi N, Sugamura K. Parvovirus B19 and the pathogenesis of anaemia. Rev Med Virol 2003;13:347-359. [PubMed]
33. Cioc AM, Sedmak DD, Nuovo GJ, Dawood MR, Smart G, Magro CM. Parvovirus B19 associated adult Henoch Schönlein purpura. J Cutan Pathol 2002;29:602-607. [PubMed]
34. Cohen BJ, Beard S, Knowles WA et al. Chronic anemia due to parvovirus B19 infection in a bone marrow transplant patient after platelet transfusion. Transfusion 1997;37:947-52. [PubMed]
35. Cohen BJ, Gandhi J, Clewley JP. Genetic variants of parvovirus B19 identified in the United Kingdom: implications for diagnostic testing. J Clin Virol 2006;36:152-155. [PubMed]
36. Crowson AN, Magro CM, Dawood MR. A causal role for parvovirus B19 infection in adult dermatomyositis and other autoimmune syndromes. J Cutan Pathol 2000;27:505-515. [PubMed]
37. Daly P, Corcoran A, Mahon BP, Doyle S. High sensitivity PCR detection of parvovirus B19 in plasma. J Clinical Microbiology 2002;40:1958-1962. [PubMed]
38. Dobec M, Kaeppeli F, Cassinotti P, Satz N. Persistent parvovirus B19 infection and arthralgia in a patient mistakenly treated for Lyme disease. J Clin Virol 2008;43:226-229. [PubMed]
39. Egbuna O, Zand MS, Arbini A, Menegus M, Taylor J. A cluster of parvovirus B19 infections in renal transplant recipients: a prospective case series and review of the literature. Am J Transplant 2006;6:225–231. [PubMed]
40. Eid AJ, Brown RA, Patel R, Razonable RR. Parvovirus B19 infection after transplantation: a review of 98 cases. Clin Infect Dis 2006;43:40-48. [PubMed]
41. Ekman A, Hokynar K, Kakkola L, Kantola K, Hedman L, Bondén H, Gessner M, Aberham C, Norja P, Miettinen S, Hedman K, Söderlund-Venermo M. Biological and immunological relations among human parvovirus B19 genotypes 1 to 3. J Virol 2007;81:6927-6935. [PubMed]
42. Enders G, Dotsch J, Bauer J et al. Life-threatening parvovirus B19-associated myocarditis and cardiac transplantation as possible therapy: two case reports. Clin Infect Dis 1998; 26: 355–358. [PubMed]
43. Enders M, Helbig S, Hunjet A, Pfister H, Reichhuber C, Motz M. Comparative evaluation of two commercial enzyme immunoassays for serodiagnosis of human parvovirus B19 infection. J Virol Methods 2007;146:409-413. [PubMed]
44. Enders M, Weidner A, Zoellner I, Searle K, Enders G. Fetal morbidity and mortality after acute human parvovirus B19 infection in pregnancy: prospective evaluation of 1018 cases. Prenat Diagn 2004;24:513-518. [PubMed]
45. Florea AV, Ionescu DN, Melhem MF. Parvovirus B19 infection in the immunocompromised host. Arch Pathol Lab Med 2007;13:799-804. [PubMed]
46. Franssila R, Hedman K. Infection and musculoskeletal conditions: Viral causes of arthritis. Best Pract Res Clin Rheumatol 2006;20:1139-1157. [PubMed]
47. Fryer JF, Hubbard AR, Baylis SA. Human parvovirus PARV4 in clotting factor VIII concentrates. Vox Sanguinis 2007;93:341-347.[PubMed]
48. Gallinella G, Bonvicini F, Filippone C, Delbarba S, Manaresi E, Zerbini M, Musiani M. Calibrated real-time PCR for evaluation of parvovirus B19 viral load. Clinical Chemistry 2004;50:759-762. [PubMed]
49. Gallinella G, Manaresi E, Venturoli S, Grazi GL, Musiani M, Zerbini M. Occurrence and clinical role of active parvovirus B19 infection in transplant recipients. Eur J Clin Microbiol Infect Dis 1999;18:811-813. [PubMed]
