Pleural Effusions
Authors: Amber Degryse, M.D., Richard W. Light, M.D.
Pleural effusions are produced by a wide variety of causes. Infectious processes including bacteria, viruses, tuberculosis, atypical mycobacterium, fungus, as well as parasites account for a substantial percentage of these effusions. This chapter will help to elucidate the broad differential diagnosis that must be entertained when a physician is faced with a pleural effusion. The etiologies of pleural effusions as a whole, and then more specifically the various specific findings of pleural effusions resulting from infectious diseases, will be examined.
PATHOPHYSIOLOGY
A pleural effusion is, simply put, an abnormal fluid collection in the chest between the visceral and pleural surfaces. A thin layer of fluid is always present in this space to allow for lubrication so that the lung may glide in the chest during inspiration and expiration. Approximately 15 mls per day of fluid enters this potential space primarily from the capillaries of the parietal pleura. This fluid is then removed by the lymphatics in the parietal pleura. At any one time there is about 20 mls of fluid in each hemithorax and the layer of fluid is 2 to 10 micrometers thick (36). However; if the normal flow of fluid is disrupted, with either too much fluid being produced or not enough being removed, fluid accumulates resulting in a pleural effusion. This regulated fluid balance can be disrupted when either local or systemic derangements occur. When local factors are altered, the fluid is rich in protein and lactate dehydrogenase (LDH) and is called an exudate. Local factors include leaky capillaries and pleural inflammation due to infection, infarction, or tumor. When systemic factors are altered producing a pleural effusion, the fluid has low protein and lactate dehydrogenase levels and is called a transudate. This can be caused by an elevated pulmonary capillary pressure with heart failure, excess ascites with cirrhosis or low oncotic pressure with the nephrotic syndrome (36).
Criteria known as Light’s criteria define the exudative and transudative effusion. An exudative effusion will have a ratio of pleural fluid protein to serum protein greater than 0.5, a ratio of pleural fluid lactate dehydrogenase to serum lactate dehydrogenase greater than 0.6 or a pleural fluid lactate dehydrogenase greater than two thirds the upper limit of normal for serum lactate dehydrogenase.
The differential diagnosis for a new pleural effusion is lengthy. The leading cause of pleural effusion in the U.S. is congestive heart failure with an estimated annual incidence of 500,000. Pneumonia is second with an incidence of 300,000 (37). Approximately 40% of the hospitalized patients with bacterial pneumonia will have an associated parapneumonic effusion (38). Other infectious causes do produce effusions but much more rarely than bacterial infections. These will be discussed in detail later in the chapter. Malignancy, pulmonary embolism, coronary artery bypass surgery, and cirrhosis account for the other top six causes of pleural effusion. In developing countries such as India and Indonesia tuberculosis (TB) is the most common cause.
The key to detecting a pleural effusion is a detailed history and physical examination. Dyspnea and cough are the most common symptoms. Pleuritic chest pain may also be present with inflammatory effusions. The typical findings on physical examination with a pleural effusion include absent breath sounds, dullness to percussion, decreased tactile fremitus, and decreased vocal transmission over the base of the lung.
DIAGNOSIS
Imaging
A posterior-anterior and lateral chest x-ray is the next step in the evaluation process. This is a sensitive test for the presence of as little as 50 mls fluid. If fluid is detected on a PA and lateral chest x-ray, a lateral decubitus film can be obtained with the patient lying on side of the effusion to assess for loculation of the fluid. Free fluid should move inferiorly and a pleural fluid line should be seen ![]() .
.
Once an effusion is identified, thoracentesis is key to identifying and diagnosing the underlying cause. A thoracentesis should be preformed on every patient with an undiagnosed pleural effusion unless there is a clear contraindication. The procedure can be done quickly and easily at the bedside with or without the use of ultrasound guidance. Ultrasound is generally more sensitive and specific than CXR as the ultrasound can identify 5-10 mls of fluid ![]() .
.
Another imaging modality that is often employed is the CT scan. The effusions are clearly defined. A CT scan is able to demonstrate anatomic details such as pleural thickening, pleural plaques seen in asbestosis, nodules on the pleura, lung masses, and pulmonary embolism ![]() (1, 60).
(1, 60).
Laboratory Testing
The pleural fluid obtained can be sent for a wide variety of tests to determine the nature of the effusion. The table below describes these tests. Not all tests will need to be sent on every effusion. The clinical situation will guide you in judicious use of resources (Tables 1 and 2).
Light's Criteria
Light's criteria are a traditional method of separating transudates and exudates that measures serum and pleural fluid protein and LDH. If at least one of the following three criteria is present, the fluid is defined as an exudate.
• Ratio of pleural-fluid protein level to serum protein level >0.5
• Ratio of pleural-fluid LDH level to serum LDH level >0.6
• Pleural-fluid LDH level > 0.67 or two thirds the upper limit of normal for serum LDH level
Very high levels of pleural fluid LDH (>1,000U/L) are found in patients with complicated parapneumonic effusion and in about 40% of those with tuberculus pleurisy (60).
Most patients who meet the criteria for an exudative effusion by LDH level but not by protein level have either parapneumonic effusions or malignancy (60). A pleural fluid absolute total protein concentrations > 3mg/dL suggests an exudate, but when taken alone this parameter misclassifies at least 10 % of exudates and 15% of transudates (60).
Protein Gradient
The difference between protein levels in the serum and pleural fluid greater than 3.1 g/dL suggests transudate.
Albumin Gradient
The difference between albumin levels in the serum and pleural fluid greater than 1.2 g/dL suggests transudate.
These are supportive tests to Light’s criteria and should not be used alone to distinguish transudates from exudates (37). Fifteen to 20% of patients with congestive heart failure or cirrhosis will meet Light’s criteria for exudative effusions. This is particularly likely if the patient has been receiving diuretics before the thoracentesis (67). The protein gradient is equally effective as the albumin gradient (40).
Adenosine Deaminase (ADA)
This test may not be routinely required in countries where tuberculosis is rare. Factors such as a positive tuberculin skin test, incarceration, foreign travel, and abnormal chest x-ray should encourage use of this test. Pleural fluid ADA greater than 40U/L has a sensitivity of 90-100% and a specificity of 85-95% for diagnosing tuberculosis pleurisy (60).
Red Blood Cell Count
Red blood cells are found in most samples of pleural effusion. Fluid for cell counts should be sent in an anticoagulant container to prevent clotting which results in inaccurate results. If fluid appears bloody, obtain hematocrit (Hct). A pleural fluid Hct greater than 50% of the peripheral Hct indicates hemothorax (40).
White Blood Cell Count and Differential
The pleural fluid needs to be sent in an anticoagulant container. If the lymphocyte count is greater than 90%, lymphoma and tuberculosis are the two most likely diagnoses (60).
Cytology
Positive cytology shows low sensitivity (54-73%) and high specificity (97%) for malignancy (59). Sensitivity will increase on multiple samples: 50%, 66.7% and 73.3% for one, two, and three samples, respectively. Malignancy should not be excluded on the basis of negative pleural cytology results.
