Prosthetic Joint Infections
Authors: Nalini Rao, M.D., FACP, FSHEA, Bruce H. Ziran, M.D.
Over one half a million people in the United States each year undergo total joint replacements ![]() , which enable them to regain physical function and be free of pain. Unfortunately, 1%-2% of joint replacements have a devastating complication of infection that can lead to poor functional outcome with a mortality approaching 8% in elderly patients. It is estimated that the cost per year to treat 3,500-4,000 total joint infections is between 150 –200 million dollars. The management of prosthetic joint infection requires pre-operative assessment of risk factors and the treatment options be individualized with a team approach between orthopaedic surgeon and musculoskeletal infectious disease specialist. Optimal management requires multiple surgical procedures with one or more hospitalizations, and 4 to 6 weeks of parenteral antibiotic therapy, often in long-term care facilities. Being in long term facilities is known to have several disadvantages including the acquisition of resistant bacteria, development of decubitus ulcers due to decreased mobility and deterioration of nutritional status, having a negative impact on the overall outcome of the patient.
, which enable them to regain physical function and be free of pain. Unfortunately, 1%-2% of joint replacements have a devastating complication of infection that can lead to poor functional outcome with a mortality approaching 8% in elderly patients. It is estimated that the cost per year to treat 3,500-4,000 total joint infections is between 150 –200 million dollars. The management of prosthetic joint infection requires pre-operative assessment of risk factors and the treatment options be individualized with a team approach between orthopaedic surgeon and musculoskeletal infectious disease specialist. Optimal management requires multiple surgical procedures with one or more hospitalizations, and 4 to 6 weeks of parenteral antibiotic therapy, often in long-term care facilities. Being in long term facilities is known to have several disadvantages including the acquisition of resistant bacteria, development of decubitus ulcers due to decreased mobility and deterioration of nutritional status, having a negative impact on the overall outcome of the patient.
PATHOGENESIS
The pathogenesis of implant-associated infection involves interaction between microorganism, the implant and the host. The minimal infecting dose decreases > 100,000 fold in the presence of a foreign body. Source of majority of infections is intraoperative contamination of air-borne pathogens in the operating room or from microorganisms from the skin of the patients or operating room staff. However, infection can spread to the prosthetic implant from a contiguous focus of overlying or adjacent skin and soft tissue, or by hematogenous route from a number of anatomic sites such as oropharynx, GI tract and GU tract. The incidence of prosthetic joint infection is highest during the first six months after implantation and declines thereafter. Once the bacteria attach to the surface of the prosthesis, a complex biofilm develops that surrounds the bacteria. This biofilm creates a unique ecosystem on the surface of the prosthesis preventing penetration of the antibiotics. The bacteria within the biofilm enter a sessile hibernating stage making it difficult to recover the organisms by intra-operative culture.
RISK FACTORS
Host factors predisposing to prosthetic joint infection are advanced age, prior arthroplasty and underlying joint disease, poor nutritional status, obesity, diabetes mellitus, malignancy remote infections, prior native joint infections and advanced HIV disease. Rheumatoid arthritis increases the risk 2.6 fold as compared to those with osteoarthritis. Revision surgery had an infection rate of 4.2% as compared to 0.54% in patients with primary arthroplasty.
Berbari and colleagues, in a retrospective case-controlled studies, of follow-up of 26,505 patients, found that development of superficial infection at the surgical wound site, a high NNIS index score, previous arthroplasty and systemic malignancy were predictors of prosthetic joint infections. The National Nosocomial Infection Score (NNIS) scoring system involves pre-operative anesthetic assessment score, duration of surgery and wound class. Staphylococcal bacteraemia is associated with a 34% risk of haematogenous prosthetic joint infection. However, McPhearson et al have outlined a more comprehensive stratification of risk factors considering systemic and local host factors which is shown in Table 1.