50. Gallinella G, Moretti E, Nardi G, Zuffi E, Bonvicini F, Bucci E, Musiani M, Zerbini M. Analysis of B19 virus contamination in plasma pools for manufacturing, by using a competitive polymerase chain reaction assay. Vox Sang 2002;83:324-331. [PubMed]
51. Gessoni G, Barin P, Marchiori G. Nucleic acids amplification technique (NAT) screening for parvovirus B19: the first Italian routine experience. Transfus Med 2007;17:417-419. [PubMed]
52. Gutersohn A, Zimmermann U, Bartel T, Erbel R. A rare case of acute 'infective' myocardial infarction triggered by acute parvovirus B19 myocarditis. Nat Clin Pract Cardiovasc Med 2005;2:167-171. [PubMed]
53. Harris JW. Parvovirus B19 for the hematologist. Am J Hematol 1992;39:119–130. [PubMed]
54. Heegaard ED, Petersen BL, Heilmann CJ, Hornsleth A. Prevalence of parvovirus B19 and parvovirus V9 DNA and antibodies in paired bone marrow and serum samples from healthy individuals. J Clin Microbiol. 2002;40:933-936. [PubMed]
55. Heegard ED, Laub Petersen B. Parvovirus B19 transmitted by bone marrow. Br J Haematol 2000;111:659-661.[PubMed]
56. Hillingsø JG, Jensen IP, Tom-Petersen L. Parvovirus B19 and acute hepatitis in adults. Lancet 1998;351:955-956.[PubMed]
57. Hokynar K, Norja P, Laitinen H, Palomäki P, Garbarg-Chenon A, Ranki A, Hedman K, Söderlund-Venermo M. Detection and differentiation of human parvovirus variants by commercial quantitative real-time PCR tests. J Clin Microbiol 2004;42:2013-2019.[PubMed]
58. Human Anti-D immunoglobulin. In: European Pharmacopoeia monograph. Document 01:557 Voluntary recall of pooled plasma, solvent-detergent treated. In: FDA, Center for Biologics Evaluation and Research. 1999. [PubMed]
59. Jacobson SK, Daly JS, Thorne GM, McIntosh K. Chronic parvovirus B19 infection resulting in chronic fatigue syndrome: case report and review. Clin Inf Dis 1997;24:1048-1051. [PubMed]
60. Jones MS, Kapoor A, Lukashov VV, Simmond P, Hecht F, Delwart E. New DNA viruses identified in patients with acute viral infection syndrome. J Virol 2005;79: 8230-8236. [PubMed]
61. Jordan J, Tiangco B, Kiss J, Koch W. Human parvovirus B19: prevalence of viral DNA in volunteer blood donors and clinical outcomes of transfusion recipients. Vox Sang 1998;75:97–102. [PubMed]
62. Jordan JA, DeLoia JA. Globoside expression within the human placenta. Placenta 1999;20:103-108. [PubMed]
63. Jordan JA. Comparison of a baculovirus-based VP2 enzyme immunoassay (EIA) to an Escherichia coli-based VP1 EIA for detection of human parvovirus B19 immunoglobulin M and immunoglobulin G in sera of pregnant women. J Clin Microbiol 2000;38:1472-1475. [PubMed]
64. Katz VL, McCoy MC, Kuller JA, Hansen WF. An association between fetal parvovirus B19 infection and fetal anomalies: a report of two cases. Am J Perinatol 1996;13:43-45. [PubMed]
65. Kelly T, Mathers A. Early presentation and spontaneous resolution of hydrops fetalis, secondary to parvovirus B19 infection. J Obstet Gynaecol 1998;18:190-191. [PubMed]
66. Kerr JR, Barah F, Chiswick ML, McDonnell GV, Smith J, Chapman MD, Bingham JB, Kelleher P, Sheppard MN. Evidence for the role of demyelination, HLA-DR alleles, and cytokines in the pathogenesis of parvovirus B19 meningoencephalitis and its sequelae. J Neurol Neurosurg Psychiatry 2002;73:739-746. [PubMed]