Culture
Pleural effusion should be injected into aerobic blood culture bottles at the bedside. Mycobacterial, fungal and viral cultures should be sent if suspicion of infection.
pH
pH must be measured with an arterial blood gas machine for accurate results. Generally, a low pH (< 7.20) will be present in conjunction with a high LDH and low glucose; this is indicative of complicated parapneumonic effusion, rheumatoid arthritis or advanced malignancy.
Glucose
Pleural glucose can be difficult to interpret in patients with hyperglycemia. Low glucose (< 60 mg/dL) is found in empyema, rheumatoid arthritis, tuberculosis and malignancy. Low glucose levels are found in almost 100% of effusions due to empyema and rheumatoid disease, complicated parapneumonic effusions (30%), tuberculosis (20%), malignancy (<10%) (60).
Triglyceride
Chylothorax is caused by lymphoma or trauma. Not all chylous pleural effusions appear milky white or whitish (60). The fluid is milky if the patient has had laceration of the thoracic duct from recent trauma or thoracic surgery. Triglyceride levels greater than 110 mg/dL are consistent with true chylothorax, whereas levels that are less than 50 mg/ dL effectively rule out this entity. For triglyceride levels of 5 to 110 mg/dL, microscopy or electrophoresis revealing chylomicrons supports a diagnosis of chylothorax (46).
Cholesterol
Optional test for exudative pleural effusion and chylothorax. Cholesterol interpreted alone as a test misclassifies 10% of exudates and 20% of transudates (60). A level greater than 200 mg/dL suggests a chyliform effusion (pseudochylothorax), which suggests chronic pleural inflammatory state including tuberculosis, solid or hematogenous malignancy, or rheumatoid arthritis (59). Less common entities include sarcoidosis and amyloidosis (5).
INFECTIONS PRODUCING PLEURAL EFFUSIONS AND ANTIBIOTIC THERAPY
Bacteria
Bacteria are the pathogens responsible for the majority of pleural effusions from infectious causes. A pleural effusion secondary to a pneumonia is termed a parapneumonic effusion. Parapneumonic effusions progress along a spectrum. In the first stage, termed the exudative phase, the pleural fluid accumulates from increased pulmonary interstitial fluid traversing the visceral pleura as well as increased permeability of the pleural capillaries from inflammation. At this stage the fluid is sterile and the effusion will resolve with proper treatment of the pneumonia. In the second stage, termed the fibropurulent stage, the fluid in the pleural space becomes infected. Gram stain and culture are positive. These effusions may have low pH, low glucose, loculations produced by fibroblasts invading the pleural space, and lactate dehydrogenase greater than three times the upper limit of normal (38). If a pleural effusion reaches the fibropurulent stage it is termed a complicated parapneumonic effusion. The pleural fluid in all stages of parapneumonic effusions is characterized by having predominantly neutrophils; if neutrophils account for less than 50% of the cells in the pleural fluid, alternate diagnoses should be sought.
An empyema, the third stage of the pleural effusion spectrum, is defined as frank pus in the pleural cavity. It will have the same laboratory characteristics as a complicated parapneumic effusion. Glucose will be less than 60ml/dl and pH less than 7.20. Loculations can develop very quickly, in a matter of 12 to 24 hours. Quick drainage for complicated parapneumonic effusions and empyema is imperative.
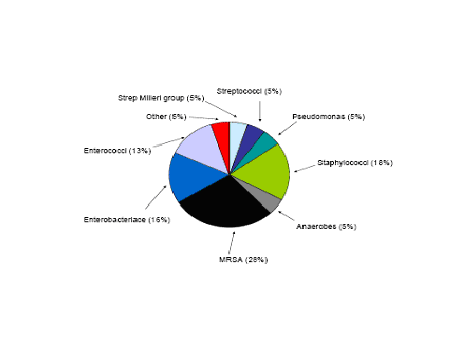
The most common bacterial causes of pleural empyema in immunocompetent adults in the community acquired setting include Streptococcus milleri group, Streptococcus pneumoniae, and Staphylococcus aureus. However, gram- negative organisms such as Escherichia coli, Pseudomonas, and Enterobacteriaceae are becoming more common but are still more rare. Anaerobic bacteria are another group that can cause empyema and these are found often in patients prone to aspiration and poor dentition. In the hospital acquired empyema the pathogens are somewhat different. The most common pathogens are S. aureus (the majority of which are MRSA), enteric gram negative bacilli and Streptococcal species. Often ventilated patients in the ICU will have a polymicrobial infection (8) (Figure 1 and 2 ).
Antibiotic choice for parapneumonic effusions and empyema is aimed at the specific pathogens’ susceptibility if this data is available. Gram staining will also help narrow the spectrum. Broad spectrum antibiotics are first employed until gram stain and culture data is available. Appropriate choices should cover gram negatives, gram positives, and anaerobic bacteria. The third generation cephalosporins, extended spectrum beta lactams, aminoglycosides, imipenem, and aztreonam will all cover gram negative bacteria well. As methicillin resistant S. aureus becomes increasingly prevalent vancomycin should be used to treat gram positive cocci. Clindamycin is the best choice for anaerobic infections.
Most all antibiotics penetrate the pleural cavity with a high enough concentration to be effective. For this reason intrapleural injection of antibiotics is not necessary. A noteworthy exception is the aminoglycoside class. This class is not only less able to penetrate the desired area but the drugs are also inactivated in acidic environments.
Treatment times for uncomplicated parapneumonic effusions should be no different than the time course to treat a pneumonia without an effusion. Generally a 7-14 day course is sufficient. For complicated parapneumonic effusions and empyemas a longer course of therapy for many weeks is indicated (8) (Table 3).

Atypical Bacteria: Mycoplasma Pneumoniae, Actinomycosis, and Nocardosis
Mycoplasma pneumoniae is an obligate intracellular bacteria that produces an illness more resembling a viral pneumonia than a bacterial infection. The pneumonia is generally a diffuse interstitial process and a pleural effusion is observed in 5-20% of infections. The effusion size is variable and the pleural fluid characteristics have not been well described. The diagnosis of M. pneumonia is established by increasing antibody titers. The presence of cold agglutinins is suggestive of the diagnosis. The effusion will resolve on its own with treatment of the infection. The treatment of choice is tetracycline or erythromycin (2, 43).
Actinomyces israelii is a thin branching filamentous gram positive anaerobic bacteria that is a common mouth flora. It produces a disease more typical of a fungal infection than a bacterial infection. This bacteria causes a chronic infiltrative pulmonary disease which classically crosses fissures. The parenchymal disease can extend through the chest wall and create chest wall abscesses and draining sinus tracts (58).
The pleural fluid can vary widely from frank pus full of neutrophils to serous fluid with lymphocytes predominating. The only finding that is characteristic of this infection is the identification of sulfur granules in the pleural fluid but this is not always present. In other words it is a specific but not a sensitive finding. The diagnosis is made with growth of the bacteria from anaerobic culture of the pleural fluid (34).
