Glycopeptides (Dalbavancin, Oritavancin, Teicoplanin, Telavancin, Vancomycin)
Authors: Françoise Van Bambeke, N. Virgincar, Alasdair MacGowan
CLASS
The glycopeptides are an expanding group of structurally complex anti Gram positive antibacterials, representatives of which have been used in human and veterinary medicine since the 1950s. Vancomycin and ristocetin were the first available, however ristocetin was associated with bone marrow and platelet toxicity, and was quickly withdrawn. Teicoplanin entered clinical use in Europe in the late 1980s and is now widely used as an alternative to vancomycin. It is not available in the USA. Other glycopeptides such as avoparcin and actaplanin have been used in veterinary practice.
Among semisynthetic derivatives of vancomycin, oritavancin (LY 333328) and telavancin (TD-2424) have undergone clinical development (till Phase III studies), and telavancin has been approved by the FDA in November 2008 for the treatment of skin and soft tissue infections, while the application at the EMEA was withdrawn in October 2008. Dalbavancin (B1 397), an amide derivative of teicoplanin, as also completed Phase III trials for the same indication but again application from both EMEA and FDA was retired in September 2008.
Ramoplanin, a lipoglycodepsipeptide has a similar microbiological spectrum to the glycopeptides but is not discussed here (239, 360).
Chemical Structure and Structure-Activity Relationships
Glycopeptides are at the original natural products, but semi-synthetic derivatives with improved activity and pharmacokinetic properties have been obtained over the last 20 years, based on the knowledge of structure-activity relationships (171, 217, 349, 350). The common core of natural glycopeptides is made of a cyclic peptide consisting in 7 amino acids, to which are bound 2 sugars, hence the name glycopeptides. Binding of the antibiotic to its target (D-Ala-D-Ala termini of peptidoglycan precursors) occurs via the formation of 5 hydrogen bonds with the peptidic backbone of the drug. The presence of an additional chlorine and/or sugar (as is the case in oritavancin) facilitates the formation of homodimers, allowing a cooperative binding to the target (7, 34). A lipophilic side chain (present in teicoplanin as well as in all the semi-synthetic derivatives enhances antibacterial potency and prolongs half-life (Figure 1).
Vancomycin
Vancomycin was isolated by Eli Lilly and Company in 1956 from a soil sample from Borneo containing a newly discovered actinomycete, Nocardia orientalis (226). In vitro studies showed the compound to have significant in vitro potency against all staphylococcal strains tested. Animal studies showed the level of toxicity to be relatively low, and the drug was approved for use in man 1958 (11). Initial lots of vancomycin contained large amounts of impurities, and it was termed “Mississippi Mud” because of its brown discoloration. The licensing of methicillin two years later, which was as effective as vancomycin against penicillin-resistant staphylococci resulted in the decline of vancomycin use. In the 1980s, methicillin-resistant Staphylococcus aureus, coagulase-negative staphylococci and Enterococci emerged as therapeutic problems, which renewed clinical interest in vancomycin. Increased usage has been accompanied by emergence of resistance first in Enterococci and subsequently in Staphylococci, threatening the future utility of vancomycin and similar glycopeptides.
Chemical structure of vancomycin was confirmed in 1978 (CAS registry number 1404-93-9, molecular formula C66H75Cl2N9O24 ; molecular weight, 1449 g/mol). The sugars substituting the hepatapeptidic core are vancosamine and glucose. It is supplied commercially as a hydrochloride salt and is most soluble at pH 3 to 5. Solubility decreases with increasing pH, and vancomycin is unstable in alkaline solutions. Vancomycin powder is generally reconstituted with sterile water and diluted with dextrose or saline to a final concentration of 2.5 to 5.0 grams per litre.
Teicoplanin
Teicoplanin (teichomycin A2) is a glycopeptide antibiotic which was obtained by fermenting the actinomyceteActinoplanes teichomyceticus. Teicoplanin was first described in 1978 and has a structure similar to that of vancomycin (258). Teicoplanin shares many chemical and microbiological properties with vancomycin, but offers advantage over vancomycin in that, it has longer elimination half life, can be administered by intramuscular injection, however resistance is much more common in coagulase negative Staphylococci and recommended doses may be too low for more severe infection. Teicoplanin is not approved for use in the United States but has been extensively used in Europe since 1988.
Teicoplanin (CAS registry number 61036-62-2; main constituent molecular formula C88H97Cl2N9O33 and molecular weight 1880 g/mol) is a complex of six analogues. As in vancomycin, the core aglycone is a cyclic heptapeptide, made in the present case of aromatic amino acids, which also bears 2 sugars, namely D-mannose, and N-acetyl-beta-D-glucosamine, and an acyl (fatty acid) moiety (51). The fatty acid component causes teicoplanin to be more lipophilic, resulting in greater tissue and cellular penetration (259). The manufactured form of teicoplanin is the sodium salt and sufficient sodium chloride to yield an isotonic solution (pH 7.5). It remains stable in solution for 48 hours at room temperature and for 7 days at 4°C (294).
Oritavancin (LY333328)
Oritavancin (CAS registry number 171099-57-3, molecular formula, C86H97Cl3N10O26, molecular weight 1793g/mol) is the p-chlorophenylbenzyl derivative of the natural glycopeptide chloroeremomycin, which itself differs from vancomycin by the presence of an additional 4-epi-vancosamine (75). It was the first clinical candidate of this new generation of glycopeptides. Discovered by Eli Lilly (Indianapolis, IL) in the late 90's, where the preclinical development and the first clinical trials were performed, it was then successively taken over by Intermune (2001; Brisbane, CA) and Targanta Therapeutics (2005, St-Laurent, QC, Canada). In January 2009, Targanta was acquired by The Medicines Company (Parsippany, NJ) after unsuccessful submission of oritavancin at the FDA in November 2008. As compared to vancomycin, the more salient features of oritavancin are the followings (see also 196, 241, 273, 349, 350, 351, 352, 363 for reviews). The cholorophenylbenzyl side chain is responsible for the prolonged half-life of oritavancin and probably also for the highly bactericidal character of this compound, by allowing anchoring and subsequent destabilization of the membrane. The additional epi-vancosamine confers a stronger ability to form dimers, which cooperatively bind to both D-Ala-D-Ala or D-Ala-D-Lac ending precursors of peptidoglycan and may explain residual activity on vancomycin-resistance strains (7).
Telavancin (TD-6424)
Telavancin (Theravance, South San Francisco, CA; CAS registry number 372151-71-8, molecular formula C80H106Cl2N11O27P; molecular weight 1756 g/mol) is another semi-synthetic derivative of vancomycin, characterized by an hydrophobic side chain on the vancomsamine sugar (decylaminoethyl) and a phosphonomethylaminomethyl substituant on the cyclic peptidic core (188), which counterbalances to some extent the hydrophobicity brought by the lipophilic side chain. Telavancin possesses therefore specific properties as compared to vancomycin or oritavancin (see also for reviews (351, 352, 22, 196, 172, 241, 253, 254, 349, 386), namely multiple modes of action including alterations of membrane integrity (149), as for oritavancin, but a markedly shorter half-life than oritavancin (188) even though it is also highly protein bound (318) and largely distributes in the organism (339).
Dalbavancin (BI 397)
Dalbavancin a semi-synthetic derivative of A40926, a teicoplanin analog (CAS registry number 171500-79-1; molecular formula C88H100Cl2N10O28 ; molecular weight:1817 g/mol). It differs from its parent compound by the replacement of the acylglucosamine on amino acid 4 and by the removal of the acetylglucosamine in the benzylic position (219). Dalbavancin was not the most active in the series, but presented the best tolerability (217). It was discovered by Biosearch Italia and out licensed to Versicor for North America. Both firms merged in Vicuron Pharmaceuticals. The later has been acquired by Pfizer (Groton, CT), which has pursued the development of dalbavancin. This molecule displays an unusually prolonged half-life (6-10 days) allowing for once weekly administration (97). Its antibacterial activity is similar to that of teicoplanin, with however lower MICs (see for reviews; 40, 49, 89, 91, 176, 218,288, 314, 332, 345, 349, 350, 351, 386). (Table 1).
ANTIMICROBIAL ACTIVITY
Spectrum and In Vitro Activity
The antibacterial spectrum of glycopeptides is limited to Gram-positive organisms, as the molecule is too voluminous to cross the external membrane of Gram-negative bacteria (163), and some anaerobic species. Current susceptibility of target organisms to glycopeptides and lipoglycopeptides is compared in Table 2.
Vancomycin
Current susceptibility breakpoints have recently been lowered b the CLSI (www.clsi.org) to S < 2 mg/L; I : 4-8 mg/L; R > 16 mg/L (343) in North America and S < 2 mg/L; R > 4 mg/L in Europe (www.EUCAST.org). This value for EUCAST is not yet official and should be confirmed very soon for EUCAST; this will be done hopefully at the stage of the proofs) to allow detection of heterogeneously resistant isolates of S. aureus. Of importance, an MIC creep was observed in MRSA over the last years, i.e. strains that remain below the susceptibility breakpoint but show increasing MICs (1-2 mg/L); (332, 362), asking question about the probability of clinical cure if using conventional dosages for such strains (see section on pharmacodynamics).
Vancomycin does not have significant activity against mycobacteria, fungi, Bacteroides spp or Gram-negative bacteria except Flavobacterium meningosepticum and some Neisseria spp (232, 278). Vancomycin is slowly bactericidal against sensitive bacteria, and there is no correlation between vancomycin concentrations in the range 2-50mg/L and killing of S. aureus (2, 187). Enterococci are inhibited but not killed by clinically achievable concentrations. Both methicillin-sensitive and resistant strains of S. aureus and most strains of coagulase-negative staphylococci have vancomycin MICs in the range 0.25 to 4.0 mg/L. The first report of a clinically significant coagulase-negative staphylococcus resistant isolate was in 1987 (316). Some strains of Staphylococcus haemolyticus and S. epidermidis with reduced susceptibility to vancomycin have also been reported (71, 121). As mentioned later in the chapter, the first S. aureus isolate with an MIC > 4mg/L to vancomycin appeared in Japan 1996 from a wound infection in a child receiving vancomycin for MRSA wound infection (150). In addition, some strains of S. aureus are deficient in autolysins and are tolerant to the bactericidal activity of vancomycin (134, 307).