67. Kerr JR, Bracewell J, Laing I, Mattey DL, Bernstein RM, Bruce IN, Tyrrell DA. Chronic fatigue syndrome and arthralgia following parvovirus B19 infection. Rheumatology 2002;29:595-602. [PubMed]
68. Kerr JR, Cunniffe VS, Kelleher P, Bernstein RM, Bruce IN. Successful intravenous immunoglobulin therapy in 3 cases parvovirus B19-associated chronic fatigue syndrome. Clin Infec Dis 2003;36:100-106. [PubMed]
69. Ki CS, Kim IS, Kim JW, Lee NY, Kim SH, Lee KW, Kim SJ, Joh JW, Huh WS, Oh HY. Incidence and clinical significance of human parvovirus B19 infection in kidney transplant recipients. Clin Transplant 2005;19:751-755.[PubMed]
70. Koenigbauer UF, Eastlund T, Day JW. Clinical illness due to parvovirus B19 infection after infusion of solvent/detergenttreated pooled plasma. Transfusion 2000;40:1203-1206. [PubMed]
71. Koppelman MH, Cuypers HT, Emrich T, Zaaijer HL. Quantitative real-time detection of parvovirus B19 DNA in plasma. Transfusion 2004;44:97-103. [PubMed]
72. Koppelman MH, Rood IG, Fryer JF, Baylis SA, Cuypers HT. Parvovirus B19 genotypes 1 and 2 detection with real-time polymerase chain reaction assays. Vox Sang 2007;93:208-215. [PubMed]
73. Krause I, Wu R, Sherer Y, Patanik M, Peter JB, Shoenfeld Y. In vitro antiviral and antibacterial activity of commercial intravenous immunoglobulin preparations – a potential role for adjuvant intravenous immunoglobulin therapy in infectious diseases. Transfusion Medicine 2002;12:133-139. [PubMed]
74. Lehamann HW, von Landenberg P, Modrow S. Parvovirus B19 infection and autoimmune disease. Autoimmun Rev 2003;2:218-223. [PubMed]
75. Lennerz C, Madry H, Ehlhardt S, Venzke T, Zang KD, Mehraein Y. Parvovirus B19-related chronic monoarthritis: immunohistochemical detection of virus-positive lymphocytes within the synovial tissue compartment: two reported cases. Clin Rheumatol 2004;23:59-62. [PubMed]
76. Liang T, Li D, Bai X, Liang L, Xu S, Wang W, Shen Y, Zhang M, Zheng S. Pure red cell aplasia due to parvovirus B19 infection after liver transplantation: a case report and review of the literature. World J Gastroenterol 2007;13:2007-2010. [PubMed]
77. Lowry SM, Brent LH, Menaldino S, Kerr JR. A case of persistent parvovirus B19 infection in bilateral cartilaginous and ligamentous damage to the wrists. Clin Inf Diseases 2005;42:42-44. [PubMed]
78. Lu J, Zhi N, Wong S, Brown KE. Activation of synoviocytes by the secreted phospholipase A2 motif in the VP1-unique region of parvovirus B19 minor capsid protein J Infect Dis 2006;193:582-590. [PubMed]
79. Lurcharchaiwong W, Chieochansin T, Payungporn S, Theamboonlers A, Poovorawan Y. Parvovirus 4 (PARV4) in serum of intravenous drug users and blood donors. Infection. 2008;36:488-491. [PubMed]
80. Manaresi E, Gallinella G, Morselli Labate AM, Zucchelli P, Zaccarelli D, Ambretti S, Delbarba S, Zerbini M, Musiani M. Seroprevalence of IgG against conformational and linear capsid antigens of parvovirus B19 in Italian blood donors. Epidemiol Infect. 2004;132:857-862. [PubMed]
81. Manaresi E, Gallinella G, Venturoli S, Zerbini M, Musiani M. Detection of parvovirus B19 IgG: choice of antigens and serological tests. J Clin Virol 2004;29:51-53. [PubMed]