Treatment of the parenchymal disease is a long term process. Six to eight weeks of 10 million units of IV penicillin daily followed by oral penicillin for 12 to 18 months is recommended. Alternatives to penicillin are tetracycline, erythromycin, and clindamycin. Treatment of the pleural effusion depends on its nature. If the pleural effusion is pus, then a chest tube and possibly surgical decortication is warranted. If the pleural fluid is serous in nature tube thoracostomy is usually not indicated. The pleural effusion usually clears with treatment of the infection (47, 76).
Nocardia is another bacteria that is more like a fungus than a bacteria. Nocardia asteroides is an aerobic, gram positive, weakly acid fast filamentous bacteria found commonly in the soil. This infection can cause radiologic findings that vary from vague pulmonary infiltrates to cavitary lesions. It is found more commonly in patients with underlying lung disease and in immunocompromised patients. With the emergence of HIV/AIDS it is becoming a more prevalent pathogen. The pleura is involved in approximately 50% of cases with pulmonary disease and like actinomycosis the characteristics of the fluid can vary widely from pus to serous fluid (58).
The diagnosis of nocardiosis is made from identification of the bacteria on acid fast stain and culturing the organism in aerobic culture. As nocardia is a very slow grower the laboratory must be aware that this diagnosis is being entertained so that the cultures can be kept for at least two weeks (22,55).
First line treatment of nocardia is bactrim at 15mg/kg/day in divided doses. Alternatives for treatment in sulfa- allergic patients include imipenem, amikacin, minocycline, and ceftriaxone (22). The treatment of the pleural effusion is the same that discussed above for actinomyces.
MYCOBACTERIA: Tuberculosis
Tuberculus pleuritis is relatively rare in the United States with an estimated incidence of 1000 cases a year (3, 48, 62). It is more common in immunocompromised patients as is pulmonary tuberculosis. In third world countries the incidence is dramatically higher and it is the most common cause of pleural effusions in some of these countries.
The pleural involvement is thought to occur from two different processes: as sequelae to a primary infection or to a reactivation of old pulmonary disease. Regardless the pleura becomes involved when a subpleural caseous focus ruptures exposing the pleural space to tuberculous protein. There is animal and human experimental evidence to suggest strongly that this is a hypersensitivity reaction.
Patients with tuberculous pleuritis are generally acutely ill with symptoms of pleuritic chest pain, non productive cough, and fever. The effusion can range from minimal to massive but is usually unilateral (79, 52, 66). Without treatment the effusion will usually resolve spontaneously but most patients will go on to develop active tuberculosis if they have not already done so. Therefore, even if there is no evidence of pulmonary tuberculosis, patients with tuberculous pleuritis should be treated. Moreover, tuberculous pleuritis should be ruled out in all patients with an undiagnosed pleural effusion with predominantly lymphocytes.
The diagnosis is confirmed by identification of the acid fast bacteria in sputum, pleural fluid, or pleural fluid biopsy. Often times; however, the pleural fluid will not have the bacteria present. The alternative method of diagnosis is to measure the adenosine deaminase (ADA) level present in the pleural. This test is quite sensitive and specific. When the cut off of 40 IU/L is used the sensitivity and specificity are 96%. The pleural fluid analysis usually reveals an exudate with a predominance of small lymphocyte. If the effusion is very early, polymorphonuclear cells may dominate but over the next few days the differential of the fluid will invariably turn to lymphocytes (26).
VIRUSES
Viruses are less common causes of pleural effusions but may very well account for more effusions than is acknowledged. The effusions are generally smaller than those encountered with bacterial pneumonia. The fluid is an exudate and lymphocytes dominate in the cell differential. The diagnosis of a viral infection is made by observing increasing titers with specific serologic tests or by a positive viral culture. As most of these pleural effusions resolve without intervention and the serologic tests can be costly the search for a viral infection is not often undertaken (35,51).
Adenovirus is the most common viral cause of atypical pneumonia. Pleural effusions occur in anywhere from 15% to 62% of patients (24, 71). The effusions are often bilateral and are accompanied by a diffuse parenchymal infiltrate typical of a viral pneumonia. There is no specific treatment.
Epstein-Barr Virus (EBV) is a common infection and there is evidence of infection in 90% of the world’s population. The primary infection is generally acquired in infancy or childhood. As with other herpes viruses this virus becomes dormant and can reactivate later in life. Infectious mononculosis is an infection caused by EBV and the symptoms include very painful sore throat with enlarged tonsils, enlarged and tender cervical lymph nodes, hepatitis, and splenomegaly. Pleural effusions were observed in 5% of 59 patients in a case series by Lander and Palayew (75). The effusions were exudative and took many months to resolve. Again there is no specific treatment.
Hantavirus pulmonary syndrome results from a relatively new member of the hantavirus family now known as the Sin Nombre virus. It was first described in the 1990s following an isolated outbreak in the southwest but now the virus has produced disease in all regions of the United States. The syndrome that results is fatal in nearly 50% of the infected. It is characterized by fever, chills, GI complaints, myalgias, and cough. The patients who experience respiratory failure generally have global bilateral infiltrates. The disease produces noncardiogenic pulmonary edema and pleural effusions were observed in 21 of 23 patients from an outbreak in New Mexico (9). The pleural effusions varied widely in size. The effusions are most likely due to interstitial fluid traversing the visceral pleura to the pleural space. The disease can be confirmed by serologic tests and prompt treatment with ribavirin may be beneficial (17).
RICKETTSIAL ILLNESSES AND EHRLICHIOSIS
Rickettsial illnesses are tick borne illnesses that can produce a wide range of disease states. Q fever and Rocky Mountain Spotted Fever are two such diseases. Both of these illnesses can have pleural effusions as disease manifestations.Coxiella burnetti is the agent responsible for Q fever and is associated with farms and stockyards. Inhalation of contaminated dust or drinking infected, unpasteurized milk is thought to be the way the disease in contracted. It generally produces a primary atypical pneumonia with high fever, cough, headache, and myalgias. In a Spanish series of 164 patients 12% had exudative pleural effusions generally lymphocyte or eosinophil dominant (19, 21, 54). Four fold increases in antibody titers are used to establish a diagnosis and the treatment of choice is doxycycline or tetracycline (54).
Rocky mountain spotted fever is caused by Rickettsia rickettsii and is characterized by fever, headache, myalgias, and a characteristic rash that appears on the soles of the hands and feet. It is transmitted to humans by the tick Ioxides scapularis. A pleural effusion is observed in 10% to 36% of the cases and can be an exudate or a transudate (53). The most likely etiology of the effusion is vasculitis of the pulmonary capillaries producing a capillary leak. Treatment again is doxycycline.
Ehrlichiosis is an illness that comes in two forms, human monocytic ehrlichiosis and human granulocytic ehrlichiosis. Humans acquire the obligate intracellular bacterial pathogen from a tick bite. The symptoms generally include similar symptoms to rocky mountain spotted fever but without the rash. That is why this illness is sometimes referred to as “rocky mountain spotless fever.” Pleural effusion is a rare complication and is generally similar to that seen in the rickettsial illnesses. Doxycycline is the treatment of choice (15, 53).