Streptococci, including viridans species, anaerobic and microaerophilic strains, and penicillin-sensitive and resistant pneumococci are susceptible to vancomycin. Most strains of Listeria monocytogenes are inhibited by clinically achievable levels of vancomycin (212), but therapeutic failures have also been reported (26, 101). Nondiphtheroid corynebacteria including C. jeikeium are susceptible in vitro (158). Vancomycin-resistant isolates of opportunistic pathogens like Lactobacillus, Leuconostoc, Lactobacillus and Pediococcus are frequently isolated (298, 340). The anaerobic spectrum of vancomycin includes anaerobic and microaerophilic streptococcus, and clostridia species, including both C. perfringens and C. difficile. The susceptibility of actinomycetes is variable (197), and Gram-negative anaerobes such as Bacteroides species are resistant. Vancomycin has no activity against Enterobacteriaceae, rickettsiae, chlamydia and mycobacteria.
Teicoplanin
Teicoplanin has slower bactericidal activity against Gram-positive organisms than vancomycin (23, 105). Bacterial killing by teicoplanin occurs in growing not resting cells (243, 268, 356). Activity against both methicillin-susceptible and resistant Staphylococcus aureus (MRSA) is comparable to that of vancomycin, with a mean MIC90 of 0.2 to 1.5 mg/L but the susceptibility of coagulase-negative staphylococci is more variable with MICs of 2 to 4 mg/L (319). Aldridge (4) reported MIC90 for teicoplanin of 16mg/L or more for Staphylococcus epidermidis, S. haemolyticus, S. hominis, S. warneri and S. xylosus. Staphylococcus saprophyticus is fully susceptible to teicoplanin (289). More recent data from the UK and Ireland where teicoplanin is widely used in clinical practice indicates 23.4 and 3.1 % of oxacillin resistant coagulase negative Staphylococci and 7.6 and 1.5 % oxacillin susceptible coagulase negative Staphylococci from blood cultures are teicoplanin intermediate and resistant (MIC = 8mg/L [I] and > 8 mg/L [R]) (154).
In vitro, teicoplanin is more potent than vancomycin against most streptococcal species, includingStreptococcus pneumoniae, with MIC50 and MIC90 in the 0.06-0.12 and 0.12-0.25 mg/L respectively, compared with values of 0.25-0.5 and 0.5-1mg/L for vancomycin (328). MIC90 values for enterococci range from 0.2 to 3.1mg/L, versus 1.56 to 4.0mg/L for vancomycin (319). Teicoplanin is only moderately bactericidal against Enterococcus faecalis (255).
Teicoplanin is active against other aerobic and anaerobic Gram-positive bacteria. Corynebacteria, Clostridia (including C. difficile), Bacillus spp, Listeria monocytogenes, and Propionibacterium acnes are inhibited by low concentrations of teicoplanin with mean MIC900.3 - 0.8 mg/L (28, 46, 244, 250, 266). It is not active against Gram-negative bacteria, Mycobacterium spp and fungi. Teicoplanin is synergistic with aminoglycosides for half the enterococcal and staphylococcal strains (90a). At equivalent concentrations, the postantibiotic effect of teicoplanin exceeds that of vancomycin for MRSA and E. faecalis (74). The clinical importance of this phenomenon is not clear.Lactobacillus spp, Pediococcus spp and Leuconostoc spp are inherently teicoplanin resistant. Acquired resistance to the glycopeptides in enterococcus species was first reported in 1988 (189, 348). Various patterns of resistance (VanA, VanB, VanC, VanD, VanE and VanG) to both vancomycin and teicoplanin are now well documented (see above). Enterococci with the VanA phenotype have transferable plasmid-mediated resistance to both vancomycin and teicoplanin. VanB inducible resistance and VanC constitutive resistance to vancomycin but teicoplanin MIC ≤2mg/L. Inducible resistance to teicoplanin has been reported in an isolate of E. faecium with the VanS phenotype (143), and VanD confirms resistance to both teicoplanin and vancomycin.
Wilson et al (373) first reported S. haemolyticus resistant to teicoplanin (MIC 16 mg/L) following cardiac surgery. Kaatz et al (170) reported development of constitutive, non-plasmid mediated resistance in serial isolated of S. aureus from a patient being treated for endocarditis. Vancomycin-intermediate S. aureus (VISA) with MIC of 8mg/L and treatment failure have been reported. In VISA strains, over production of PBP2 and PBP2’ with thickening of the bacterial cell wall is seen, limiting access of the antibiotic to its target site (151). These strains have higher MICs to teicoplanin (MIC 8-32mg/L than vancomycin (MIC 8mg/L).
Oritavancin
MIC of oritavancin need to be determined in the presence of 0.002 % polysorbate 80 to prevent the adsorption of the drug on plastic surfaces (15). Clinical and Laboratory Standards Institute (CLSI) guidelines have been modified accordingly, but all data published prior this revision of standard protocols underestimate the potency of this drug, since MICs are 4- to 64-fold lower in the presence of polysorbate 80 for staphylococci and enterococci. In contrast, they remain unaffected against streptococci (15).
Oritavancin shows a very potent activity against staphylococci, enterococci and streptococci, regardless their resistance phenotype (81). Of interest, it remains active against VISA and VRSA strains, with, however, MICs slightly higher than against most of the vancomycin-susceptible strains. Oritavancin is also active in vitro against other Gram-positive pathogens, like Listeria monocytogenes or Bacillus spp (47, 80).
Oritavancin is also highly active on some important anaerobic species, like Clostridium pefringens, Clostridium difficile and Propionibacterium acnes, with MICs 2-4 fold lower than those of vancomycin and metronidazole against C. difficile. Strains that are intrinsically resistant to vancomycin such as Leuconostoc spp, Pediococcus spp andLactobacillus spp had MICs to oritavancin equal or less to 8mg/L (81).
Telavancin
Telavancin shows lower MICs than vancomycin (2-4 dilutions) against S. aureus and S. epidermidis, whether methicillin susceptible or resistant. MICs are higher against VISA strains as well as MRSA (1mg/L) (198). Telavancin also demonstrates potent activity against various species of streptococci. Its activity is comparable to that of vancomycin against vancomycin-susceptible enterococci, but MICs are much higher against vancomycin-resistant strains. Telavancin also possesses activity against a broad range of anaerobic Gram-positive bacteria and Corynebacterium spp. (most strains with MIC90 < 1 mg/L).
Dalbavancin
As for oritavancin, the addition of 0.002 % polysorbate 80 is now recommended by the CLSI for susceptibility testing, as it prevents the adsorption of the drug to plastic surfaces, resulting in more consistent and reproducible MIC values (281). Due to the high protein binding of dalbavancin, MICs are markedly increased in the presence of serum (3-5 dilutions), bringing them back close to vancomycin values, but the drug keeps its bactericidal character (207).
In general, dalbavancin is more potent than vancomycin and teicoplanin against staphylococci (including coagulase-negative species, that are usually less susceptible to teicoplanin), and against S. pyogenes and S. pneumoniae(91, 333, 387). Dalbavancin has been tested only against a few strains of glycopeptide-intermediate S. aureus, with MICs of 1 mg/L at least (129, 207). Dalbavancin is active against vancomycin-susceptible enterococci, but not against glycopeptide-resistant strains, with MIC50 only marginally (4-fold) lower than those of teicoplanin (382). As teicoplanin, it is inactive on vanA enterococci (334, 382), and therefore also against VRSA.
Subpopulations of staphylococci with low level of resistance (2- to 4-fold increase in MIC) have been selected upon serial passage at sub-MIC of dalbavancin (208), but in vivo selection of such mutants may however be more difficult since trough levels of dalbavancin are above the current MIC of the targeted microorganisms. Dalbavancin shows MICs ≤1 mg/L for Corynebacterium spp and MIC90 ≤0.125 mg/L against anaerobes including Clostridium spp and anaerobic gram-positive cocci (130).
Pharmacodynamic Effects
Vancomycin and Teicoplanin
In vitro studies suggest that vancomycin is slowly bactericidal, and is therefore primarily considered as time-dependent (2, 187). Investigations using broad ranges of concentrations shows however that its activity also develops on a concentration-dependent manner, as for any antibiotic (29, 30). Vancomycin has a number of persistent antibiotic effects. A post antibiotic effect has been shown in vitro and in animals (147, 288). Sub MIC drug concentrations prolonge the PAE (299). Against VISA strains, the rate of killing is slowed down, but the extent of killing and the PAE remain unaffected (3). Morover, the antibacterial effect in time-kill curves towards vancomycin susceptible or hVISA strains is much reduced by increasing the inoculum from 106 to 108 cfu/ml (290, 382).
Vancomycin-aminoglycoside combinations were synergistic against most methicillin-susceptible and resistant strains of S. aureus by the time-kill curve method (365) and also in animal models (32). However, some controversy surrounds the efficacy of the combination of vancomycin and rifampin. When used against coagulase-negative staphylococci, vancomycin and rifampin are often synergistic and rarely demonstrate antagonism (107, 210). But, in S. aureus, vancomycin-rifampin synergy is found inconsistently, and antagonism has been reported (366).
In animal models of clinical infection of methicillin-resistant S. aureus (MRSA) endocarditis, the response to combination therapy has varied. In an experimental model of MRSA endocarditis (32), vancomycin-rifampin proved more effective in eradicating organisms from the valve and causing clinical cure than vancomycin alone. Similar data was reported in a rabbit endocarditis model (122) and a chronic MRSA bone infection model (147). However, in a randomized trial of vancomycin alone versus vancomycin-rifampin for treatment of MRSA endocarditis in humans (200) slow clinical response was found in both groups, with no advantage to combination treatment. Vancomycin has also been shown to increase the bactericidal activity of linezolid in in vitro pharmacokinetic models (8) and of quinupristin/dalfopristin in in vitro models and animal infective endocarditis (173, 260).
The combination of vancomycin and an aminoglycoside is bactericidal against enterococci unless high level aminoglycoside resistance is present (142, 367).
Oritavancin
Oritavancin is highly bactericidal in vitro (47). In killing curve experiments, oritavancin shows a very rapid and highly concentration-dependent bactericidal activity (3-log reduction in bacterial counts after 1 to 8 hours) in conditions where vancomycin requires at least 8 to 24 hours to reach the same effect (47,79). Oritavancin however acts more slowly against vancomycin-resistant enterococci (230). These properties are related to the multiple modes of action of this drug. Oritavancin also displays a concentration-dependent post-antibiotic effect, increasing from ~ 2 h at 1 X MIC to 4-8 h at 4 X MIC against MRSA and VRE, respectively (230). Oritavancin combinations with gentamicin, linezolid, moxifloxacin, and rifampin, are synergistic against MSSA, VISA and VRSA (36). The combination with ampicillin enhances the bactericidal activity of oritavancin, without being truly synergistic and prolongs its postantibiotic effect against vancomycin resistant enterococci from 18 to 23 h at 10 X its MIC (27). Oritavancin activity is negatively affected by large inocula (230), but not by acid pH or by the growth phase of the bacteria (231); is effectively kills bacteria in stationary phase and in biofilms (37).