82. Manaresi E, Gallinella G, Zuffi E, Bonvicini F, Zerbini M, Musiani M. Diagnosis and quantitative evaluation of parvovirus B19 infections by real-time PCR in the clinical laboratory. Journal of Medical Virology 2002;67:275-281.[PubMed]
83. Manaresi E, Zuffi E, Gallinella G, Gentilomi G, Zerbini M, Musiani M. Differential IgM response to conformational and linear epitopes of parvovirus B19 VP1 and VP2 structural proteins. J Med Virol 2001;64:67-73. [PubMed]
84. Manning A, Willey SJ, Bell JE, Simmond P. Comparison of tissue distribution, persistence and molecular epidemiology of parvovirus B19 and novel human parvovirus PARV4 and human bocavirus. J inf Dis 2007;195:1345-1352. [PubMed]
85. Mari G. Middle cerebral artery peak systolic velocity for the diagnosis of fetal anemia: the untold story. Ultrasound Obstet Gynecol 2005;25:323-330. [PubMed]
86. Matsuda H, Sakaguchi K, Shibasaki T, Takahashi H, Kawakami Y, Furuya K. Intrauterine therapy for parvovirus B19 infected symptomatic fetus using B19 IgG-rich high titer gammaglobulin. J Perinat Med 2005;33:561-563. [PubMed]
87. Mehraein Y, Lennerz C, Ehlhardt S, Venzke T, Ojak A, Remberger K, Zang KD. Detection of parvovirus B19 capsid proteins in lymphocytic cells in synovial tissue of autoimmune chronic arthritis. Mod Pathol 2003;16:811-817. [PubMed]
88. Miyagawa E, Yoshida T, Takahashi H, Yamaguchi K, Nagano T, Kiriyama Y, Okochi K, Sato H. Infection of the erythroid cell line, KU812Ep6 with human parvovirus B19 and its application to titration of B19 infectivity. J Virol Methods 1999;83:45–54.[PubMed]
89. Modrof J, Berting A, Tille B, Klotz A, Forstner C, Rieger S, Aberham C, Gessner M, Kreil TR. Neutralisation of human parvovirus B19 by plasma and intravenous immunoglobulins. Transfusion 2008;48:178-186. [PubMed]
90. Moore TL. Parvovirus-associated arthritis. Curr Opin Rheumatol 2000;12:289-294. [PubMed]
91. Morey AL, Keeling JW, Porter HJ, Fleming KA. Clinical and histopathological features of parvovirus B19 infection in the human fetus. Br J Obstet Gynaecol 1992;99:566-574. [PubMed]
92. Mossong J, Hens N, Friederichs V, Davidkin I, Broman M, Litwinska B, Siennicka J, Trzcinska A, VAN Damme P, Beutels P, Vyse A, Shkedy Z, Aerts M, Massari M, Gabutti G. Parvovirus B19 infection in five European countries: seroepidemiology, force of infection and maternal risk of infection. Epidemiol Infect 2008;136:1059-1068. [PubMed]
93. Mouthon L, Guillevin L, Tellier Z. Intravenous immunoglobulins in autoimmune- or parvovirus B19-mediated pure red-cell aplasia. Autoimmun Rev 2005;4:264-269. [PubMed]
94. Munakata Y, Saito Ito T, Kumura-Ishii K, Huang J, Kodera T, Ishii T, Hirabayashi Y, Koyanagi Y, Sasaki T. Ku80 autoantigen as a cellular coreceptor for human parvovirus B19 infection. Blood 2005;106:3449-3456. [PubMed]
95. Murer L, Zacchello G, Bianchi D, Dall'Amico R, Montini G, Andreetta B, Perini M, Dossi EC, Zanon G, Zacchello F. Thrombotic microangiopathy associated with parvovirus B 19 infection after renal transplantation J Am Soc Nephrol 2000;11:1132-1137.[PubMed]
96. Musiani M, Manaresi E, Gallinella G, Cricca M, Zerbini M. Recurrent erythema in patients with long-term parvovirus B19 infection. Clin Infect Dis 2005;40:117-119.[PubMed]