FUNGAL INFECTIONS
Aspergillus
While fungal infections are undoubtedly rare causes of pleural effusions, accounting for less than 1% of all pleural effusions, it is important to recognize them. The fungus that will cause the greatest amount of damage and will require the most aggressive therapy is the Aspergillus species. Aspergillus fumigatus and Aspergillus niger can cause pleural disease and have devastating consequences. Aspergillus is important to have as a differential in a patient who has had past treatment with artifical pneumothorax therapy for tubercuslosis (24, 33, 65). These patients will be chronically ill with weight loss, low grade fever, and a productive cough. The post operative period after pneumonectomies and lobectomies is another situation in which aspergillus is frequently encountered. The pleura is generally thickened and a bronchopleural fistula is often present as indicated by air-fluid levels in the pleural space (23).
Aspergillus can be diagnosed with fungal culture of the pleural fluid but fungal cultures can take weeks and time is of the essence. If this diagnosis is seriously entertained then radioimmunoassay of the pleural fluid for aspergillus antigens is performed. At times the diagnosis will be obvious as brown clumps of fungal hyphae can be seen in the pleural fluid. In addition the niger variety can impart a black pigmentation to the pleural fluid (33, 49).
The treatment will require surgical intervention. The involved pleura must be excised along with any involved lung tissue. Voriconazole or Itraconazole must also be administered as soon as the diagnosis is made and continued through the post operative period. If a patient is too ill to undergo surgery then intrapleural amphotericin B or nystatin are instilled daily through a chest tube (11, 33, 70). Treatment will usually go on for many months but is generally successful.
Blastomycosis
Blastomyces dermatitidis is a fungus that has been associated with pleural effusions in 3% to 21% of patients with pulmonary blastomycosis in different case series (11, 69). The pleural effusions are most commonly small and cause only blunting of the costphrenic angle on x-ray. The pleural fluid is an exudate with polymorphonuclear cells or lymphocytes predominating. The diagnosis can be made with pleural fluid cytology if the characteristic broad based budding yeasts are seen. A complement-fixation test is also possible but is not very reliable (68). Fungal cultures of the pleural fluid are more reliable but take time. Treatment consists of itraconazole, ketoconazole, or amphotericin B. Treatment duration is generally 6 months (32).
Crypotococcosis
Cryptococcus neoformans is a common fungus found in the environment, most often in bird or bat droppings. Before the emergence of the AIDS virus this fungus was a very rare cause of serious disease. However, now it is seen much more commonly. Pulmonary cryptococcocis presents often as a lung nodule. When the subpleural nodule extends into the pleural space pleural effusions are seen. There is again a widely varying percentage of pleural involvement in case series: 5% to 25% have been reported (6, 10). The pleural effusion itself can vary from minimal to massive. Diagnosis is made with fungal culture, histologic/cytologic examination, and increased cryptococcal antigen titers in the pleural fluid or serum. There is debate in the literature in regards to treatment. If the disease is isolated to the pleural space, the cryptococcal antigen tests in the serum and cerebrospinal fluid are negative, and the patient is immunocompetent and asymptomatic then no systemic therapy needs to be employed as spontaneous resolution is likely. If crytpococcus is isolated in the pleura or lungs, immunocompetent patient then a search for systemic infection is warranted with collection of blood and cerebrospinal fluid for analysis. If the serum cryptococcal antigen is positive then fluconazole is recommended for six months. If there is evidence of CSF involvement then amphotericin B and 5-fluorocytosine needs to be used for optimal treatment.Immunocompromised patients should always be treated (20, 80).
Histoplasmosis
Histoplasmosis capsulatum is endemic to the central United States and most residents in this area have evidence of histoplasmosis exposure. Pleural effusions secondary to histoplasmosis are exceedingly rare and will only briefly be discussed. Only twenty pleural effusions due to histoplasmosis have been reported. The fungus can be identified on cytologic examination or cultured. There are titers for histoplasmosis in the serum but this would only establish circumstantial evidence for pleural involvement. No treatment is generally employed as the effusions are generally small and resolve spontaneously (12, 20).
Coccidioidomycosis
Coccidioides immitis is a fungus found in the southwest region of the United States. Pleural involvement with this fungus is much more common than with the other fungi discussed in this chapter. There are two types of pleural disease caused by this organism. One is associated with primary benign infection and the lung parenchyma is not always involved. The second type of pleural involvement occurs when a cavity secondary to coccidiiodmycose ruptures into the pleural space creating a hydropneumothorax and a bronchopleural fistula.
In primary benign infection nearly 20% of patients have only blunting of the costophrenic angles on chest x-ray but some of the pleural effusions can be quite large occupying 50% of the hemithorax (41). The pleural fluid is an exudate and lymphocytes dominate. Eosinophilia is also possible. Most patients will not require treatment but if any sign of systemic dissemination exists, treatment with amphotericin B or itraconazole is advised. Systemic dissemination is often indicated by high complement fixation titers (>1:16) (32, 41).
With pleural involvement from cavity rupture the patient will generally appear acutely ill and toxic. The pleural fluid analysis can show very low glucose levels and very high lactic acid dehydrogenase (LDH) levels (up to10 times the upper limit of normal) (13). A chest tube should be inserted to remove the air and fluid from the pleural space. Itraconazole or amphotericin B are the antifungals of choice and surgery is usually required to remove the cavitated area of lung (81).
PARASITIC INFECTIONS
Clearly parasitic infections in the United States are rare occurrences. However as the world becomes a more global community and travel and immigration increase, U.S. physicians are apt to see more pleural effusions due to parasites that are common in third world countries. Amebiasis, Echinococcosis, and Paragonimiasis are able to cause pleural effusion.
Amebiasis
Entamoeba histolytica is a parasite seen in areas of poor sanitation. The life cycle is complicated and beyond the scope of this chapter. It suffices to say that during the life cycle the trophozoite form of the parasite migrates to the liver and causes liver abscesses. The liver abscess can cause diaphragmatic irritation and a sympathetic pleural effusion can result. Antibody tests and PCR techniques are available for diagnosis. It is important to obtain CT scans to evaluate for liver abscesses when this disease is suspected. Treatment consists of metronidazole 500mg to 750 mg for ten days. These pleural effusions generally do not require chests tubes as a single therapeutic thoracentsis is generally sufficient (42, 72).
Secondarily, the liver abscess can rupture through the diaphragm into the pleural space. The fluid that is removed is described as “chocolate sauce or anchovy paste” that is composed of blood and liver tissue. When this occurs the patient is septic and may be in shock. The pleural effusion is often massive. A chest tube is needed in this circumstance as well as percutaneous drainage of the liver. Bacterial infections of the pleural space concomitantly exist in 30% of these patients and broad spectrum antibiotics are recommended in addition to the metronidazole. Decortication is frequently required if the lung does not reexpand properly (7, 25, 64).