MICROBIOLOGY
Gram-positive bacteria account for the majority of infections. In our experience over a two-year period of 95 cases (unpublished data) that were judged to be infected with positive intraoperative cultures and/ or positive histopathology with associated serologic markers of inflammation, Staphylococcal species accounted for 62% of cases while gram-negative organisms accounted for 8 % of cases. In 17 % of cases more than one organism was recovered. Powers and associates evaluated 51 episodes of infections in 38 elderly patients. Staphylococcal species accounted for 60 % of episodes while Pseudomonas and other gram-negative organisms were the cause in a third of the episodes and anaerobic organism were noted in one fourth of the episodes.
DIAGNOSIS
Clinical
Fever and chills with acute pain in the prosthesis is generally indicative of hematogenous seeding. Wound drainage, presence of effusion or sinus tract ![]() into the prosthesis is also suggestive of infection. Pain, especially at rest is suggestive of infection, however, a variety of extrinsic and intrinsic factors do cause pain. Therefore, a painful prosthesis does not always mean infection.
into the prosthesis is also suggestive of infection. Pain, especially at rest is suggestive of infection, however, a variety of extrinsic and intrinsic factors do cause pain. Therefore, a painful prosthesis does not always mean infection.
Laboratory Diagnosis
Elevated white count is found only in 15% of patients with an acute infection. Most patients with Type 2 or 4 infections (Table 2) have a normal white count. Erythrocyte Sedimentation Rate (ESR) is notoriously unreliable and non-specific for a variety of reasons. ESR averages around 64 mm/h on post-operative day 14 and often remains elevated for four months. C - reactive protein (CRP) on the other hand is found in only trace amounts under normal conditions. CRP level of over 10 mg/L was considered to be indicative of infection with sensitivity ranging from 91% and specificity 88% to 92%.
Cultures of Joint Aspirates
The major problem with joint aspirates is false negatives. The reported rates of sensitivity and specificity again varied widely with sensitivity ranging from 50% to 93% and specificity from 82% to 97%. No single routinely used clinical or laboratory test has been shown to achieve ideal sensitivity, specificity, and accuracy for the diagnosis of prosthetic joint infection.
Gram Stains
Intra-operative gram stain is notoriously unreliable with sensitivity ranging from 0% to 23%. When positive, it is highly specific for infection.
Histological Examination of Frozen Sections of Tissue
Frozen section technique to detect the prosthetic joint infection has been widely used. Feldman and associates studied the association of the surgeons’ intra-operative opinion and pathological diagnosis (frozen section of over 5 PMN/hpf). They found that a sensitivity of 70% and specificity of 87%.
Intra-operative Cultures
Prophylactic antibiotics, if given at all, should not be administered until after tissue samples have been taken. Positive intra-operative cultures are considered the gold standard for diagnosis of most infections, especially if pathogens such as Staphylococcus aureus or gram-negative bacilli are isolated; the likelihood of a false positive resulting from external contamination by these organisms is minimal. Prosthetic joint infections, in contrast, are often caused by skin flora such as Staphylococcus epidermidis and Propionibacterium acnes. Recovery of such organisms in culture can be difficult to interpret as they are more easily introduced as external contaminants. An implanted joint, like any foreign body allows smaller number of organisms to cause infection than in normal tissue. Infection in prosthetic joints is also patchy and irregular, often requiring multiple sample sites. The culture results of a series of nearly 300 patients by Atkins et al led the authors to recommend five or six tissue samples obtained at surgery.
Cultures are essential to document active infection. Recovery of the same organism on multiple intraoperative tissue or fluid cultures, taken from different areas as well as from the same area at different points in time is useful for validation. A number of innovative attempts have been to improve the sensitivity of culture including sonication of surgically removed implants, use of monoclonal or polyclonal organism-specific antigens for immunofluorescence, use of scanning electron microscopy for detection of bacteria in biofilms, and PCR methods. These interesting ideas need to be standardized and validated.
Criteria for the Diagnosis of a Prosthetic-Joint Infection
There are no generally accepted criteria to diagnose prosthetic joint infection. One common definition is the presence of at least one of the following four criteria: 1) growth of an identical microorganism in two or more cultures of synovial fluid or periprosthetic tissue or > 20 CFU per 10 ml of sonicate fluid or both; 2) purulence of synovial fluid or at the implant site; 3) presence of granulocytes on histopathological examination of periprosthetic tissue; and 4) presence of a sinus tract communicating with the device.