In a model of infected human macrophages, oritavancin displays a remarkable intracellular activity, reaching a maximal effect of –2 to –3 log against MSSA, MRSA or VISA (30, 308). This activity develops on a bimodal concentration-dependent manner, which may correspond to the multiple modes of action of this drug. Oritavancin is also as active against extracellular and intracellular SCV as against the normal phenotype, and is highly synergistic with rifampin or moxifloxacin against intracellular SCV (245, 246).
In an in vitro model of human gut, oritavancin instillation markedly and rapidly reduced C. difficile vegetative numbers and spores as well as cytotoxin titres that persist after oritavancin cessation (25). In a rat central venous catheter model of infection by vancomycin-resistant E. faecium, a single dose of oritavancin prevented the dissemination of the infection (299). In a rabbit model of experimental endocarditis by a susceptible strain of E. faecalis and two glycopeptide-resistant transconjugants, the combination of oritavancin with gentamicin was the only regimen bactericidal against the three strains (191). In a rabbit model of cephalosporin-resistant pneumococcal meningitis, oritavancin caused a rapid decrease in colony counts and no therapeutic failures. In combination with ceftriaxone, activity was improved but no synergistic effect was observed (62). In an animal model of inhalated anthrax, oritavancin proves efficacious and shows a low propensity to select resistance (146).
Telavancin
Telavancin shares with oritavancin a high a rapid bactericidal activity (31). In an in vitro kinetic model, telavancin was bactericidal against MSSA and MRSA at concentrations higher than MIC at 24 h, even in the presence of human albumin, with no regrowth. In contrast to the methicillin-susceptible strain, the methicillin-resistant strain in 40 g/L human albumin showed a regrowth at concentrations of 0.5x MIC and 1x MIC at 24 h. Telavancin was also kill these strains when in a nongrowing phase (252). Telavancin also shows a concentration-dependent bactericidal effect against GISA, hGISA and VRSA at concentrations equal to or above 4x MIC, which again was diminished but remained cidal in the presence of serum (198).
Telavancin shows PAE of 0.9 to 3.9 h, 0.4 to 6.7 h and 0.3-2.2 h, and PA-SME (0.4 x MIC) of 6.7 to >10.7 h, >10.7 to >11.0 h, and >10 to >10.8 h against staphylococci, streptococci, and enterococci, respectively, which support once-daily dosing (257).
In a murine model of MRSA pneumonia, telavancin (AUC ~ 780 mg•h/L) showed higher reduction in bacterial count and lower mortality than vancomycin or linezolid at human doses equivalent (282). In a model of localized osteomyelitis by MRSA in rabbits, telavancin has comparable efficacy to vancomycin and linezolid (385). In a model of MSSA pneumonia in neutropenic mice simulating human exposures at therapeutic doses, telavancin (total (free) drug AUC of 747 (52.3) mg•h/L) was more efficient than vancomycin (total (free) drug AUC of 225 (37) mg•h/L), nafcillin (t > MIC for 50% of the dosing interval), or linezolid (total (free) drug AUC of 160 (365) mg•h/L) (144). In rabbit experimental endocarditis by MRSA and VISA, showed a higher efficacy, with more vegetations sterilized and greater reduction in vegetations bacterial counts in non sterilized animals (146, 234). In a model of rabbit meningitis, telavancin proved superior to vancomycin combined with ceftriaxone against a penicillin-resistant pneumococcal strain, but equivalent to vancomycin against a methicillin-sensitive staphylococcal strain (338).
As oritavancin, televancin is highly active against intracellular S. aureus, whatever their resistance phenotype (MSSA, MRSA, VISA or VRSA), with bimodal concentration –effect relationships against MSSA and MRSA (30). In contrast with oritavancin, however, it is poorly active against intracellular SCV, possibly in relation with its lower cellular concentration (246).
Dalbavancin
Dalbavancin is bactericidal, with MBC/MIC ratios close to 1, even in the presence of 30 % serum (331). It is synergic with ampicillin, including against VanA-type enterococci.
Using the broth microdilution checkerboard method, dalbavancin shows synergistic or partially synergistic effects with oxacillin for staphylococci (including MRSA and VISA) and enterococci (164).
In the rat granuloma pouch infection model, a single intravenous dose of 10 mg/kg reduced bacterial counts of 2 log for MRSA and below the limit of detection for MSSA, and prevented regrowth for up to 120 h. This was associated with a good penetration in the exudates (plasma/AUC ratio of 1.01) (157). In rabbit endocarditis, 10 mg/kg for 4 days dalbavancin or as a single 40-mg/kg dose caused a 2 log decrease in S. aureus counts (190).
Pharmacodynamic Correlates with Outcomes
Vancomycin and Teicoplanin
In the murine thigh infection model, AUC/MIC is the most predictive PD parameter for vancomycin efficacy against S. aureus, whether MSSA or MRSA, with AUC24h/MIC ranging from 86 to 460 to reach 50% of the maximal effect. In an in vitro pharmacodynamic model simulating human dosages, development of heteroresistance in agr-null group II of S aureus was observed for vancomycin exposure corresponding to freeAUC/MICs lower than 112 to 169 against 28 only in agr-positive isolates (347). Still in vitro, vancomycin simulated doses of 750 to 2250 give raise to fAUC/MIC of 105 to 317 and had poor activity against clinical strains of hVISA, suggesting these patients may be at increased risk of treatment failure (290). Moreover, for VISA stains, Peak/MIC ranging from 4.4 to 9.5 better correlate with efficacy (80). Likewise, in a non-neutropenic mouse model of Streptococcus pneumoniae peritonitis, the peak serum concentration divided by the MIC (peak/MIC) was most predictive (261). Time of exposure seems however to be also an important determinant, since a maximal killing rate is obtained when vancomycin concentration remains constantly above the MIC but was not influence by concentration itself (103, 187).
Based on the study of Moise-Broder et al. (374), who examined the relationship between the vancomycin AUC/MIC and the outcomes of 108 patients with MRSA pneumonia, an AUC/MIC > 400 is considered as the best parameter to predict clinical success and bacterial eradication. Other studies, however, failed to determine the best parameter predictive of vancomycin efficacy in bacteremia or endocarditis (300).
Because of the long half-life and post-antibiotic effect of vancomycin, both an intermittent continuous infusion of vancomycin will insure a prolonged time of exposure, with concentrations remaining above an MIC of 4 mg/L for the whole dosage interval upon administration of a conventional dose of 1 g twice-a-day (148). In contrast, an AUC/MIC ratio of 400 with the same dose is reached for MIC < 0.5 mg/L (148). Continuous infusion could facilitate dosage adaptation to reach pharmacodynamic targets, with steady state concentration of 15 mg/L enabling the target AUC/MIC ratio for MICs of < 1 mg/L (263). A clinical study comparing continuous infusion (targeted plateau drug serum concentrations of 20 to 25 mg/L) and intermittent infusions of vancomycin (targeted trough drug serum concentrations of 10 to 15 mg/L) in 119 critically ill patients with MRSA infections failed to show any difference in microbiological and clinical outcomes (382)). Overall, less vancomycin was given in the continuous infusion arm, contributing to cost savings. Yet, no data on the susceptibility of offending organisms were reported in this work. A recent study suggests however that increasing serum concentrations to 28 mg/L or more for sustained periods of time (as would be needed to reach target for higher MICs) is accompanied by higher risk of nephrotoxicity (140).
Teicoplanin was studied in fewer details. Based on its prolonged half-life, it is administered once a day. A recent work suggests that a twice daily administration for 48 h may be useful to reach rapidly target trough values of 10 to 20 mg/L (54). Moreover, higher doses than 6 mg/kg may be requested for deep compartments or serious infections, including pneumonia or bone infections (222, 233). In an in vitro pharmacodynamic model, and as opposed to vancomycin, suboptimal doses of teicoplanin (< 4 mg/kg) were associated with selection of resistance whatever the agr group of the Staphylococcus strain. Doses of 7.5 mg/kg resulted in variable changes in susceptibility in the agr group I strains; higher doses (15-30 mg/kg) did not cause any change in MIC values (291).
Oritavancin
In the neutropenic mice infection model, dose fractionation studies suggest that Cmax/MIC ratios better correlate with bactericidal activity than the time during which the concentration in plasma exceeds the MIC (T>MIC) and AUC/MIC (53).
A population pharmacokinetic model for 55 patients with S. aureus bacteremia rather determined a probability for success of 93% as soon as free drug concentrations levels remain above the MIC for 22% of the time (287). A Monte Carlo simulation was used to assess PK-PD target attainment for different MICs, using population pharmacokinetics from Phase II/III studies and CFU reduction from the thigh infection model in neutropenic mice (44). Probabilities of target attainment reached almost 1 for MIC ≤ 0.12 mg/L and decreased to 50 % or less for higher MICs suggesting a PD breakpoint of 0.25 mg/L. In a small group (24) of diabetic patients, clinical success was higher for those with AUC/MIC ratios ≥ 2253 h, suggesting that greater oritavancin exposure may be required in the presence of this comorbidity, but this needs to be further investigated with larger groups (135).
Telavancin
In an in vitro model of S. aureus infection, comparable effects were observed against MRSA for simulated doses of 10 mg/kg telavancin and 2x1 g vancomycin with AUC/MIC of 3400 and 500 h, respectively. However, a 1.6-fold greater effect was obtained with telavancin against GISA Mu-50 (AUC)/MIC of 1700 vs 130 h, respectively. Moreover, no mutant of either strain with increased MICs was selected with telavancin (211). In the mouse neutropenic thigh and mouse subcutaneous infection models, telavancin shows higher potency than vancomycin, nafcillin, and linezolid; free AUC/MIC ratio was the best predictor of efficacy, with maximal effect reached for values of ~ 10 h (145).