Echinococcosis
The tape worm Echinococcus granulosus is responsible for this disease. The definitive host for this parasite is the dog or sheep and it is therefore found in areas of the world where sheep herding is commonplace. It is most prevalent in Turkey, Lebanon, and Greece. The disease is often called hydatid disease in humans. When humans are infected the parasitic cysts grow very slowly over years in the lung, liver, and sometimes spleen. These cysts can rupture once they reach a large enough size and cause devastating disease in the host. The liver cyst can rupture into the pleural space or the lung cyst itself can rupture. Regardless of the etiology sepsis and shock generally develop. Simultaneous rupture of the cyst into the tracheobronchial tree can occur and and the patient will cough up large amount of purulent appearing material that contains membranes of the cyst (29, 31, 56, 63, 79).
The diagnosis can be established with visualization of the echinococcal scolices with hooklets in the pleural fluid. The fluid will be eosinophil rich and is often superinfected with bacteria. A CT scan is also useful in these patients as it was with amoebiasis.
Surgery is needed in these patients to carefully remove the cysts. Immediate thoracotomy is called for. Antiprotozoal therapy with albendazole 400mg BID for a month is recommended as adjuvant therapy (16).
Paragonimiasis
Paragonimus westermani and Paragonimus miyazakii are lung flukes with a very complicated lifecycle most often found in Asian countries. The human host acquires the fluke in the larvae form when eating infested seafood. The larvae bore through the intestinal wall and into the peritoneal cavity. They can then bore through the diaphragm, the pleura, and into the lung. These larvae mature into adult lung flukes that live in the airways of the lung producing eggs. When the larvae enter the pleural space pleural disease can develop.
The diagnosis is made by detecting the eggs of the lung fluke in sputum or stool. An anti-Paragonimus antibody test is also available (28). The pleural fluid is very specific. It is an exudate with a very low glucose (<10 mg/dl), a low pH (<7.10), and a high lactate dehydrogenase (>3 times the upper normal limit for serum) Cholesterol crystal are frequently present and the cell count will have predominantly eosinophils. Interleukin 5 levels are also markedly elevated (73). Importantly the only other disease that will exhibit a pleural fluid that is an exudate with a low glucose and pH with predominantly eosinophils is the Churg Strauss syndrome (18).
The treatment is praziquantel, 25 mg/kg TID for three days. An alternative second line therapy is Bithionol 35 to 50 mg/kg on alternate days for 10 to 15 doses (27). Occassionally in long term pleural infestation the pleura will be sufficiently thickened as to necessitate decortication (50,65).
TREATMENT OPTIONS FOR PLEURAL EFFUSIONS
All unexplained pleural effusions deserve a thoracentesis. This can easily and safely be done at the bedside by a physician. The risks of the procedure include bleeding, infection and pneumothorax ![]() . The thoracentesis needle should be inserted at the upper border of the rib to avoid the neurovascular bundle that runs along the bottom of the ribs. To reduce complications a needle-less catheter system should be used since, as the lung expands, the needle can lacerate the lung leading to a pneumothorax.
. The thoracentesis needle should be inserted at the upper border of the rib to avoid the neurovascular bundle that runs along the bottom of the ribs. To reduce complications a needle-less catheter system should be used since, as the lung expands, the needle can lacerate the lung leading to a pneumothorax.
Once the nature of the effusion is determined additional management decisions need to be made. Often drainage of the pleural effusion with thoracentesis will be adequate. However, there are occasions when more invasive and definitive therapy is required.
In patients with parapneumonic effusions, the recommended initial invasive procedure should be a therapeutic thoracentesis rather than a diagnostic thoracentesis. The reason for this is that if all the fluid is removed and it never returns then the definitive therapy has been performed. If the fluid returns, a repeat therapeutic thoracentesis should be performed if the initial pleural fluid had a low glucose, low pH, high lactate dehydrogenase (>3 times the upper normal limit for serum) or positive gram stain or culture. If the fluid recurs a second time and the biochemical parameters are still indicative of a complicated parapneumonic effusion or empyema, a chest tube should be inserted. An alternate option is to place a pigtail catheter (small bore chest tube) for a few days.
Fibrous loculations represent a complication in pleural effusions with significant inflammation such as bacterial parapneumonic effusions. When loculations are present a single chest tube is inadequate for drainage. In these circumstances multiple chest tubes are often used. If interventional radiology is available it is often possible to place smaller bore tubes into the specific areas of loculation and avoid multiple large bore tubes (40).
Instillation of fibrinolytics is one possible alternative to drain the effusion when fibrous loculations are present. Fibrinolytics such as urokinase, streptokinase, and DNase are used to dissolve the bands of fibrous tissue and improve drainage. The benefits are controversial. While this treatment option has been available since 1949, no well-performed randomized clinical trial has shown any benefit. The most recent and best-done trial (MIST1) failed to show any difference in mortality and the need for surgical intervention at 3 months (44).
A video assisted thoracoscopic surgery (VATS) with lysis of adhesions is also a viable option for loculated effusions. If it is clear that there are multiple loculations then it is wise to avoid delay and proceed directly to this procedure.
REFERENCES
1. Arenas-Jimenez J, Alonso-Charterina S, Sanchez-Parva J, et al. Evaluation of the CT finding for diagnosis of pleural effusions. Eur Radiology 2000;10:681-690. [PubMed]
2. Alptekin F. An epidemic of pleurisy with effusion in Bitlis, Turkey: study of 559 cases. US Armed Forces Med J 1958;9:1–11. [PubMed]
3. Baumann MH, Nolan R, Petrini M, et al. Pleural Tuberculosis in the United States: Incidence and Drug Resistance. Am Rev Resp Dis Crit Care Med (in press)
4. Berger HW, Mejia E. Tuberculous pleurisy. Chest 1973;63:88–92. [PubMed]
5. Berger HW, Seckler SG. Pleural and pericardial effusions in rheumatoid disease. Ann Intern Med 1966;64(6):1291-7.[PubMed]
6. Cadranel JL, Chouaid C, Denis M, et al. Causes of pleural effusion in 75 HIV-infected patients. Chest 1993;104:655. [PubMed]
7. Cameron EWJ. The treatment of pleuropulmonary amebiasis with metronidazole. Chest 1978;73:647-650. [PubMed]
8. Chapman SJ, Davies RJ. Recent advances in parapneumonic effusion and empyema. Curr Opin Resp Med 2004; 10: 299-304 [PubMed]
9. Charles RE, Katz RL, Ordonez NG, et al. Varicella-zoster infection with pleural involvement. Am J Clin Pathol 1986;85:522–6. [PubMed]
10. Chechani V, Kamholz SL. Pulmonary manifestations of disseminated cryptococcosis in patients with AIDS. Chest 1990;98:1060–5.[PubMed]
11. Colp CR, Cook WA. Successful treatment of pleural aspergillosis and bronchopleural fistula. Chest 1975; 68:96–8.[PubMed]
12. Connell JV, Muhm JR. Radiographic manifestations of pulmonary histoplasmosis: a 10-year review. Radiology 1976;121:281–5. [PubMed]
13. Cunningham RT, Einstein H. Coccidioidal pulmonary cavities with rupture. J Thorac Cardiovasc Surg 1982;84:172–7. [PubMed]
14. Dietrick RB, Sade RM, Pak JS. Results of decortication in chronic empyema with special reference to paragonimiasis. J Thorac Cardiovasc Surg 1981;82:58-62. [PubMed]
15. Donohue JF. Lower respiratory tract involvement in Rocky Mountain spotted fever. Arch Intern Med 1980;140:223-7. [PubMed]