Radiographs
Plain radiographs often show a variety of changes that are neither sensitive nor specific for infection. Radionucleotide imaging also shows wide range of sensitivity (38% to 100%) and specificity (41% to 100%) with various scans. Technetium-99m (99mTc) bone scan following total joint arthroplasty is sensitive but not specific. Difficulty associated with 99mTc is that it can remain positive up to one year after uncomplicated joint replacement and for more than two years after insertion of prosthesis without cement. Some investigators have found that a negative bone scan rules out infection. Indium-111-labeled white blood cells (111In-WBC) scanning appears to be dependent upon activity of the infection with reduced sensitivity in chronic and indolent infection. This method has replaced the use of gallium-67 citrate scintigraphy. The use of sequential 99mTc and 111In-WBC is often used. However, triple scan with 99mTc, 111In-WBC and sulfur colloid marrow scintigraphy is 95% accurate. MRI can be of value after an infection has been diagnosed in a patient with total joint arthroplasty performed with use of radiolucent cement, to determine the extent of the cement mantle prior to revision procedure. CT scan has limited role in the diagnosis of prosthetic joint infection. Soft tissue abscesses or thickened capsule may indicate infection. Therefore, a combination of clinical, laboratory, imaging, microbiology and histopathological studies are employed for the diagnosis of prosthetic joint infection.
MANAGEMENT
The goal of the treatment is to restore a pain-free, functioning prosthesis and to eradicate or control infection. A combined medical and surgical approach is necessary. The individual treatment protocol including the type of surgical procedure performed depends upon the systemic and local host factors, type of prosthetic joint infection, pathogen causing infection and the experience of the orthopaedic surgeon. The approach to treatment includes:
• Systemic antibiotics plus debridement with retention of the prosthesis.
• Systemic antibiotics plus debridement with removal of all the components of
the prosthesis and the cement with the placement of an antibiotic cement spacer
and delayed re-implantation.
• Systemic antibiotics plus debridement and permanent removal of all the
components of the prosthesis and the cement (resection arthroplasty).
• Joint arthrodesis after removal of all the components of the prosthesis.
• Amputation.
• Chronic antibiotic suppression.
Surgical Treatment
Systemic antibiotics plus debridement with retention of the prosthesis 
It is generally accepted that debridement with retention of the prosthesis may be an option in post-operative infection diagnosed within 30 days of arthroplasty or acute hematogenous prosthetic joint infection in a well-fixed and functioning prosthesis. Tsukayama and associates who reported the largest series with above criteria for selection had an overall success rate of 68% at two-year follow up. Others have reported varying rates of success ranging from 14 to 83%. Despite the differences in various studies from different centers and different countries, an important observation is that the successful outcome of prosthetic joint infection treated with debridement and retention of the prosthesis is possible if the timing of the debridement after the symptoms is preferably between 2 and 7 days. Prosthesis salvage from infection with Staphylococcus aureus is associated with a greater than 50% chance of cure if surgical debridement occurs within two days of the onset of symptoms. Intervention occurring after one to two weeks is unlikely to be successful. None of the above studies addresses the chronic suppressive antibiotic therapy in conjunction with debridement and retention of the prosthesis.
Re-implantation Arthroplasty
This is a widely accepted treatment option. It involves complete removal of the infected prosthesis and the cement. The one-stage or direct exchange arthroplasty entails insertion of a new prosthesis ![]() at the same time as the removal of the infected prosthesis followed by four to six weeks of appropriate systemic antibiotic therapy.
at the same time as the removal of the infected prosthesis followed by four to six weeks of appropriate systemic antibiotic therapy.
The delayed two-stage arthroplasty involves debridement, removal of the infected prosthesis and cement followed by the insertion of a new prosthesis after eight to twelve weeks interval. An antibiotic-impregnated cement spacer is placed to manage dead space and deliver local antibiotic in conjunction with six weeks of systemic antibiotic therapy (Figure 1). Antibiotic cement is often used with the new prosthesis. Overall success rates with one and two-stage reimplantation arthroplasty is outlined in Table 3.