Dalbavancin
In the thigh neutropenic mice infection model, dalbavancin single doses of 2.5 mg/kg of greater reduced S. pneumoniae bacterial counts and prevents regrowth in a dose-dependent fashion for up to 96 hours; doses of 20 mg/kg or greater resulted in less killing of S. aureus but were still followed by a prolonged suppression of regrowth. Multiple-dosing-regimen studies with the same organisms were used to determine the pharmacodynamic indices predictive of efficacy. Both the 24-h AUC/MIC and the C(max)/MIC parameters correlated well with the efficacy in thigh and lung infection models. Free AUC/MIC of 17 and 265 were required for static effect against S. pneumoniae and S. aureusrespectively (10). Since a single dose of 1 g produces a free-drug AUC of more than 1,500 mg•h/L and trough free-drug concentrations exceeding 2 mg/L at the end of the treatment, adequate pharmacodynamic target attainment is probable in view of the current susceptibility profiles of target organisms. In accordance with this study, Monte Carlo simulations using population pharmacokinetic data and MICs from clinical isolates allowed to calculate susceptibility breakpoint for dalbavancin of < 0.5 mg/L using as target an AUC(14 days)/MIC 1000 for S. aureus of < 1 mg/L for S. aureus and < 2 mg/L for streptococci using a time-dependent target of maintenance of free drug concentrations above the MIC for 14 days (t>MIC). Based on current MIC distributions, this also supports the use of once-weekly dosing regimens of dalbavancin in the treatment of complicated skin and skin structure infections (98).
MECHANISM OF ACTION
Vancomycin and Teicoplanin
Conventional glycopeptides act on the late stage of cell wall synthesis in dividing organisms. They interfere with the formation of peptidoglycan, the major structural polymer of the bacterial cell wall, by inhibiting the transpeptidation reaction necessary to the elongation of the peptidoglycan backbone (265, 320, 372, 373). The molecular mechanism of action has been best characterized for vancomycin. The primary target of vancomycin was shown to be the D-Alanyl-D-Alanine terminus of pentapeptidic precursors. Molecular modeling and experimental studies (19, 206, 283) indicate that vancomycin forms a stoechiometric complex with the D-Ala D-Ala dipeptide via the formation of five hydrogen bounds with the peptidic backbone of the glycopeptide. The formation of this complex prevents the transpeptidation reactions by steric hindrance. Recent studies have shown the importance of the protonation state of vancomycin and of the formation of dimmers of antibiotic molecules in this interaction (249, 383, 384).
In protoplasts, vancomycin also alters the permeability of cytoplasmic membranes and may impair RNA synthesis (167, 168), but these mechanisms are probably not relevant in vivo, as the drug does not have access to membrane or intracellular compartments of the bacteria.
Like vancomycin, teicoplanin inhibits cell wall synthesis in susceptible bacteria. It inhibits polymerisation of peptidoglycan in bacterial cell walls by binding non-specifically to saturate the outer layers of the bacterial peptidoglycan. It then binds to the terminal amino acyl- D-alanyl-D-alanine precursor, which fits into a cleft in the teicoplanin molecule (283, 326).
Oritavancin and Telavancin
These molecules present additional modes of action that explain their highly bactericidal character (352).
For oritavancin, an improved inhibition of peptidoglycan synthesis could be ascribed to (a) a cooperative binding to the pentapeptidic target, which is made possible by the capacity of the molecule to dimerize (7), and (b) an increased steric hindrance around peptidoglycan precursors due to the presence of a bulky substituant on its dissacharide moiety, which allows for a potent inhibition of both transglycosylation and transpeptidation reactions (175). As a result, aberrations in the formation of septa were observed in electron microscopy (38). More importantly, oritavancin shows a rapid bactericidal effect on both exponential phase and stationary phase of vancomycin-susceptible or vancomycin-resistant staphylococci as well as on bacteria growing in biofilms; these effects develop in parallel with membrane permeabilization and depolarisation (37), which is most probably favored by the anchoring of the lipophilic chain of oritavancin in the membrane.
Telavancin also displays multiple modes of action, which include the depolarization and permeabilization of the bacterial membrane (149) and a potent inhibition of transglycosylation and transpeptidation reaction through a tight interaction with lipid II (41, 355).
MECHANISMS OF RESISTANCE
Organisms Commonly Resistant
Acquired resistance to vancomycin was unusual until the late 1980s, when first reports of enterococci resistance to glycopeptides began to occur in UK (348) and other European countries. This increase in resistance coincided with a significant rise in the use of vancomycin to treat MRSA and coagulase-negative staphylococcal infections as well as C. difficile colitis in many countries. Most vancomycin-resistant enterococcal isolates have been E. faecium, but glycopeptide resistance has also been seen in E. faecalis, E. gallinarum, E. casseliflavus, E. avium, E. durans, E hirae, andE. raffinosus.
The prevalence of vancomycin-resistant enterococci isolated from intensive care units in the USA increased from 0.4% in 1989 to 13.6% in 1993 (67), and 26% in 2000 (50). This increase was accompanied over the last years by a higher incidence of hospitalizations, witnessing the poor outcome of these infections (280). In Europe, figures are highly variable for E. faecium among countries (292, 370), from very low rates (< 1 %) in most countries to 1-5% in Austria, Croatia, France, Slovenia, Spain, Switzerland, 5-10% in Czech republic and Turkey but 10-25% in Germany, Italy, and UK, and even 25-50% in Greece, Ireland, or Portugal in 2007. The proportion of resistant E. faecalis is globally much lower, with a maximum of 5-10 % in Greece (http://www.rivm.nl/earss/database/).
More recently resistance of moderate or of high level emerged in S. aureus raising concern for the treatment of infections for which glycopeptides often appeared as last resort drugs. In 1997, the first clinical strain of S. aureus with reduced susceptibility to vancomycin (Mu50) and teicoplanin (VISA) was reported from Japan (150) followed by two additional cases from United States (254).
In 1997, a resistance phenotype of hetero-resistance to vancomycin (Mu3) (hVISA) was isolated in Japan (90). VISA and hVISA have been described from many countries all over the world though VISA (Mu50 like) strains remain rare. The incidence of hVISA is unknown because of variability in detection techniques and their sensitivity and specificity. Using the most reliable methodology – population analysis – profiles then the true incidence of hVISA is probably <5%. A trend to increase over time seems however quite clear (301). There are a number of definitions of vancomycin reduced susceptibility in S. aureus which makes discussion difficult. CLSI and the European breakpoint committees regard S. aureus strains with vancomycin MIC ≤ 2 mg/L as susceptible. CLSI regards strains with MICs of 4 or 8 mg/L as intermediate and ≥ 16 mg/L as resistant. European Committee on Antimicrobial Susceptibility Testing (EUCAST) regards strains with MIC ≥ 4 mg/L as resistant. Importantly also, VISA strains appear to also loose they susceptibility to daptomycin, because their thickened cell wall (see below) prevents the access of daptomycin to its membrane target (86).
Most recently a few strains of MRSA have been described in the USA with vancomycin MICs above the resistance breakpoint (VRSA). These strains are in addition resistant to a wide range of antibiotic classes so that therapeutic options are limited, but hopefully they remain anecdotal so far (321). Isolated cases have also been reported in Iran and India (6, 308).
Disc diffusion tests have been shown to be unreliable for detection of VRSA, VISA or hVISA (344). The first screening method for VISA was described by Hiramatsu et al (152) and was based on simplified population analysis. It involves inoculating 10µL of a 108 CFU/ml on brain heart infusion agar (BHIA) containing 4ug of vancomycin per ml. Growth at 24h was considered potential VISA. Subsequently other methods have been used to determine MIC like, macro Etest, broth dilution, agar dilution and MicroScan, generating unreliable results, so that visa are probably underreported (14, 274). Population analysis profiling (42) has been proposed as the most precise method of determining heteroresistance (203). Wootton et al (379) have modified this method a step further and calculated the area under the population analysis curve and compared it to Mu3 as a control. The resulting ratios were 0.90-1.3 for hVISA and >1.3 for VISA. However, Centers for Disease Control and Prevention has adopted three criteria to identify VISA strains: broth microdilution MIC of 8-16mg/L, Etest MIC of >6mg/L and growth on BHIA screen plates containing 6mg/L vancomycin at 24h (345). This method will detect VISA but not hVISA which is not really recognized in the USA as a microbiological or clinical entity.
A glycopeptide tolerant phenotype has also been described in clinical S. aureus isolates in which the MICs remain ≤ 4mg/L but MBC are raised. The clinical significance of these strains is unknown.
Mechanisms of Resistance
Glycopeptide resistance in enterococci is mediated by the acquisition of a gene operon, located on a mobile genetic element, which codes for the concerted production of enzymes involved in the synthesis of low-affinity precursors termini ending in D-Alanyl-D-lactate or D-Alanyl-D-serine, and in the elimination of high-affinity precursors ending in D-Alanyl-D-alanine, as well as for a regulatory system allowing induction by glycopeptides (19). Six types of vancomycin resistance have been differentiated on a phenotypic and genotypic basis. 3 summarizes their main features in terms of location and transferability of the operon, regulation of the expression, level of resistance to both vancomycin and teicoplanin, and peptidic precursor produced (78, 228). The name of the resistance type is determined by the ligase produced.
The VanA-type of resistance is the most spread and was also the most studied so far; it is characterized by inducible and high levels of resistance to both vancomycin and teicoplanin. Resistance genes are present on the transposon Tn1546 (18), which codes for 7 proteins with complementary functions. Thus, three enzymes are required for glycopeptide resistance, namely the dehydrogenase VanH, which reduces pyruvate in D-lactate (58), the ligase VanA, which catalyses the formation of an ester bond between D-alanine and D-lactate (59), and the DD-dipeptidase VanX, which hydrolyzes the D-alanyl – D-alanine dipeptide produced by the host D-alanyl – D alanine ligase (284). Two accessory enzymes can increase the level of resistance. The DD-carboxypeptidase VanY removes the C-terminal D-alanine residue of late peptidoglycan precursors (and contributes therefore to the elimination of high affinity precursors when the hydrolysis of D-alanyl – D-alanine by VanX is incomplete) (20). The function of the VanZ protein is still unclear, but it is involved in teicoplanin resistance (21). A two-component regulatory system, made of the membrane-bound kinase sensor VanS and of the cytoplasmic regulator VanR that acts as a transcriptional activator, allows for induction of the transcription of the operon upon exposure to glycopeptides (17a). As VanA (59), VanB- (110), and VanD- (92) types of resistance result from the preferential incorporation of D-alanyl – D-lactate precursors in peptidoglycan, the main differences between these three phenotypes consisting in their inducibility and in the level of resistance conferred (see Table 3). More important differences are observed for VanC (16), VanE (1) and VanG (93). In these cases, the ligase produces D-alanyl – D-serine rather than D-alanyl – D-lactate (285). Therefore, a membrane-bound serine racemase VanT replaces the dehydrogenase VanH (17). Moreover, a unique VanXY protein possessing both DD-dipeptidase and DD-carboxypeptidase activities replaces VanX and VanY and allows hydrolysis of precursors ending in D-Alanine (267).