97. Musiani M, Gallinella G, Venturoli S, Zerbini M. Competitive PCR-ELISA protocols for the quantitative and the standardized detection of viral genomes. Nat Protoc 2007;2:2511-2519. [PubMed]
98. Musiani M, Venturoli S, Gallinella G, Zerbini M. Qualitative PCR-ELISA protocol for the detection and typing of viral genomes. Nat Protoc 2007;2:2502-2510. [PubMed]
99. Nagel HT, de Haan TR, Vandenbussche FP, Oepkers D, Walther LJ. Long-term outcome after fetal transfusion for hydrops fetalis associated with parvovirus B19 infection. Obstet Gynecol 2007;109:42-47. [PubMed]
100. Naides SJ. Infection with parvovirus B19. Curr Infect Dis Rep 1999;1:273-278. [PubMed]
101. Norbeck O, Papadogiannakis N, Petersson K, Hirbod T, Broliden K, Tolfvenstam T. Revised clinical presentation of parvovirus B19-associated intrauterine fetal death. Clin Infect Dis 2002;35:1032-1038. [PubMed]
102. Norja P, Hokynar K, Aaltonen LM, Chen R, Ranki A, Partio EK, Kiviluoto O, Davidkin I, Leivo T, Eis-Hübinger AM, Schneider B, Fischer HP, Tolba R, Vapalahti O, Vaheri A, Söderlund-Venermo M, Hedman K. Bioportfolio: lifelong persistence of variant and prototypic erythrovirus DNA genomes in human tissue. Proc Natl Acad Sci U S A 2006;103:7450-7453.[PubMed]
103. Ogawa E, Otaguro S, Murata M, Kainuma M, Sawayama Y, Furusyo N, Hayashi J. Intravenous immunoglobulin therapy for severe arthritis associate with human parvovirus B19 infection. J Infect Chemother 2008;14:377-382. [PubMed]
104. O'Malley A, Barry-Kinsella C, Hughes C, Kelehan P, Devaney D, Mooney E, Gillan J. Parvovirus infects cardiac myocytes in hydrops fetalis. Pediatr Dev Pathol 2003;6:414-420. [PubMed]
105. Ozawa K, Kurtzman G, Young N. Productive infection by B19 parvovirus of human erythroid bone marrow cells in vitro. Blood 1987;70:384-391. [PubMed]
106. PA/PH/OMCL (03) 38 DEF: OMCL Guideline for validation of nucleic acid amplification techmniques (NAT) for quantitation of B19 virus DNA in plasma pools; in Biological Substances Submitted to the Official Control Authority Batch Release. Strasbourg, France, Council of Europe, 2006. [PubMed]
107. Pallier C, Greco A, Le Junter J, Saib A, Vassias I, Morinet F. The 3’ untraslated region of B19 parvovirus capsid protein mRNAs inhibits its own mRNA translation in non-permissive cells. J Virol 1997;71:8482-8489. [PubMed]
108. Papadogiannakis N, Tolfvenstam T, Fischler B, Norbeck O, Broliden K. Active, fulminant, lethal myocarditis associated with parvovirus B19 infection in an infant. Clin Infect Dis 2002;35:1027-1031. [PubMed]
109. Pasquinelli G, Bonvicini F, Foroni L, Salfi N, Gallinella G. Placental endothelial cells can be productively infected by parvovirus B19. J Clin Virol 2009;44:33-38. [PubMed]
110. Pattison JR, Jones SE, Hodgson J et al. Parvovirus infections and hypoplastic crisis in sickle-cell anaemia. Lancet 1981; 1:664–665. [PubMed]
111. Pfrepper KI, Enders M, Motz M. Human parvovirus B19 serology and avidity using a combination of recombinant antigens enables a differentiated picture of the current state of infection. J Vet Med 2005;52:362-365. [PubMed]
112. Plachouras N, Stefanidis K, Andronikou S, Lolis D. Severe nonimmune hydrops fetalis and congenital corneal opacification secondary to human parvovirus B19 infection. A case report. J Reprod Med 1999;44:377-380. [PubMed]