16. Drugs for parasitic infections. The Medical Letter 1998;40:1-7.
17. Duchin JS, Koster FT, Peters CJ, et al. Hantavirus pulmonary syndrome: a clinical description of 17 patients with a newly recognized disease. The Hantavirus Study Group. N Engl J Med 1994;330:949–55. [PubMed]
18. Erzurum SE, Underwood GA, Hamilos DL, et al. Pleural effusion in Churg-Strauss syndrome. Chest 1989;95:1357-1359. [PubMed]
19. Esteban C, Oribe M, Fernandez A, et al. Increased adenosine deaminase activity in Q fever pneumonia with pleural effusion. Chest 1994;105:648. [PubMed]
20. Goodwin RA, DesPrez RM. Histoplasmosis. Am Rev Respir Dis 1978;117:929–56. [PubMed]
21 Gordon JK, MacKeen AD, Marrie TJ, et al. The radiographic features of epidemic and sporadic Q fever pneumonia. J Can Assoc Radiol 1984;35:293–6. [PubMed]
22. Green M, Avery RK. Nocardiosis. Am J Transplant 2004; 4(suppl. 10)47-50.
23. Hillerdal G. Pulmonary aspergillus infection invading the pleura. Thorax 1981;36:745–51. [PubMed]
24. Hooper JW, Larsen T, Custer DM, et al. A lethal disease model for hantavirus pulmonary syndrome. Virology 2001;289:6-14. [PubMed]
25. Ibarra-Perez C. Thoracic complications of amebic abscess of the liver. Chest 1981;79:672-677. [PubMed]
26. Ibrahim WH, Ghadban W, Khinji A, et al. Does pleural tuberculosis disease pattern differ among developed and developing countries. Respir Med 2005;99:1038-45. [PubMed]
27. Ikeda T, Oikawa Y, Owhashi M, et al. Parasite-specific IgE and IgG levels in the serum and pleural effusion of paragonimiasis westermani patients. Am J Trop Med Hyg 1992;47:104-107. [PubMed]
28. Im JG, Whang HY, Kim WS, et al. Pleuropulmonary paragonimiasis: radiologic findings in 71 patients. AJR Am J Roentgenol 1992;159:39-43 [PubMed]
29. Jacobson ES. A case of secondary echinococcosis diagnosed by cytologic examination of pleural fluid and needle biopsy of pleura. Acta Cytol 1973;17:76-79. [PubMed]
30. Jimenez D, Diaz G, Garcia-Rull S, et al. Routine use of pleural fluid cultures. Are they indicated? Limited yield, minimal impact on treatment decisions. Respir Med. 2006;100:2048-2052. [PubMed]
31. Keramidas D, Mavridis G, Soutis M, et al. Medical treatment of pulmonary hydatidosis: complications and surgical management. Pediatr Surg Int 2004; 19:774-6. [PubMed]
32. Klein NC, Cunha BA. New antifungal drugs for pulmonary mycoses. Chest 1996;110:525–32. [PubMed]
33. Krakowka P, Rowinska E, Halweg H. Infection of the pleura by Aspergillus fumigatus. Thorax 1970;25: 245–53. [PubMed]
34. Kwong JS, Muller NL, Godwin JD, et al. Thoracic actinomycosis: CT findings in eight patients. Radiology 1992;183:189–92. [PubMed]
35. Lababidi HMS, Gupta K, Newman T, et al. A retrospective analysis of pleural effusion in human immunodeficiency virus infected patients. Chest 1994;106:86S.
36. Light RW. Pleural Diseases Third Edition. Baltimore, MD: Williams and Wilkins; 1995.
37. Light RW. Clinical practice. Pleural effusion. N Engl J Med. 2002;346:1971-1977. [PubMed]
38. Light RW. Parapneumonic effusions and empyema. Proc Am Thorac Soc 2006;3:75-80. [PubMed]
39. Light RW. The undiagnosed pleural effusion. Clin Chest Med. 2006;27(2):309-19. [PubMed]
40. Light RW. Pleural Diseases Fifth Edition. Philadelphia, PA: Lippincott Williams & Wilkins; 2007.
41. Lonky SA, Catanzaro A, Moser KM, et al. Acute coccidioidal pleural effusion. Am Rev Respir Dis 1976;114:681–8. [PubMed]
42. Lyche KD, Jensen WA. Pleuropulmonary amebiasis. Semin Respir Infect 1997;12:106-112. [PubMed]
43. Mansel JK, Rosenow EC, Smith TF, et al. Mycoplasma pneumoniae pneumonia. Chest 1989;95:639–46. [PubMed]
44. Maskell NA, Davies CW, Nunn AJ, et al. U.K. Controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med 2005;352:865-874 [PubMed]
45. Maskell NA, et al. The bacteriology of pleural infection by genetic and standard methods and its mortality significance. Am J Resp Crit Care Med 2006;174:817-823 [PubMed]
46. Maskell NA, Butland RJ. BTS guidelines for the investigation of a unilateral pleural effusion in adults." Thorax 2003; 58 Suppl 2: ii8-17. [PubMed]
47. McQuarrie DG, Hall WH. Actinomycosis of the lung and chest wall. Surgery 1968;64:905–911. [PubMed]
48. Mehta JB, Dutt A, Harvill L, et al. Epidemiology of extrapulmonary tuberculosis. Chest 1991;99:1134–8. [PubMed]
49. Metzger JB, Garagusi VF, Kerwin DM. Pulmonary oxalosis caused by Aspergillus niger. Am Rev Respir Dis 1984;129:501–2. [PubMed]
50. Minh VD, Engle P, Greenwood JR, et al. Pleural paragonimiasis in a southeast Asian refugee. Am Rev Respir Dis 1981;124:186-188. [PubMed]
51. Monath TP, Maher M, Casals J, et al. Lassa fever in the Eastern Province of Sierra Leone, 1970-1972. II. Clinical observations and virological studies on selected hospital cases. Am J Trop Med Hyg 1974; 23: 1140-9. [PubMed]
52. Moudgil H, Sridhar G, Leitch AG. Reactivation disease: the commonest form of tuberculous pleural effusion in Edinburgh, 1980–1991. Respir Med 1994;88:301–4. [PubMed]
53. Murphy PP, Richardson SG. Q fever pneumonia presenting as an eosinophilic pleural effusion. Thorax 1989;44:228–9. [PubMed]
54. Nagayama Y, Sakurai N, Tamai K, et al. Isolation of Mycoplasma pneumoniae from pleural fluid and/or cerebrospinal fluid: report of four cases. Scand J Infect Dis 1987;19:521–4. [PubMed]
55. Neu HC, Silva M, Hazen E, et al. Necrotizing nocardial pneumonitis. Ann Intern Med 1967;66:274–84. [PubMed]
56. Ozvaran MK, Ersoy Y, Uskul B, et al. Pleural complications of pulmonary hydatid disease. Respirology 2004;9:115 9. [PubMed]
57. Palmer DL, Harvey RL, Wheeler JK. Diagnostic and therapeutic considerations in Nocardia asteroides infection. Medicine 1974;53:391–401. [PubMed]
58. Peabody JW, Seabury JH. Actinomycosis and nocardiosis: a review of basic differences in therapy. Am J Med 1960;28:99–115. 12728146
59. Pendharkar S. Guideline on how to identify the cause: A diagnostic approach to pleural effusion. J Resp Dis 2007;28(12): 565-581.