The mechanism of resistance of intermediate level in Staphylococci (VISA or GISA) is not yet fully understood and is probably multifactorial. A global proteomics and transcriptomics approach evidenced a series of proteins or genes overexpressed in resistants trains, among which regulators, attenuator or hypermutability factors (315). Their role in the resistance phenotype needs to be further explored. VISA strains show lower growth rates and thicker cell walls (323). Both VISA and hVISA produce three to five-fold greater quantities of penicillin-binding proteins 2 and 2' and of cell wall precursors (46), but in contrast to hVISA, VISA show an increased amidation of glutamine residues in cell-wall muropeptides, which reduces the cross-linking within the cell wall and increases the amount of vancomycin bound to peptidoglycan precursors. This impairs the capacity of vancomycin to reach the bacterial surface where primary targets of the antibiotic are situated (28). A marked decrease or no PBP4 activity was also observed in clinically-derived VISA, which again reduces cross-linking and susceptibility (118). Moreover, VISA strains often have an impaired acetate catabolism, which may affect a series of physiological function like growth yield, antibiotic tolerance, regulation of cell death or plysaccharide intracellular adhesin synthesis, but again the link with resistance is not clearly established (242).
Importantly also, the emergence of VISA phenotype has been associated with a loss of function of the accessory gene regulator agr and with agr II polymorphism (309, 310). Of importance, this agr II group has been associated with therapeutic failures of vancomycin treatment (238). The role of agr in the VISA phenotype is not yet understood, but agr is known to control the expression of exotoxins, exoproteins and adhesions, and agr mutants as well as VISA strains show reduced autolysis and virulence (261, 311). Of importance, vancomycin treatment failure has been associated with the agr group II.
VRSA strains result from the acquisition of the VanA gene cluster by conjugative transfer from Enterococci toS. aureus (68, 69). While some of these strains have high vancomycin MIC ≥ 32mg/L, others do not. This is thought to be related to the stability of the resistance genes post transfer (264).
Methods to Overcome or Prevent Resistance
Lipoglycopeptides bring only a partial response to resistance. Dalbavancin remains susceptible to teicoplain resistance mechanism. Telavancin shows improved activity on VISA strains, but MICs of VRSA or VRE, although lower than for vancomycin, remain high. Oritavancin is the most active against VRSA and VRE, probably because of its capacity to form dimers that can bind with higher affinity to modified peptidoglycan precursors (352).
As explained in the pharmacodynamic section, optimizing vancomycin dosage so as to obtain an AUC/MIC ratio > 400 h is predictive of efficacy. Increasing doses for coverage of higher MIC is limited by the risk of nephrotoxicity which increases as soon as concentrations are maintained above 28 mg/L (156).
The best way to avoid MIC increase therefore remains the prudent and limited use of vancomycin (9).
PhARMACOKINETICS and dosage
The main pharmacokinetic properties of glycopeptides and lipoglycopeptides are compared in Table 4.
Vancomycin
Vancomycin is administered intravenously for systemic infections. Intramuscular administration is not recommended as it causes severe pain. Oral administration is used when a local action on the digestive tract is looked for, as systemic absorption of the oral form is minimal and serum levels are negligible even in anephric patients (56). The coexistence of bowel inflammation and renal failure may however very rarely result in potentially toxic concentration when vancomycin is given orally (329). When taken orally, vancomycin is excreted in stool in high concentrations that far exceed the MIC for C. difficile (87). The significance of these observations is however unclear as much of the vancomycin will be bound and the free fraction (microbiologically active) is unknown.
Systemic infections are treated with vancomycin administered by slow (60 min or 1mg/min) intravenous therapy to avoid infusion related side effects. In subjects with normal kidney function, multiple 15mg/Kg i.v., doses of vancomycin produce the following mean plasma concentration: 63 mg/L after the completion of the infusion, 18-26mg/L two hours after infusion, and 8mg/L eleven hours after infusion (data on file).
Distribution of vancomycin is a complex process consistent with two or three compartment pharmacokinetic model. An initial rapid distribution phase of about ten minutes is followed by an intermediate half-life of approximately one hour. The elimination half-life varies between three to eleven hours in patients with normal renal function (293, 235). Vancomycin has a volume of distribution of about 0.3L/kg, a total clearance of 0.06 L/hr/kg of which renal excretion accounts for 80-90%. The calculated AUC is about 250-300mg.hr/L and protein binding (albumin) around 50%. The pharmacokinetic parameters are different in obese patients as these patients have larger volume of distribution and increased rates of glomerular filtration, justifying dosage adaptation (140). With multiple dosing, levels above 75% of those in serum are attainable in ascitic, pericardial, and synovial fluid, 50% in pleural fluid, and 30-50% in bile (223). Vancomycin does not significantly penetrate the cerebrospinal fluid (CSF) in the absence of meningeal inflammation. The degree of vancomycin penetration of the CSF increases in direct proportion to the severity of meningeal inflammation, but is unpredictable, with reported ranges of 1-37% of the serum levels (76, 139, 358). Higher doses of 15mg/Kg every 6 h have been used to treat patients with meningitis (177), or continuous infusions with doses as high as 60 mg/kg. This scheme allowed for a sufficient penetration of vancomycin in CSF of patients suffering from streptococcal meningitis and receiving dexamethasone (CSF concentration of 7-25 mg/L; 250). Some physicians have chosen to administer the drug by the intrathecal route. Following intra-peritoneal instillation of vancomycin, 54-65% of the dose is detectable in the serum (256). CNS vancomycin concentrations in children receiving a prophylactic dose of vancomycin prior CNS shunt insertion is minimal (< 1mg/L) and highly variable (< 1-18 % penetration) in children receiving multiple-dose vancomycin therapy for shunt infection (169). Penetration into bone is more variable even in patients with osteomyelitis, but adequate cancellous and cortical levels were obtained (136). The lung penetration of vancomycin is controversial with a range of different findings. This is probably related to different methodologies used (83, 287). Employing lung tissue homogenates tissue vancomycin concentrations in some patients were undetectable (83). In contrast when epithelial lining fluid (ELF) and alveolar macrophages (AM) in patients undergoing bronchial alveolar lavage were studied, ELF concentrations were 2.4mg/L and AM levels 45.2mg/L 12 hours after a standard dose giving serum concentrations of 5 10mg/L.
Vancomycin is excreted primarily unchanged by the kidneys by glomerular filtration, 80-90% of an administered dose appears in urine within 24 hours (138). As renal function declines, elimination half-life of vancomycin increases and in anephric patients may exceed seven days (235).
In ICU patients, conventional dosages often lead to suboptimal serum level, with an increasing risk of not achieving the recommended AUC24h/MIC breakpoint. This results from increased Vd and clearance variability depending on renal function, APACHE II score, age and serum albumin levels (95).
In non elderly adult patients with normal renal function, 1g (15mg/Kg) every 12h is the usual dose of vancomycin. Peak serum levels with a dose of 1g every 12h is usually between 20-40mg/L and trough levels are between 5-10mg/L. For S. aureus infections, a recent consensus from the American Society of Health-System Pharmacists, the Infectious Diseases Society of America and the Society of Infectious Diseases Pharmacists indicates that trough levels should exceed 10 mg/L to avoid development of resistance and even 15-20 mg/L for complicated infections like bacteremia, endocarditis, osteomyelitis, meningitis, and hospital-acquired pneumonia to improve penetration (302). Monitoring of trough levels is recommended in these patients to avoid nephrotoxicity.
In elderly patients the elimination half-life of vancomycin was prolonged (12.1h) compared to that of young male patients (7.2 h) receiving the same dose (313). Vancomycin dosing in the elderly usually required dose modification.
In children with normal renal function, fixed dosage can be based on age as follows: <1 week, 15 mg/Kg every 12h; 8-30 days, 15 mg/L every 8h; >30 days, 10mg/Kg every 6h (313). Vancomycin doses in premature infants are based on gestational age as follows: <27 weeks, 27 mg/Kg every 36h; 27-30 weeks, 24mg/Kg every 24h; 31-36 weeks, 18mg/Kg every 24h, >37 weeks, 22.5mg/Kg every 12h (159). In a pharmacodynamic perspective, dosages need to be adapted to reach trough/MIC > 8. In a context of increasing MICs (> 1 mg/L), a trough < 10 mg/L is frequently obtained (55% of children and 43% of neanates in a study examining 3759 subjects) (220). Regardless of the dosing regimen, vancomycin dose should thus be adjusted according to the serum levels of the drug, as there is significant inter individual pharmacokinetic variation in these age groups. This also applies in neonates, for whom dosage needs to be individualized based on first dose pharmacokinetics and not based on normograms (85).
In burn patients and intravenous drug abusers, the half-life is shorter and dose requirements are higher (303).
Vancomycin is virtually completely eliminated by the kidney, and since vancomycin elimination correlates directly with creatinine clearance, the dosage can be based on creatinine clearance rather than on a simple determination of serum creatinine level (236). Creatinine clearance can be estimated by means of the following formula (72): CrCl (males) = [Wt (Kg) x (140-age)] / [72 x Scr (mg/dl)]; CrCl (females) = 0.85 x CrCl (males). A number of nomograms have been developed by North American specialists to determine vancomycin dose based on estimates of renal function from creatinine clearance (224, 236). In Moellering’s method, vancomycin is dosed daily according to creatinine clearance value; however, by Matzke’s method the dose of the vancomycin remains constant at about 15mg/Kg but the dosing interval increases with declining renal function. There have been no good quality evaluations of these normograms in improving clinical outcomes and reducing toxicity and simpler approaches may be justified.
Very little vancomycin is cleared from the body by haemodialysis or peritoneal dialysis (202, 221). Patients on haemodialysis should be given 1g i.v., weekly. Vancomycin dose can be repeated if necessary by monitoring serum vancomycin levels. Hemodialysis with the newer, more-permeable, high-flux membranes or continuous a-v and v-v hemodialysis results in more rapid vancomycin clearance and more frequent dosing is necessary (271). Continuous hemofiltration removes large amounts of vancomycin and monitoring of serum levels is necessary (39). Patients on CAPD can receive a loading dose of 30mg/Kg IP, followed by 1.5 mg/Kg in each peritoneal exchange or 7 mg/Kg once daily. If the i.v., route is chosen, a loading dose of 15mg/Kg, followed by an additional dose of 15mg/Kg every seven days depending on serum vancomycin levels (61).