113. Reid DM, Reid TM, Brown T, Rennie JA, Eastmond CJ. Human parvovirus-associated arthritis: a clinical and laboratory description. Lancet 1985:422–425. [PubMed]
114. Röhrer C, Gärtner B, Sauerbrei A, Böhm S, Hottenträger B, Raab U, Thierfelder W, Wutzler P, Modrow S. Seroprevalence of parvovirus B19 in the German population. Epidemiol Infect 2008;136:1564-1575. [PubMed]
115. Rugolotto S, Padovani EM, Sanna A, Chiaffoni GP, Marradi PL, Borgna-Pignatti C. Intrauterine anemia due to parvovirus B19: successful treatment with intravenous immunoglobulin. Haematologica 1999;84:668-669. [PubMed]
116. Schneider B, Fryer JF, Oldenburg J, Brackmann HH, Baylis SA, Eis-Hübinger AM. Frequency of contamination of coagulation factor concentrates with novel human parvovirus PARV4. Haemophilia 2008;14:978-986. [PubMed]
117. Schwarz TF, Serke S, Hottentrager B, von Brunn A, Baurmann H, Kirsch A, Stolz W, Huhn D, Deinhardt F, Roggendorf M. Replication of parvovirus B19 in hematopoietic progenitor cells generated in vitro from normal peripheral blood. J Virol 1992; 66:1273-1276. [PubMed]
118. Segata M, Chaoui R, Khalek N, Bahado-Singh R, Paidas MJ. Fetal thrombocytopenia secondaru to parvovirus B19 infection. Am J Obstet Gynecol 2007;61:61-64. [PubMed]
119. Simchen MJ, Toi A, Bona M, Alkazaleh F, Ryan G, Chitayat D. Fetal hepatic calcifications: prenatal diagnosis and outcome. Am J Obstet Gynecol 2002;187:1617-1622. [PubMed]
120. Simmonds P, Manning A, Kenneil R, Carnie FW, Bell JE. Parenteral transmission of the novel human parvovirus PARV4. Emerg Infect Dis 2007;13:1386-1388. [PubMed]
121. Souliè JC. Cardiac involvement in fetal parvovirus infection. Pathol Biol 1995;43:416-419.[PubMed]
122. Srivastava A, Bruno E, Briddell R, Cooper R, Srivastava C, van Besien K, Hoffman R. Parvovirus B19-induced perturbation of human megakaryocytopoiesis in vitro. Blood 1990;76:1997–2004. [PubMed]
123. Srivastava A, Lu L. Replication of B19 parvovirus in highly enriched hematopoietic progenitor cells from normal human bone marrow. J Virol 1988;62:3059-3063. [PubMed]
124. Srivastava CH, Zhou S, Munshi NC, Srivastava A. Parvovirus B19 replication in human umbilical cord blood cells. Virology 1992;189:456-461. [PubMed]
125. Stahl HD, Pfeiffer R, Emmrich F. Intravenous treatment with immunoglobulins may improve chronic undifferentiated mono- and oligoarthritis. Clin Exp Rheumatol 2000;18:515-517. [PubMed]
126. Tang WJ, Lau JSM, Wong SYN, Cheung JKO, Chan CH, Wong KF, Wong A, Chan PKS. Dose-by-dose virological and hematological responses to intravenous immunoglobulin in an immunocompromised patient with persistent parvovirus B19 infection. J Med Virol 2007;79:1401-1405. [PubMed]
127. Tattersall P, Bergoin M, Bloom ME, Brown KE, Linden RM, Muzyczka N, Parrish CR, Tijssen P. Parvoviridae; in Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA (eds):Virus Taxonomy: Eighth report of the International Committee on Taxonomy Viruses. Academic Press 2005:353-369. [PubMed]
128. Thomas HIJ, Barrett E, Hesketh LM, Wynne A, Morgan-Capner P. Simultaneous IgM reactivity by EIA against more than one virus in measles, parvovirus B19 and rubella infection. J Clin Virol 1999;14:107-108. [PubMed]