60. Porcel JM, Light RW. Diagnostic approach to pleural effusion in adults. Am Fam Physician 2006;73:1211-1220. [PubMed]
61. Porcel JM, Madronero AB, Pardina M, Vives M, Esquerda A, Light RW., Analysis of pleural effusions in acute pulmonary embolism: radiological and pleural fluid data from 230 patients. Respirology. 2007;12:234-9. [PubMed]
62. Qiu L, Teeter LD, Liu Z, et al. Diagnostic associations between pleural and pulmonary tuberculosis. J Infect 53:377-86. [PubMed]
63. Rakower J, Milwidsky H. Hydatid pleural disease. Am Rev Respir Dis 1964;90:623-631. [PubMed]
64. Rasaretnam R, Paul ATS, Yoganathan M. Pleural empyema due to ruptured amoebic liver abscess. Br J Surg 1974;61:713-715. [PubMed]
65. Reyes CV, Kathuria S, MacGlashan A. Diagnostic value of calcium oxalate crystals in respiratory and pleural fluid cytology: a case report. Acta Cytol 1979;23:65–8. [PubMed]
66. Richter C, Perenboom R, Mtoni I, et al. Clinical features of HIV-seropositive and HIV-seronegative patients with tuberculous pleural effusion in Dar es Salaam, Tanzania. Chest 1994;106:1471–5. [PubMed]
67. Romero-Candeira S, Hernandez L, et al. Is it meaningful to use biochemical parameters to discriminate between transudative and exudative pleural effusions? Chest 2002;122(5): 1524-9. [PubMed]
68. Sarosi GA, Armstrong D, Davies SF, et al. Laboratory diagnosis of mycotic and specific fungal infections. Am Rev Respir Dis 1985;132:1373–1380.
69 Sheflin JR, Campbell JA, Thompson GP. Pulmonary blastomycosis: findings on chest radiographs in 63 patients. AJR Am J Roentgenol 1990;154:1177–80. [PubMed]
70. Shirakusa T, Ueda H, Saito T, et al. Surgical treatment of pulmonary aspergilloma and Aspergillus empyema. Ann Thorac Surg 1989;48:779–82. [PubMed]
71. Simila S, Ylikorkala O, Wasz-Hockert O. Type 7 adenovirus pneumonia. J Pediatr 1971;79:605–11. [PubMed]
72. Talaat KR, Nutman TB. Parasitic diseases. In: Mason RJ, Broaddus VC, Murray JF, et al, eds. Textbook of respiratory medicine, 4th ed. Philadelphia: Elsevier Saunders, 2005:1083-113.
73. Taniguchi H, Mukae H, Matsumoto N, et al. Elevated IL-5 levels in pleural fluid of patients with paragonimiasis westermani. Clin Exp Immunol 2000;123:94-8. [PubMed]
74. Teixeira, LR, Maria Villarino. Antibiotic treatment of patients with pneumonia and pleural effusion. Curr Opin Pulm Med 1998;4: 230-234 [PubMed]
75. Thijsen SF, Luderer R, van Gorp JM, et al. A possible role for Epstein-Barr virus in the pathogenesis of pleural effusion. Eur Respir J 2005;26:662-6. [PubMed]
76. Varkey B, Landis FB, Tang TT, et al. Thoracic actinomycosis: dissemination to skin, subcutaneous tissue and muscle. Arch Intern Med 1974;134:689–93. [PubMed]
77. Warr W, Bates JH, Stone A. The spectrum of pulmonary cryptococcosis. Ann Intern Med 1968;69:1109–16. [PubMed]
78. Wex P, Utta E, Drozdz W. Surgical treatment of pulmonary and pleuro-pulmonary Aspergillus disease. Thorac Cardiovasc Surg 1993;41:64–70. [PubMed]
79. Xanthakis DS, Katsaras E, Efthimiadis M, et al. Hydatid cyst of the liver with intrathoracic rupture. Thorax 1981;36:497B501. [PubMed]
80. Young EJ, Hirsh DD, Fainstein V, et al. Pleural effusions due to Cryptococcus neoformans: a review of the literature and report of two cases with cryptococcal antigen determinations. Am Rev Respir Dis 1980;121: 743–7. [PubMed]
81. Youssef SS, Ramu V, Sarubbi FA. Unusual case of pyopneumothorax in Tennessee. South Med J. 2005;98:1139-41.[PubMed]
Tables
Table 1: Common Laboratory Tests for Diagnosis or Pleural Effusion
Test Name |
Test Value |
Suggested Diagnosis |
When To Use |
|---|---|---|---|
Lactate dehydrogenase (LDH) |
> Two thirds of upper limit of normal for serum LDH (Light criteria) |
Any condition causing an exudate |
Routine |
|
LDH fluid to serum ratio > 0.6 (Light criteria) |
Any condition causing an exudate |
Routine |
Protein |
Protein fluid to serum ratio > 0.5 (Light criteria) |
Any condition causing an exudate |
Routine |
Protein gradient |
> 3.,1 g/dL |
Transudate |
Calculate when Light’s criteria of LDH and protein are borderline |
Adenosine deaminase (ADA) |
> 40 U/L (667 nkat/L) |
Tuberculosis (>90%), empyema (60%), complicated parapneumonic effusion (30%), malignancy (5%) rheumatoid arthritis |
Request if pleural fluid appears bloody |
Red Blood Cell |
>10,000/mm3 (100 x106/L) |
Malignancy, trauma, parapneumonic effusion, pulmonary embolism |
Request if tuberculosis effusion is suspected |
White blood cell (WBC) count and differential |
>100,000/mm3 (100 x109/L) |
Empyema, other exudates (uncommon) |
Routine |
Lymphocytes |
> 50% |
Malignancy, tuberculosis or pulmonary embolism, coronary artery bypass surgery (60) |
Send as a routine test in all unexplained effusions |
Neutrophils |
> 50% |
Paraneumonic effusion, pulmonary embolism, abdominal abscess (60) |
Routine |
Cytology |
Present |
Malignancy |
Routine |
Culture |
Positive |
Infection |
Request if parapneumonic effusion is suspectged or if fluid is purulent (30) |
pH |
< 7.20 |
Complicated parapneumonic effusion or empyema, malignancy (< 10%), tuberculosis (<10%), esophageal rupture |
Routine |
Glucose |
< 60mg/dL (3.3 mmol/L) |
Complicated parapneumonic effusion of empyema, tuberculosis (20%), malignancy (<10%), rheumatoid arthritis |
Routine |
Triglyceride |
>110 mg/dL with milky appearance (1.24 mmol/L) <110 mg/dL with milky appearance |
Chylothorax
Chyliform effusion (pseudochylothorax), malnourished |
Request when pleural fluid is cloudy or milky (61). |
Cholesterol |
> 200 mg/dL with milky appearance > 45 to 60 mg/dL (1.16 to 1.55 mmol/L) |
Chyliform effusion (pseudochylothorax) |
Request if chylothorax or pseudochylothorax is suspected (61) |
Table 2: Causes of Pleural Effusions
| Common Causes of Effusion | Transdate/Exudate | Laboratory Test Features (pleural effusion and others) | Clinical Pearls |
|---|---|---|---|
| Pneumonia | Exudate | Low glucose level (< 60 mg/DL).