In recent times, administration of vancomycin 2.0 – 2.5g/day by continuous infusion has become practice in some countries. In addition sometimes vancomycin is infused to achieve a desired steady state concentration often in the range 10-15mg/L. In a series of 102 patients receiving receiving continuous vancomycin infusion as outpatient parenteral antibiotic therapy, The likelihood of developing nephrotoxicity during therapy was higher (~ 21 times) if their steady-state vancomycin concentration was 28 mg/L or greater (156). The exact role of this form of administration needs to be established, but it is comparable to standard dosing in efficacy and tolerance, facilitates the monitoring, and may be more cost effective (160, 382). For patients on continuous renal replacement, dosages should be modified (Table 5).
Teicoplanin
Teicoplanin is poorly absorbed from the gastrointestinal tract of healthy volunteers (60) but can be administered by either intravenous or intramuscular route. Teicoplanin is not recommended for oral administration for the treatment of systemic infections, however, it can be given orally for the treatment of pseudomembranous colitis (294). Unlike vancomycin, intravenous injections can be given as bolus over 5 minutes. Intravenous administration of 3 and 6 mg/kg in healthy volunteers results in peak plasma levels of 53.5 and 111.8mg/L, respectively, with concentrations of 2.1 and 4.2 mg/L at 24 h (357). By the intramuscular route, a dose of 6mg/Kg i.m, was associated with a peak serum concentration of 12mg/L after 4 hours (13). Systemic availability after i.m. administration approaches 100% and clearance is similar to that of the intravenous route. After intravenous administration, teicoplanin has an elimination half-life of up to 170h in patients with normal kidney function. This prolonged half-life is likely due to its high degree of protein binding (90%) (259). Therapeutic levels have been found in the heart (375) and blister and peritoneal fluid (377). Teicoplanin does not penetrate cerebrospinal fluid well even in the presence of inflamed meninges (330), but intraventricular administration has been used. Teicoplanin pharmacokinetics is best described by a three compartment model with the elimination serum half life being 80 170h depending on the sampling protocol. Teicoplanin is 90% protein bound and has a volume of distribution of 0.9 1.6L/kg and total plasma clearance of 0.01 (L/hr/kg). 80% of teicoplanin is renally excreted and the AUC (mg/L.hr) is 500 600 after a 6mg/kg 24 hourly dose.
Teicoplanin is excreted almost completely by the kidneys, 80% of the dose being recovered in urine and 3% in stool in 16 days (60), without significant metabolism. Teicoplanin is excreted almost entirely by glomerular filtration, with minimal renal secretion. Elimination half-life is prolonged in patients with renal insufficiency, and dosage adjustments are necessary as renal function declines. Clearance of teicoplanin declines as renal function decreases and is correlated linearly with creatinine clearance (111, 183). Lam and colleagues designed a dosage nanogram based on the relationship between teicoplanin clearance and creatinine clearance and an average desired steady-state concentration of 20mg/L (183). Teicoplanin is not removed by hemodialysis. Measurement of serum concentration helps in determining the appropriate dose and dosing interval in severe infections and renal impairment.
Due to its prolonged terminal half-life, teicoplanin can be administered intravenously or intramuscularly every 24 hours. Occasionally, a 48 hour or 3 times per week dosing is used in non hospitalized patients. Unlike vancomycin, dosage adjustments are made on the basis of the severity of infection as well as renal function. To achieve adequate serum levels rapidly, a loading dose of 400mg (6 mg/kg) 12-hourly on the first day is essential. This dose is usually dissolved in 100ml of infusion fluid and administered as a 30-min i.v. infusion. For less serious infections such as those involving the urinary tract, and skin and soft tissue, a lower dose of the usual 200mg (3 mg/kg) daily may be used. Treatment of serious Gram-positive infections such as septicemia, infective endocarditis, and osteomyelitis requires a higher loading dose of 400 to 800 mg (6-12 mg/kg) every 12h for two to three doses, followed by a maintenance dose of 400 to 800 mg (48, 319). There is no established range for therapeutic serum concentrations of teicoplanin, but it has been suggested that trough levels of at least 10 mg/L are necessary for treatment of severe infections (373). However others have shown that therapy for endocarditis with teicoplanin as a single agent has been problematic, and maintenance of trough levels of >20 is recommended (213, 275). Harding et al (141) reviewed 92 patients with S. aureus bacteremia using a multivariate analysis to relate age, weight, dose, loading dose, combination therapy and serum concentrations to outcome and has shown that only trough concentrations and age were significantly related to outcome.
In patients with renal failure, it is suggested giving a normal dosage regimen until the fourth day (216). Then, for creatinine clearance between 40-60mL/min half the normal dose of teicoplanin once every day should be given. If creatinine clearance is below 40mL/min, maintenance doses of 1/3 the normal dose is given (294). Alternatively, higher maintenance doses (6 mg/kg) can be given at less frequent intervals (every 2-3 days). Nomograms and serum drug monitoring may be useful in determining the appropriate dose of teicoplanin in critically ill patients with renal failure.
Teicoplanin is not removed by haemodialysis. Patients on hemodialysis are generally treated with a loading dose of 800mg followed by 400mg weekly (35). Peritoneal dialysis eliminates very little teicoplanin. Patients with Gram-positive peritonitis can be successfully treated by giving teicoplanin intraperitoneally. The UK data sheet recommends 20mg/L per bag in the first week, 20mg/L per alternate bag in the second week and 20mg/L in the overnight bag in the third week (216).
Oral teicoplanin may be an effective alternative treatment for colitis caused by C. difficile. In small studies, no dosage regimen has been established for therapy of C. difficile colitis, but doses of 100 to 400mg/day for 10 days have been used effectively (94).
Teicoplanin can be used in the treatment of pediatric patients. A dose of 10mg/Kg iv for children and 6mg/Kg for neonates once-daily is appropriate (194, 262, 341). Intravenous drug abusers have highly variable renal clearance of teicoplanin (304). In severe infections the dosage has to be adjusted according to the serum levels.
Potel et al. (272) found that terminal half-life of teicoplanin was not significantly affected by the extent of the burn, but the trough concentrations were. Patients with burns excreted teicoplanin more quickly and less predictably than other patients.
Neurosurgical shunt infections by staphylococci can be treated with intraventricular teicoplanin. A dose of 20mg in 5-10ml, every 1-2 days for adults and 5mg in 2-4ml per day for children, a similar volume of cerebrospinal fluid first being aspirated has been used (84).
Oritavancin
In healthy volunteers, oritavancin shows a linear pharmacokinetic profile, with Cmax increasing linearly (range 13.1 to 23.6mg/L) after intravenous administration of single dose of 0.5 to 3mg/kg (70). Changes in plasma clearance (range 0.0547-0.138 ml/min/kg), volume of distribution at steady state (Vss, 0.65-1.92 L/kg), and terminal disposition half-life (t1/2, 132-356 hrs) are dose independent over the range studied. The excretion of the drug in urine in 336 hrs was less than 6%. After a single dose of 800mg, Cmax, t1/2, AUC0-24 and AUC0-t (AUC from time zero until the last measurable concentration) are 137 ± 29mg/L, 204 ± 162, 1111 ± 316 mg/L/hour, and 2267 ± 762mg/L/hour respectively, and 46 ± 11mg/L, 151 ± 39 hrs, 457 ± 99 mg/L/hour, and 1146 ± 277mg/L/hour after multiple dosing of 200mg/day i.v. for 3 days. Protein binding is 90% (117, 350).
Oritavancin diffuses in skin structures, reaching in blister fluid an AUC of about 20% that measured in serum, and concentrations remaining above 2 mg/L (MIC90 of S. aureus) during 24 h (117). Oritavancin accumulates to high levels within the cells, as demonstrated in in vitro models of phagocytic and non phagocytic cells (accumulation levels, up to 300 times (353), and in vivo, in alveolar macrophages (accumulation levels, up to 50 times 255). Mechanistic studies in cultured macrophages have shown that oritavancin enters cell by adsorptive endocytosis, and is concentrated in the lysosomes from where its efflux is slow. This accumulation and retention within the cells (together with the high level of protein binding) may contribute to the prolonged half-life of the drug.
In clinical trials, oritavancin was used at doses ranging from to 1.5 to 3 mg/kg. Phase III trials were performed with a daily dose 3 mg/kg or 200 mg of with treatment duration of 3-7 days (126, 364).
Telavancin
In healthy volunteers, telavancin pharmacokinetics was linear, with Cmax of 85-97, 151.3, and 202.5 mg/L and steady-state AUC values of 600-700, 1033, and 1165 mg/L/h after the administration of doses of 7.5, 10, and 15 mg/kg, with no gender difference (318, 339, 380).
Despite a high level of protein binding, the elimination half-life is much shorter than that of oritavancin (6.9 to 9.1 h; 31), due to the presence of a polar phosphonate substituant (188, 351).
In the blister fluid, telavancin reaches a Cmax of 16.0 ± 2.0 mg/L, and a AUC of 241 ± 33 mg/L/h, with an half-life of 6.91 ± 0.53 h after administration of 7.5 mg/kg. Over the dosing interval, the mean ratio of AUC obtained in blister fluid compared to that in plasma is about 40% (339).
In volunteers treated with 10 mg/kg, telavancin reaches concentrations of 3.73 + 1.28 mg/L at 8 h and 0.89 +1.03 mg/L at 24 h in ELF, and of 19.0 + 16.8 mg/L at 8 h, 45.0 + 22.4 mg/L at 12 h, and 42.0 + 31.4 mg/L at 24 h in alveolar macrophages, corresponding to a macrophage/plasma ratio of 6.67 at 24 h. Over the entire dosing interval, telavancin is therefore present in ELF and alveolar macrophages at concentrations up to 8-fold and 85-fold, respectively, above its MIC90 for MRSA (0.5 mg/L) (135). A population modeling and Monte Carlo simulation estimate an AUC in the ELF / free plasma ratio of 73 % (204). In models of cultured cells (macrophages and fibroblasts), telavancin accumulates to lower extents (5 times) than oritavancin, but is also located within lysosomes (31).
Telavancin is excreted by renal route for 2/3 and partially metabolized by the liver (major metabolite: hydroxylated form) (104, 380). In patients with renal impairment, exposure to telavancin is increased 2 to 3-fold, justifying dosage adjustments, while no difference in pharmacokinetic profile were noticed in subjects with mild hepatic impairment or in older patients (102, 104, 380).
Phase II clinical trials allowed to set the dose of telavancin to 10 mg/kg administered once daily by intravenous route (335). This dose was used in subsequent phase III trials having led to the approval by the FDA for skin and skin structure infections (336). In patients with impaired renal function, telavancin dosage should be reduced to 75% (7.5 mg/kg) when creatinine clearance is ranging from 30 to 50 ml/min, and to dosage interval should be increased to 48 h when creatinine clearance is < 30 ml/kg (102).