Resection Arthroplasty
Resection arthroplasty entails complete removal of all the prosthetic components, infected bone and cement with no intention of re-implantation at a later date. This is reserved for patients who are debilitated with major bone loss, poor tissue, multiple prior infections and minimum ambulatory demands.
Arthrodesis
This is a viable option for infected total knee arthroplasty (Figure 1). After resection arthroplasty, arthrodesis involves fusion of tibia and femur to near full extension allowing weight bearing on the affected limb. This is reserved for younger patients who have high functional demand, single joint disease, rupture of the extensor mechanism, poor soft tissue envelope, immunocompromised host or resistant infection.
Amputation
This is an infrequent option chosen for infected total knee arthroplasty with life threatening uncontrollable sepsis, massive bone loss and intractable pain.
ANTIMICROBIAL THERAPY
Systemic antimicrobial treatment is used in conjunction with surgical intervention. Empiric antibiotic therapy should not be given prior to obtaining an etiologic diagnosis. It is critical to identify the offending pathogen given the duration of antibiotic (months) therapy and the insidious effects of the damage caused by an untreated microorganism. The choice of the antibiotics must be individualized based upon culture and sensitivity of the infecting organism. Intravenous route is preferred unless the drug has 100% oral bioavailability. Average duration of treatment is six weeks. The choice of the specific antimicrobial agent based upon the pathogen (Table 4).
Role of Rifampin
Rifampin has excellent antistaphyloccal activity and penetration into bone. Rifampin has been combined with either fluroquinolones or vancomycin or flucloxacillin in the treatment of chronic bone sepsis. In a randomized, placebo controlled, double blind trial, a cure rate with retention of prosthesis of 100% was obtained for early onset, short duration staphylococcal prosthetic joint infection in all of 12 patients treated with a combination of ciprofloxacin plus rifampin, compared with a cure rate of 58% (7/12) patients with ciprofloxacin alone. Other studies comparing oral rifampin combined with either fusidic acid or ofloxacin for staphylococcal implant infection; there was a similar rate of cure in the two groups.
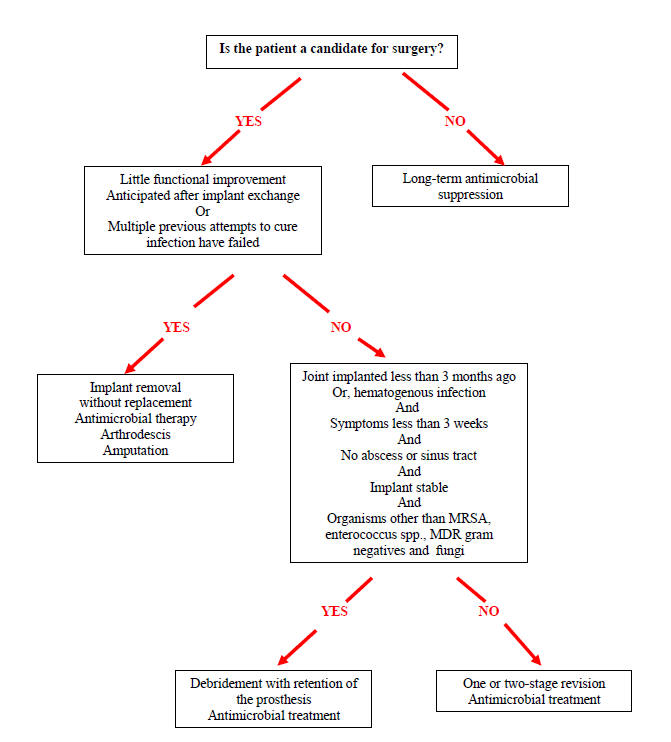
Chronic Antibiotic Suppression
Elderly patients who are too frail to undergo surgery with insufficient bone stock or who refuse to undertake surgery may be candidates for chronic antibiotic suppression (Figure 2). We again emphasize that the infecting pathogen should be microbiologically documented; prosthesis should be well fixed and functional. The oral antibiotics to which the microorganism is susceptible, should be inexpensive, preferably given once or twice a day with minimal or no drug/drug interaction. The patient should be compliant, able to tolerate long-term therapy and be followed closely as an outpatient.