pH < 7.20, pleural neutrophilia (>50%) |
This effusion is called parapneumonic. It is the second most common cause of pleural effusion. Occurs in up to 40% of patients hospitalized with pneumonia (37) |
| Empyema | Exudate | LDH > 1000 U/ml
Very low glucose level (< 40 mg/dL)pH < 7.20 (exception: Proteus mirabilis, pH 8.0), pleural neutrophilia positive bacterial culture, ADA (if tuberculosis) (59) |
The yield of culture is increased if blood-culture bottles are inoculated with the pleural fluid
Request fungal and mycobacterial culture |
| Malignancy | Almost always exudate | Low glucose level (< 60 mg/dL), pH variable (may be < 7.30), pleural lymphocytosis, positive cytology (low sensitivity: 54-73%; high specificity: 97%). | Third most common cause of pleural effusion and the most common cause of an exudate effusion in patients older than 50 years of age (37). Sensitivity of cytology 50% if one sample obtained, 66.7% if two samples and 73.3% is three samples. Malignancy should not be excluded on the basis of negative pleural cytology results. |
| Congestive cardiac failure | Transudate | Light criteria (see text) SEAG > 1.2 g/dL, Protein gradient > 3.1 g/dL | Most common cause of pleural effusion, it will commonly recur with heart failure, decompensation (Light 2002) |
| Hepatic hydrothorax Nephrotic syndrome | Transudate | Light criteria (see text), SEAG > 1.2 g/dL | |
| Hypothyroidism | Exudate, can present with transudate | Myxoedema heart disease could be transudate | |
| Recent myocardial infarction | Exudate | Light criteria (see text)
SEAG < 1.2 g/dL |
Patients with post coronary injury syndrome (Dressler’s syndrome) can develop fever, pleuropericarditis, and pulmonary infiltrates. The pleural effusion is often bloody. |
| Recent coronary artery bypass graft surgery (CABG) | Exudate | Light criteria (see text)
SEAG < 1.2 g/dLProtein gradient < 3.1 g/dL |
10% of post CABG patients may develop pleural effusion occupying >25% of the hemithorax. Early the pleural fluid is bloody and eosinophilic while > 4 weeks, the fluid is clear yellow with a high lymphocyte percentage |
| Rheumatoid arthritis | Exudate | Very low glucose levels (< 40 mg/dL)
pH < 7.00 (59)LDH > 1000 U/mLLow yield of rheumatoid factor in pleural effusion (37) |
A low pH and glucose in the pleural fluid labs are key findings. These two elements are also found in empyema but the management is quite different |
| Systemic Lupus Erythematosis | Exudate | Glucose level (< 60 mg/dL)
pH < 7.2LDH > 1000 U/ml |
In patients who present with a pleural effusion secondary to a pulmonary embolus, consider a procoagulant state particularly in patients with lupus. Check for antiphospholipic antibodies such as the lupus anticoagulant and anticardiolipin antibodies |
| Renal failure | Exudate | Light criteria
SEAG < 1.2 g/dLProtein gradient < 3.1 g/dL |
Uremia can cause pleural effusion. The effusion may be bilateral |
| Drug-induced | Exudate | Eosinophilic effusion | The most common drugs include nitrofurantoin, dantrolene, ergot alkaloids, valproate, propylthioruacil, and isotretinoin. |
| Pulmonary embolism | Exudate almost always | Serum D-dimer (highly sensitive) for screening: can be ruled out if negative | Reported incidence in pleural effusion ranges from 23-48%. Exudative in nature but are often too small to be obtained by thoracentesis (61). |
| Sarcoidosis | Exudate can present with transudate | Elevated serum angiotensin-converting enzyme level | Variable findings with eosinophils in pleural effusions, chylothorax. Noncaseating granulomas in the pleura by biopsy |
| Chylothorax | Exudate | Triglyceride level > 100 mg/dL,
Cholesterol level < 200 mg/dL |
Can occur following trauma or cardiothorax surgery. Fluid often appears milky. |
| Pseudochylothorax | Exudate | Cholesterol crystals in the sediment (37) | Longstanding (> 5 years) pleural effusion. High levels of triglycerides are not usually present (37) |
| Urinothorax | Transudate | Pleural fluid/serum creatinine > 1,
Low glucose, low pH (38) |
Presence of urine in the pleural spaces secondary to obstructive uropathy. Fluid looks and smells like urine. |
Table 3: Empiric Therapy for Various Pleural Effusions
Type of Effusion |
First line Abx |
Second Line Abx |
Treatment time |
|---|---|---|---|
Community Acquired Uncomplicated Effusion |
Third Generation Cephalosporin or Clindamycin
|
Augmentin |
7-14 days |
Community Acquired Complicated Effusion or empyema |
Third Generation Cephalosporin or Clindamycin |
Augmentin |
4-6 weeks |
Hospital Acquired Uncomplicated Effusion |
Extended spectrum beta lactam with vancomycin |
Imipenem or Aztreonam |
7-14 days |
Hospital Acquired Complicated Effusion or Empyema |
Extended spectrum beta lactam with vancomycin |
Imipenem or Aztreonam |
4-6 weeks |
Figure 1: Etiology of 336 Community Acquired Pleural Infections
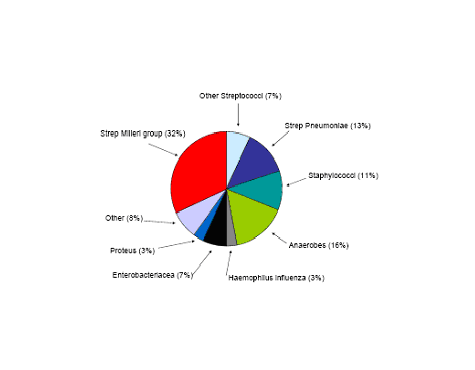
Figure 2: Etiology of 60 Hospital Acquired Pleural Infections
Guided Medline Search For:
Guided Medline Search For Recent Reviews
Guided Medline Search For Historical Aspects
Table of Contents
- Pathophysiology
- Diagnosis
- Infections Producing Pleural Effusions and Antibiotic Therapy
- Treatment Options for Pleural Effusions