Dalbavancin
In the phase I study, 4 healthy volunteers received a single dose of 140, 350, 500, 630, 840, or 1,120 mg iv infusion and in the multiple dose section, 300 and 30, 400 and 40, 600 and 60, 800 and 80, and 1,000 and 100 mg (193). Cmax and AUC increased in proportion to the dose and the terminal half-life was 181h. In a phase I study, dalbavancin up to 1120 mg/day or 500mg twice a day as a loading dose, followed by 100 mg as a maintenance dose for 6 days was well tolerated, no ototoxicity was observed (63). Dalbavancin has a long elimination half-life of 9-12 days in humans.
In healthy volunteers, dalbavancin Cmax and AUC increase proportionally with the dose (range investigated: 140-1120 mg), with values of 312 mg/L (125) and 27,103 mg/L/h (22,967-27,299) at the highest dose tested. Half-life was 181 h and overall clearance 47.2 ml/h, with about 1/3 excreted in the urine (193). 42% of the unchanged drug is excreted in the urine after 42 days, suggesting that no dosage adjustment is needed in case of renal insufficiency (99). Volume of distribution at steady state is 15.7 L. Dalbavancin is > 90% protein bound (66). Bactericidal activity remained detectable after 7 days in the serum of those receiving doses of 500 mg or more.
The tissue distribution of dalbavancin was studied in the rat. The highest concentrations were recovered in the kidney and the liver after 24 h, but most of the tissues showed concentrations higher than in the plasma after 3 days, with measurable levels still found in the kidneys, liver, brown fat, and skeletal muscle after 14 days (66).
As the other lipoglycopeptides, dalbavancin penetrates well in the blister fluid, reaching maximal concentration of 67.3 mg/L and a AUC7 days of 6438 mg/L/h , corresponding to a mean penetration of 60%. These concentrations are well above the MIC90 of target organisms during 7 days (247). The elimination of dalbavancin has been characterized in a population pharmacokinetic analysis performed on 532 patients, the majority of which (78.4%) received dalbavancin intravenously as a 1000-mg dose on day 1 and a single 500-mg dose on day 8. A 2-compartment model with first-order elimination provided the best fit to the data. The clearance of dalbavancin was influenced by body surface area (as a predictor of central volume of distribution) and creatinine clearance, but these two parameters described less than 25% of the interpatient variability. Dalbavancin clearance was not influenced by metabolic substrates, inhibitors, or inducers of cytochrome P450 or concomitant medications (57). The elimination of dalbavancin is neither modified in patients with variable level of hepatic impairment (100).
Dalbavancin is not removed by hemodialysis, asking the question of the way to eliminate a molecule with such a prolonged half-life from the organism in case of severe allergy or adverse reaction.
The selection of a weekly dosage regimen for dalbavancin was based on pharmacokinetic and pharmacodynamic data from animal models and humans studies. Animal data suggested that dalbavancin concentrations> 5 mg/L are necessary for extended in vivo activity (157). In humans, bactericidal activity of the serum was demonstrated for concentrations > 20 mg/L (193). Together with Monte-Carlo simulations of pharmacokinetics based on data collected in healthy volunteers, these data have led to propose a therapeutic scheme consisting in a dose of 1000 mg at day one and of 500 mg at day 8 (97). This dose was evaluated in phase II trial in comparison with a single dose of 1100 mg (317) and then selected for Phase III trial (162).
ADVERSE EFFECTS
Vancomycin
Adverse events were more common in the past, but now the modern purified formulation of vancomycin has an improved safety profile. Red-man syndrome is the most frequently reported dose and infusion rate related event associated with vancomycin. It occurs 10-20 minutes after the start of infusion. Patients usually complain of pruritus and flushing of skin over the upper part of the body. Hypotension and musculoskeletal pain is also reported. Polk et al (269) found that this syndrome was a result of non-immunologically mediated, vancomycin–induced histamine release. Symptoms resolve within 20 min of the infusion being stopped but may persist for several hours (76). These events can be prevented by administering vancomycin over at least 60 minutes in dilute solution and is not a contraindication for future use of the antibiotic (112, 227). Life-threatening allergic reactions although rare, have been reported (76, 113). So, when hypotension and urticarial rash develops, hypersensitivity should be considered.
The incidence of nephrotoxicity reported was as high as 25% in the initial clinical trials (167, 349), but these studies were complicated by the number of confounding variables such as concomitant use of nephrotoxic drugs, hypotension and heart failure. In later studies of patients receiving vancomycin alone, the rate of reversible nephrotoxicity was found to be 0-5% (113, 327). However, the risk of nephrotoxicity of aminoglycosides is enhanced by vancomycin when the two are given together (305). Goetz et al (128) performed a meta-analysis of studies published between 1966 and 1991 of adult patients receiving vancomycin plus an aminoglycoside or either drug alone. They concluded that the incidence of nephrotoxicity with combination therapy is 13.3% greater than with vancomycin alone and 4.3% greater than with aminoglycoside alone. In a retrospective case review of > 200 patients with proven Gram-positive bacteraemia patients who developed nephrotoxicity had higher vancomycin levels before the onset of toxicity (388) and a retrospective review of cancer patients receiving vancomycin associated troughs of >15mg/L with greater toxicity (180). Vancomycin dose is also predictive of toxicity. In a population of 246 patients stratified according to the daily dose of vancomycin received, patients taking ≥ 4 g vancomycin/day, having a total body weight of ≥ 101.4 kg, an estimated creatinine clearance of ≤ 86.6 ml/min, and residing in the intensive care unit developed more rapidly nephrotoxocity (205). In patients receiving a continuous infusion of vancomycin, the probability of developing nephrotoxicity was associated to sustain serum levels > 28 mg/L (156). It therefore appears that dose escalation in response to MIC increase should be undertaken with caution in both the discontinuous or continuous modes of administration, to avoid excessive risk of toxicity.
The balance of evidence suggests that vancomycin can cause renal impairment. Vancomycin is eliminated by glomerular filtration, as renal function declines vancomycin levels increase, so it may be important to monitor serum levels of the drug but dose adjustment is central.
The molecular mechanism of vancomycin nephrotoxicity has on been poorly explored. The drug accumulates in the lysosomes (33) and causes oxidative stress and activates an inflammatory response (96, 248).
Vancomycin associated ototoxicity like tinnitus, vertigo and hearing loss have been reported, but these were associated with high serum levels (> 40mg/L) of the drug or concurrent use of ototoxic drugs, conditions such as meningitis that can cause hearing loss or high serum levels in older patients (119, 123, 327, 346). Importantly, a review of the literature by Brummett et al (55) concluded that the ototoxicity of vancomycin has been overrated, and very few cases of ototoxicity due to vancomycin have occurred.
Vancomycin associated neutropenia has been reported after patients have been treated for several weeks. Reversal of neutropenia occurs after stopping the antibiotic (113, 52, 371). There is no clear relationship between serum levels and neutropenia. Cross reaction with teicoplanin is possible (155). Thrombocytopenia have also been reported in patients receiving vancomycin (359).
Narita, M. Vancomycin-Induced Neutropenia.
Phlebitis occurs commonly when vancomycin is infused through peripheral veins. Drug fever and immunologically mediated rashes have also been reported (113, 327). Cases of severe hypotension and cardiac arrest after bolus administration of vancomycin appear in the literature (127, 225). Diarrhea, nausea, vomiting and abnormal liver function tests have also been reported in clinical trials involving vancomycin (373).
Teicoplanin
Teicoplanin is generally well tolerated by intramuscular or intravenous use. Clinical trials and postmarketing experience in Europe indicate that one or more adverse events were experienced by 10.3% of 3,377 patients treated with teicoplanin (90). The most common adverse events were hypersensitivity (2.6%), abnormal liver function (1.7%), fever (0.8%), local intolerance (1.6%), abnormal renal function (0.7%) and ototoxicity (0.3%). Thrombophlebitis during intravenous administration is common at higher doses of teicoplanin, and pain with intramuscular injection is minimal. In a meta-analysis of 11 comparative clinical trials, adverse events occurred significantly more often with vancomycin (21.9%) than with teicoplanin (13.9%) (381).
In humans nephrotoxicity is less common with teicoplanin than with vancomycin when administered with an aminoglycoside. At a dose of 6mg/Kg/day, clinical trials show a lower incidence of nephrotoxicity with teicoplanin plus an aminoglycoside than with vancomycin plus an aminoglycoside (225, 346). However, in comparative US trials of vancomycin and teicoplanin (n=823 patients), both agents had equivalent nephrotoxicity (14).
The incidence of ototoxicity associated with teicoplanin is extremely low. Greenberg, 1990 reported a few patients who developed tinnitus or a mild loss of high-frequency hearing, but these patients received 15mg per Kg per day of teicoplanin and the trough serum levels in these patients was 41mg/L.
Patients developing red man syndrome with intravenous vancomycin tolerate teicoplanin well (270, 324). However allergic cross-reactivity may occur between vancomycin and teicoplanin (137). Since uncertainty exists about cross-reactivity and because of its long half-life, teicoplanin should probably not be administered to patients with serious hypersensitivity reactions to vancomycin. Thrombocytopenia due to teicoplanin use have been reported (342) which is more likely with high prolonged doses and is associated with trough serum concentrations of >60mg/L (13% vs 5%; (373). A number of other adverse events have been reported in clinical trials, the most common being rash, diarrhea, nausea and vomiting.
Oritavancin
Data from clinical trials did not evidence any significant difference in the safety profile of oritavancin versus its comparators. Specific investigations have been conducted and published as abstracts. Single doses of oritavancin (200 or 800 mg) did not cause any significant change in cardiac repolarization as assessed by electroradiography (120).
Telavancin
In clinical trials, telavancin was generally well tolerated. In the ATLAS phase II study, with incidence of adverse effects leading to treatment discontinuation of 8% vs 6% and of serious adverse effects of 7% vs 4% for telavancin versus vancomycin, respectively (336). Mild taste disturbance described as metallic or soapy taste (33% vs 7%), nausea (27% vs 15%), and vomiting (14% vs 7%) were more frequent in the telavancin treatment group and pruritus (6% vs 3%) in the vancomycin group. Only one patient experienced a QTc prolongation of more than 500 msec and 10 patients, of > 60 msec, in the telavancin group, with however no associated cardiac events. Serum creatinine concentration elevation at least 50% higher than baseline was observed in 6% vs 2% of the patients treated by telavancin vs. vancomycin. On this basis EMEA stated that current data with telavancin are insufficient to conclude to a positive benefit-risk balance for telavancin in the cSSTI indication.
Studies in cultured cells showed that telavancin did not induce more deposition of lipidic material in the lysosomes than vancomycin, despite a higher degree of accumulation (31).