PREVENTION
Pre-operative optimization of the host includes correction of malnutrition, obesity, good control of blood glucose, lower dosage of immunosuppressive drugs, treatment of remote site infection and pre-operative dental and urinary tract evaluation. Pre-operative antiseptic shower and application of mupirocin to nares has been shown to reduce postoperative Staphyloccus aureus infection. Pre-operative antibiotic prophylaxis give within two hours of incision is crucial. Good incision, appropriate size of the prosthesis, wound hemostasis, decreased operative time, ultra clean air, and minimal OR traffic decrease the risk of postoperative wound infection.
American Dental Association in collaboration with American Academy of Orthopaedic Surgeons recommends antibiotic prophylaxis in patients with prosthetic joints undergoing dental procedures under the following circumstances:
- Patients receiving immunosuppressive agents.
- Patients with insulin-dependent diabetes, hemophilia, systemic lupus erythematosis, rheumatoid arthritis.
- Patients with previous prosthetic joint infection.
- Patients with joint replacement within last two years.
CONCLUSION
Infection of prosthetic joints is a serious and a challenging disease that can often lead to poor functional outcome. It is essential to stage the prosthetic joint infection based on systemic and local host factors for optimal management. The device needs to be removed to clear the infection, however when that is not feasible due to host factors, medical and surgical treatment decisions are to be individualized in each case. Optimal nutritional status plays a key role in successful management. Adverse effects to medications are more common in elderly patients due to endorgan dysfunction and drug-drug interaction.
READING LIST
1. Anguita-Alonso P, Hanssen AD, Patel R. Prosthetic joint infection. Exp Rev Anti-Infective Therapy. 2005;3:797-804. [PubMed]
2. Atkins BL, Athanasou N, Deeks JJ, Crook DW, Simpson H, Peto TE, McLardy-Smith P, Berendt AR. Prospective evaluation of criteria for microbiological diagnosis of prosthetic joint infection at revision arthroplasty. J Clin Microbiol 1998;36:2932-2929. [PubMed]
3. Berbari EF, Hanssen AD, Duffy MC, Steckelberg JM, Ilstrup DM, Harmsen WS, Osmon DR. Risk factors for prosthetic joint infection: case-control study. Clin Infect Dis 1998;27:1247-1254. [PubMed]
4. Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilm: A common cause of persistent infections. Science 1999;284:1318-1322. [PubMed]
5. Darouiche RO. Device-associated infection: a macroproblem that starts with microadherence. Clinical Infectious Diseases 2001;33(9):1567-1572. [PubMed]
6. Duggan JM, Georgiadis GM, Klenshinski JF. Management of prosthetic joint infection. Infect Med 2001;18:534-541.
7. Garcia-De La Torre I. Advances in the management of septic arthtitis. Rheum Dis Clin N Am 2003;29:61-75. [PubMed]
8. Gernaat-van der Sluis AJ, Hoogenboom-Verdegaal AM, Edixhoven PJ, Spies-van Rooijen NH. Prophylactic mupirocin could reduce orthopedic wound infection. Acta Orthop Scand 1998;69:412-414. [PubMed]