129. Tiessen RG, van Elsacker-Niele AM, Vermeij-Keers C, Oepkes D, van Roosmalen J, Gorsira MC. A fetus with a parvovirus B19 infection and congenital anomalies. Prenat Diagn 1994;14:173-176. [PubMed]
130. van Elsacker-Niele AM, Salismans MM, Weiland HT, Vermeij-Keers C, Anderson MJ, Versteeg J. Fetal pathology in human parvovirus B19 infection. Br J Obstet Gynaecol 1989;96:768-775. [PubMed]
131. Venturoli S, Cricca M, Bonvicini F, Gallinella G, Gentilomi G, Zerbini M, Musiani M. Detection of adeno-associated virus DNA in female genital samples by PCR-ELISA. J Med Virol 2001;64:577-582. [PubMed]
132. Voluntary recall of pooled plasma, solvent-detergent treated. In: FDA, Center for Biologics Evaluation and Research. 1999.[PubMed]
133. Walz CM, Correa-Ochoa MM, Müller M, Schlehofer JR. Adenoassociated virus type 2-induced inhibition of the human papillomavirus type 18 promoter in transgenic mice. Virology 2002;293:172-181. [PubMed]
134. Wang X, Zhang G, Liu F, Han M, Xu D, Zang Y. Prevalence of human parvovirus B19 DNA in cardiac tissues of patients with congenital heart diseases indicated by nested PCR and in situ hybridisation. J Clin Virol 2004;31:20-24.[PubMed]
135. Wegner CC, Jordan JA. Human parvovirus B19 VP2 empty capsids bind to human trophoblast cells in vitro via the globoside receptor. Infect Dis Obstet Gynecol 2004;12:69-78. [PubMed]
136. Weigel-Kelley KA, Yoder MC, Srivastava A. Alpha5beta1 integrin as a cellular coreceptor for human parvovirus B19: requirement of functional activation of beta1 integrin for viral entry. Blood . 2003;102:3927–3933. [PubMed]
137. White DG, Woolf AD, Mortimer PP, Cohen BJ, Blake DR, Bacon PA. Human parvovirus arthropathy. Lancet 1985:419–421.[PubMed]
138. Wicki J, Samii K, Cassinotti P, Voegeli J, Rochat T, Beris P. Parvovirus [correction of Parovirus] B19-induced red cell aplasia in solid-organ transplant recipients. Two case reports and review of the literature. Hematol Cell Ther 1997;39:199-204. [PubMed]
139. Yango Jr, Morrissey P, Gohh R, Wahbeh A. Donor-transmitted parvovirus infection in a kidney transplant recipient presenting as pancytopenia and allograft dysfunction. Transpl Infect Dis 2002;4:163-166. [PubMed]
140. Zerbini M, Gentilomi GA, Gallinella G, Morandi R, Calvi S, Guerra B, Musiani M. Intrauterine parvovirus B19 infection and meconium perotinitis. Prenatal Diagnosis 1998;18:599-606.[PubMed]
Table 1: Main Diagnostic Approaches in Clinical Manifestations Associated with B19V Infection
| Clinical Manifestations | Clinical Specimens | Laboratory Diagnosis |
|---|---|---|
| Erythema infectiosum | serum | IgG/IgM |
| Arthropathy | serum | IgG/IgM |
| Transient aplastic crisis | serum | DNA by PCR |
| Pure red cell aplasia | serum/bone marrow aspirates | DNA by PCR |
| Hydrops fetalis | maternal serum | DNA by PCR, IgG/IgM |
| fetal specimens (amniotic fluid cells/cord blood, bioptic tissues) | DNA by PCR and ISH | |
| Chronic syndromes and persistent B19V infection | serum | DNA by quantitative PCR |
Table 2: Comparison of Major Commercial Assays for the Detection of B19V DNA and Antibodies
| Real Time PCR assays | Assay characteristics | References |
|---|---|---|
| Roche LightCycler Parvovirus B19 Quantification Kit (Roche Diagnostics, Germany) | Quantitative detection of B19V genotype 1. Genotype 2 and 3 are not detected | 8, 21, 35, 57 |