9. Goldenberg DL. Septic arthritis. Lancet 1998, 351:197-202. [PubMed]
10. Hasan S, Smith JW. Septic arthritis. Curr Treat Options Infect Dis 2001;3:279-286.
11. Ho G. Bacterial arthritis. Curr Opin Rheumatol 2001;12:310-314. [PubMed]
12. Kocher MS, Mandiga R, Murphy JM, Goldmann D, Harper M, Sundel R, Ecklund K, Kasser JR. A Clinical Practice guideline for Treatment of Septic Arthritis in Children. J Bone Joint Surg Am 2003;85-A(6):994-999.[PubMed]
13. Kothari NA, Pelchovitz DJ, Meyer JS. Imaging of musculoskeletal Infections Radiol Clin North Am 2001, 39:653-671. [PubMed]
14. Lentino JR. Prosthetic joint infections. Clin Inf Dis 2003;36:1157-1161. [PubMed]
15. Mader JT, Shirtliff ME, Bergquist S, Calhoun JH. Bone and Joint Infections in the Elderly. Drugs Aging 2000;16:67-80. [PubMed]
16. Mariani BD, Tuan RS. Advances in the diagnosis of infection in prosthetic joint implant. Molecular Med Today 1998;4:207-213. [PubMed]
17. McPherson EJ, Tontz W Jr, Patzakis M, Woodsome C, Holtom P, Norris L, Shufelt C. Outcome of infected total knee utilizing a staging system for prosthetic joint infection. Am J Ortho 1999;161-165. [PubMed]
18. Murdoch DR, Roberts SA, Fowler VG, Jr. Infection of orthopedic prostheses after Staphylococcus aureus bacteremia. Clin Infect Dis 2001;32(4):647-648. [PubMed]
19. Perry CR. Septic Arthritis. Am J Orthop 1999;28:168-178. [PubMed]
20. Poss R, Thornhill TS, Ewald FC. Factors influencing the incidence and outcome of infections following total joint arthroplasty. Clinical Orthopedics and Related Research 1984;(182):117-126. [PubMed]
21. Ragni MV, Crossett LS, Herndon JH. Postoperative infection following orthopedic surgery in HIV infected hemophiliacs with CD4 counts < or = 200/mm3. The Journal of Arthroplasty 1995;10(6):716-721. [PubMed]
22. Rao N. Septic Arthritis. Current Treatment Options in Infectious Diseases 2002; 4:279-287.
23. Rao N, Crossett LS, Sinha RK, Le Frock JL. Long-term suppression of infection in total joint arthroplasty. Clin Orthop 2003;414:55-60. [PubMed]
24. Ross JJ. Septic Arthritis. Infect Dis Clin N Am 2005;19:799-817. [PubMed]
25. Segreti J. Prosthetic joint infections. Curr Opinion Infect Dis 2000;2:200-207.
26. The Management of Septic Arthritis. Drug Ther Bull 2003;41:65-8. [PubMed]
27. Tsukayama DT, Estrada R, Gustilo RB. Infection after total hip arthroplasty: a study of the treatment of one hundred and six infections. J Bone Joint Surg 1996;78:512-523. [PubMed]
28. Tunney MM, Patrick S, Gorman SP, Nixon JR, Anderson N, Davis RI, Hanna D, Ramage G. Improved detection of infection in hip replacements a currently underestimated problem. J Bone Joint Surg 1998;80:568-572.[PubMed]
29. Virk A, Osmon DR. Prosthetic Joint Infection. Current Treatment Options in Infectious Diseases 2001;3:287-300.
30. Wilson MG, Kelly K, Thornhill TS. Infection as a complication of total knee arthroplasty. Risk factors and treatment in sixty-seven cases. The Journal of Bone and Joint Surgery. American Volume 1990;72(6):878-883.[PubMed]
31. Zimmerli W, Waldvogel FA, Vaudaux P. Pathogenesis of foreign body infection: description and characteristics of an animal model. The Journal of Infectious Diseases 1982;146(4):487-497. [PubMed]
32. Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. Role of Rifampin for the treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. JAMA 1998;279:1537-1541. [PubMed]
33. Zimmerli W, Trampuz A, Ochsner PE. Prosthetic joint infections. N Eng J Med 2004;351:1645-1654. [PubMed]
Tables
Table 1: Systemic Host (Medical/Immune)
| Systemic inflammatory disease (e.g. rheumatoid arthritis, SLE) |
|---|
|
| Local Extremity Grade (Wound) |
|
HIV = human immunodeficiency virus
ABG = arterial blood gases
Adapted from McPherson et al
Table 2. Classification of Infected Prosthetic Joints
Type |
Definition |
Timing |
|---|---|---|
| I | Positive intra-operative culture Equal to or more than 2 specimens |
Intra-operative |
| II | Early postoperative infection | First month |
| III | Acute hematogenous infection | Late/acute onset |
| IV | Late chronic infection | Chronic clinical course |
Table 3: Success rates of exchange arthroplasty in the treatment of prosthetic joint infection
|
Total hip Arthroplasty |
Total Knee Arthroplasty | ||
|---|---|---|---|---|
|
One-stage exchange |
Two stage exchange |
One stage exchange |
Two stage exchange |
| Without antibiotic- impregnated cement | 57% to 92%; seven studies; 214 PJI; mean 83% | 53% to 100%; eight studies; 246 PJI; mean 79% | 25% to 100%; seven studies; 60 PJI; mean 80% | 53% to 100%; 11 studies; 216 PJI; mean 88% |
| With antibiotic- impregnated cement only | 38% to 100%; 12 studies; 1666 PJI; mean 83% | 90% to 96%; five studies; 187 PJI; mean 92% | 63% to 100%; three studies; 37 PJI; mean 89% success | |
| With antibiotic- impregnated cement and spacers or beads | 73% to 100%; nine studies; 282 PJI; mean 92% | 60% to 100%; 10 studies; 246 PJI; mean 88% | ||
Adapted from: Virk A, et al
Table 4. Antibiotic Treatment in Prosthetic Joint Infections
Microorganism |
Antimicrobial agent |
Alternative |
Duration |
|---|---|---|---|
| Streptococcus species | Penicillin
Ampicillin |
Cefazolin
ClindamycinVancomycin |
4 -6 weeks |
| Methicillin-sensitiveStaph. aureus | Nafcillin
Cefazolin |
Vancomycin
DaptomycinLinezolid |
4 -6 weeks |
| Methicillin-resistantStaph. aureus | Vancomycin | Daptomycin
Linezolid |
4 -6 weeks |
| Enterococcus spp, Penicillin-susceptible | Penicillin Ampicillin | Vancomycin
Daptomycin |
4 -6 weeks |
| Enterococcus spp Penicillin- resistant | Vancomycin | Linezolid
Daptomycin |
4 -6 weeks |
| Propionibacterium acnes | Penicillin Ceftriaxone | Clindamycin
Vancomycin |
4 -6weeks |
| N. gonorrheae | Ceftriaxone | Fluoroquinolone | 4 -6 weeks |
| Gram-negative bacilli (other than Pseudomonas aeruginosa)< | Ceftriaxone | Extended spectrum penicillin
CarbapenemFlouroquinoloneTMP/Sulfa |
4 -6 weeks |
| Pseudomonas aeruginosa | Cefepime Meropenem | Ciprofloxacin | 4 -6 weeks |
Figure 1. Example of A Patient with An Infected Knee Replacement Who Underwent A Staged Reconstruction. (A-B). Anteroposterior and lateral views of the infected total knee arthroplasty. (C-D). Anteroposterior and lateral views of the cement-antibiotic spacer fashioned in-situ. (E-F). Anteroposterior and lateral views of the reconstructed total knee arthroplasty.
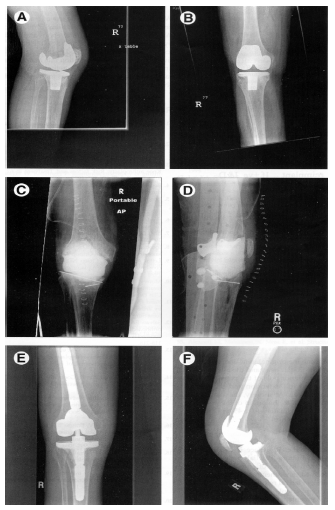
Figure 2: Algorithm for the Treatment of Infection Associated with Prosthetic Joint
Gómez J, et al. Linezolid plus rifampin as a salvage therapy in prosthetic joint infections treated without removing the implant. Antimicrob Agents Chemother. 2011;55:4308-10
Martínez-Pastor JC, Muñoz-Mahamud E, et al. Outcome of Acute Prosthetic Joint Infections Due to Gram-Negative Bacilli Treated with Open Debridement and Retention of the Prosthesis. Antimicrob Agents Chemother. 2009 Nov;53:4772-7.
Guided Medline Search For:
Osmon DR, et al. Diagnosis and Management of Prosthetic Joint Infection: Clinical Practice Guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2013;56:e1-e25.