| RealArt Parvo B19 LC PCR Kit (Artus GmbH, Germany) | Quantitative detection of B19V genotype 1 and 2 (underestimation of genotype 3) | 8, 35, 57 |
| EIA assays |
|
|
| Parvovirus B19 EIA IgG/IgM (Biotrin, Ireland) | Baculovirus-produced VP2 antigens for detection of B19V IgG Baculovirus-produced VP2 antigens in capture-format for detection of B19V IgM | 29,43, 63, 81, 92 |
| Parvovirus B19 EIA IgG/IgM Denka Seiken (Japan) or Medac Diagnostika (Germany) | Baculovirus-produced VP1 and VP2 antigens for detection of B19V IgG Baculovirus-produced VP1 and VP2 antigens in capture-format for detection of B19V IgM | 29, 81 |
| recomWell Parvovirus B19 IgG/IgM (Mikrogen Germany) | Escherichia coli expressed VP1 and Saccharomyces cerevisiae produced VP2 antigens for the detection of B19V IgG and IgM | 29, 43, 92 |
| NovaLisaTM Parvovirus B19 recombinant (NovaTec Immundiagnostica, Germany) | Escherichia coli expressed VP1 and baculovirus-produced VP2 antigens for detection of B19V IgG and IgM | 89 |
| RidaScreen Parvovirus B19 IgG/IgM (R-Biopharm, Germany) | Escherichia coli expressed VP1 and VP2 antigens for detection of B19V IgG and IgM | 38 |
| Immunofluorescence assays |
|
|
| Parvovirus B19 IFA (Biotrin, Ireland) | Baculovirus-produced VP1 antigens for detection of B19V IgG and IgM | 38 |
| Immunoblot assays |
|
|
| recomLine Parvovirus B19 IgG [Avidity]/IgM (Mikrogen Germany) | Escherichia coli expressed VP1, VP2, NS antigen fragments and yeast-produced VP2 for detection of B19V IgG and IgM. Optionally available additional reagent for IgG Avidity | 43, 92, 111
|
| RidaBlot Parvovirus B19 IgG/IgM (R-Biopharm, Germany) | Escherichia coli expressed VP1, VP2, NS antigen fragments for detection of B19V IgG and IgM | 38 |
Table 3: Treatment for B19V Infection
Clinical Manifestations |
Treatments |
References |
|---|---|---|
| Arthropathy | Anti-inflammatory drug | 22, 77 |
| Transient aplastic crisis (TAC) | Blood transfusion | 53 |
| Pure red cell aplasia (PRCA) | IVIG | 76, 126, 93, 40 |
| Hydrops fetalis | Blood transfusion | 44 |
| IVIG | 86, 115 | |
| Chronic arthritis and persistent B19V infection | IVIG | 103, 125, 77 |
| Chronic fatigue syndrome and persistent B19V infection | IVIG | 68 |
| Meningoencephalitis | IVIG | 66, 58 |
The current recommendation is IVIG 0,4g/Kg per 5 days in immunocompetent patients. An empirical maintenance treatment with a single –day infusion
of 0.4g/Kg IVIG every 4 weeks may control B19V in immunosuppressed patients (27).
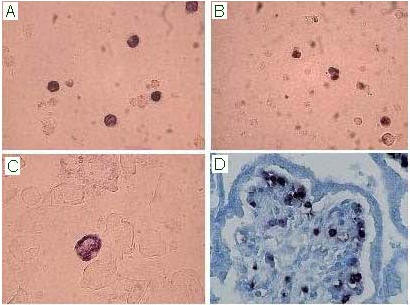
Figure 1: In situ hybridisation assay for the detection of B19V nucleic acids on bone marrow (A), fetal cord blood (B), amniotic fluid cells (C) and placental bioptic sample (Methyl Green counterstain; D). B19V infected cells show a nuclear purple/blue precipitate.
What's New
Douvoyiannis, M. Neurologic Manifestations Associated with Parvovirus B19 Infection. Clin Infect Dis 2009;48:1713-1723.
GUIDED MEDLINE SEARCH FOR
Review articles
Eid, A. Parvovirus B19 Infection in Transplant Recipients.GUIDED MEDLINE SEARCH FOR RECENT REVIEWS
History
Bassols, AC. Parvovirus B19 and the New Century. Clin Infect Dis. 2008 Feb 15;46(4):537-9.