Dalbavancin
The safety profile of dalbavancin has been evaluated in more than 1000 patients in Phase II/III trials, and was found comparable with that of the comparators (317, 162, 277), with in particular no evidence of renal or hepatic toxicity. A small percentage of patients (3.9% for the dalbavancin arm and 3.2% for the linezolid arm) discontinued treatment because of adverse events. Serious adverse events were reported by 8% of patients overall (7.5% in the dalbavancin arm and 8.5% in the linezolid arm). Nausea and diarrhea were the most frequently reported (about 3% of the patients in Phase III study) adverse effects. In a small group of volunteers, dalbavancin did not significantly modify the intestinal flora (251).
MONITORING REQUIREMENTS
Monitoring serum vancomycin concentrations is well entrenched but perhaps unjustified in clinical practice. When vancomycin was first used in the 1950’s, due to impure drug formulations, hearing loss was noted in a patient with renal dysfunction who had serum vancomycin concentration of 80-95µg/ml (123), the authors suggested monitoring serum concentrations to maintain them below 40mg/L to prevent drug toxicities. Since then vancomycin serum concentrations are measured routinely to avoid toxic concentrations and to maintain therapeutic levels. The utility of routine vancomycin therapeutic monitoring has been questioned since the early 1990s (65).
The two forms of toxicities which could be avoided by monitoring serum vancomycin concentrations are nephrotoxicity and ototoxicity. There are reports of reversible ototoxicity associated with serum drug concentrations of greater than 40mg/L and often pre-existing renal impairment but the relationship between vancomycin use and ototoxicity is unclear (see above). Reversible nephrotoxicity has been reported in 5% of treated cases (113, 108, 153,346), but these findings were from uncontrolled observational studies and should be considered as evidence of a weak association between vancomycin and toxicity.
Cantu et al (65) have suggested that vancomycin should be dosed on the basis of patient’s ages, weight and estimated renal function. Subsequently, a study by Saunders (312) showed that peaks were below 40mg/L when troughs were less than 15mg/L, and suggested monitoring only trough concentrations, and that levels of 10-12mg/L be considered early signs of accumulation and may need dose modifications. Two prospective studies of the impact of therapeutic drug monitoring (TDM) on nephrotoxicity have been published. A randomized trial in hematology patients indicated reduced nephrotoxicity in the therapeutic drug monitoring group and a cohort study in non neutropenic, non ICU patients support this finding (116, 369). The therapeutic trough levels of 5-10mg/L or 5-15mg/L and peak of 20-30mg/L (76, 124, 214, 276, 306) currently recommended are not based on controlled trials in which serum concentrations have been related to clinical response. However the initial vancomycin dose should be selected using guidelines based on age and renal function. Some believe the best approach should be based on population pharmacokinetics and clearance (182, 236, 276, 306). In general, serum concentrations should be monitored after 4-5 days of therapy in non-renal patients and earlier if patient is receiving other nephrotoxic drugs (115, 214). The recent guidelines from the American Society of Health-System Pharmacists, the Infectious Diseases Society of America and the Society of Infectious Diseases Pharmacists indicate that there are limited data suggesting a direct correlation between specific serum levels and toxicity, because of variable definition of toxicity in the literature and of the frequent coadministration of other nephrotoxic drugs. They therefore recommend considering as vancomycin-induced nephrotoxicity the occurrence of two or three consecutive high serum creatinine levels (> 50 % baseline) after several days of vancomycin therapy in the absence of alternative explanation (302). Furthermore, they recommend trough level monitoring only, especially in patients receiving doses producing trough > 15-20 mg/L, or other nephrotoxic drugs, as well as in patients with unstable renal function. All patients receiving prolonged therapy (> 3-5 days) should be monitored after the fourth dose, or once-a-week if vancomycin is administered in continuous infusion. Frequent monitoring is required in hemodynamically instable patients. In contrast, monitoring is useless for prevention of ototoxicity.
DRUG INTERACTIONS
With glycopeptides, the main risk of interaction relies on the coadministration of nephrotoxic drugs. This is clearly demonstrated with aminoglycosides (128). However, animal models suggest that cisplatin or fosfomycin might reduce vancomycin nephrotoxicity (240). Only a few studies have examined the possibility of drugs interactions with lipoglycopeptides. No interactions were found between dalbavancin and inhibitors or inductors of cytochromes P450 (57,193).
Telavancin was shown to interfere with coagulation assays and dye test for urinary proteins (186).
CLINICAL INDICATIONS
Vancomycin
Vancomycin is used in the parenteral form to treat serious infections caused by Gram-positive organisms. It is the main stay for intravenous treatment of infections caused by methicillin resistant S. aureus (MRSA). It is also used to treat Gram-positive infections in patients who are unable to tolerate beta-lactam antibiotics but this may not represent best practice. Vancomycin was used to treat infections caused by methicillin-sensitive S. aureus (MSSA) prior to the discovery of penicillinase-resistant penicillins. Later studies showed that the success rate of vancomycin treatment of MSSA endocarditis is 60-75% (5) and also, the duration of bacteremia was prolonged when vancomycin was used to treat MSSA compared to beta-lactams (200).
If vancomycin is used alone to treat MRSA endocarditis clinical response is slow and mortality was increased in MSSA pneumonia with bacteremia when cloxacillin and vancomycin were compared (199). Combination therapy has been used to improve the efficacy of vancomycin and the addition of aminoglycosides, fusidic acid, or dalfopristin/quinupristin, or rifampicin should be considered (368).
Coagulase-negative staphylococci are the most common causes of infection of prosthetic devices like cardiac valves, artificial hips, CSF shunts and vascular grafts. The combination of vancomycin, rifampicin and an aminoglycoside is recommended for the treatment of prosthetic valve endocarditis caused by methicillin-resistant coagulase-negative staphylococci (107, 174).
Vancomycin can also be used to treat endocarditis caused by susceptible strains of enterococci in penicillin-allergic patients, but it must be used in combination with an aminoglycoside. It is the drug of choice for infections caused by resistant organisms like Corynebacterium spp (131, 158).
Vancomycin has been used to treat CNS shunt infections caused by susceptible organisms. However, removal of the device and intrathecal administration of the drug is generally needed for cure (133, 139).
Vancomycin is used for treatment of pseudomembranous colitis caused by C. difficile. Oral administration of 125 to 250 mg results in luminal levels of 500-2000 mg/L, which is high above the MIC for C difficile [0.2 mg/L] (106). Coagulase negative staphylococci and other Gram-positive organisms, is the most common cause of infections in neutropenic patients, due to the use of more intensive chemotherapy regimens and long-term indwelling central venous catheters. Vancomycin is used for treatment of proven Gram-positive infections but the use of vancomycin in the initial empirical treatment of febrile neutropenic patients is controversial. Several randomized trials showed no decrease in morbidity or mortality when vancomycin was not used as part of initial therapy (109, 279, 295).
Vancomycin is useful for the prevention of bacterial endocarditis in high risk, penicillin-allergic patients undergoing dental, oral or upper respiratory tract procedures and American Heart Association also recommends vancomycin in combination with gentamicin for gastro-intestinal and genitourinary procedures. Vancomycin is also used for surgical prophylaxis when prosthetic materials are being implanted into patients known or suspected of being colonized with MRSA. Operations which may be considered for vancomycin prophylaxis are vascular graft, prosthetic joint and neurosurgical implants.
Teicoplanin
Teicoplanin can be used as an alternative to vancomycin in the treatment of many Gram-positive infections, but its use is limited to a greater degree than vancomycin by bacterial resistance, especially in coagulase negative Staphylococci.. It can be used as empirical treatment, in immunocompromised patients and in bacteremias secondary to invasive devices like intravascular catheters, which are usually caused by coagulase-negative staphylococci. It is a safe alternative for prophylaxis and therapy of infections in patients with serious allergic reactions to beta-lactam antibiotics.
Two large, open, multicenter studies have evaluated the efficacy of teicoplanin in the treatment of a variety of Gram-positive infections including bone and joint, skin and soft tissue, lung and endocarditis. In these studies, clinical efficacy was 87 to 92% and bacteriologic efficacy was 79 to 85% (184, 201). Teicoplanin has been used effectively in the treatment of staphylococcal, streptococcal and enterococcal endocarditis when combined with an aminoglycoside (275). However, substantial failure rates have been seen in the treatment of S. aureus endocarditis and intravenous drug abusers, especially when lower doses of the drug are used (125, 304). This problem may be partially overcome by using higher maintenance doses (12-30mg/kg/day) or by addition of aminoglycosides (373). Treatment is given for 4-6 weeks. Randomized trials have failed to show any difference in efficacy of teicoplanin at a daily dose of 6mg/Kg/day and vancomycin either as monotherapy in immunocompromised (354) or in combination with other antibiotics in febrileneutropenic patients with suspected vascular catheter infections (73, 181, 325). Teicoplanin is also an effective treatment for Gram-positive bacteremia.
Lewis et al (201) reported a cure rate of 96% in skin and soft tissue infections with Gram-positive organisms using a daily dose of 400mg teicoplanin. In In septic arthritis and osteomyelitis a dose of 12mg/kg/day may be needed (192).
Oral teicoplanin is a possible alternative for treatment of C. difficile colititis. Teicoplanin 100mg twice-daily orally for 10 days appeared to be equally effective to vancomycin (94). However more comparative studies are needed before recommendations can be made about the utility of teicoplanin in the treatment of C. difficile colitis.
Teicoplanin can be used for prophylaxis of endocarditis in penicillin-allergic patients or when vancomycin or other antibiotics are not tolerated (322). Wall et al (361) used a single dose of 400mg teicoplanin in orthopedic surgical wound prophylaxis and found it to be as effective as two doses of cefuroxime. However, in cardiac surgery flucloxacillin and tobramycin was found to be superior to teicoplanin (375). Teicoplanin has been used in the treatment of pediatric patients with septicemia, lung infections, skin and soft tissue, bone and joint infections and in patients with neutropeniaand fever with high clinical response rate (88, 262). Teicoplanin is suitable for outpatient treatment of some community-acquired Gram-positive skin and soft tissue infections (88), or initially hospitalized patients subsequently treated as outpatients for acute and chronic osteomyelitis (192, 82).
Oritavancin
Published Phase II trial document the efficacy of oritavancin in S. aureus blood stream infections (209). This open-label randomized trial showed equivalence between oritavancin (5-10 mg/kg once daily) and comparators (vancomycin 15 mg/kg twice daily or a beta-lactam) for 10-14 days, with higher clinical and bacteriological success in the 10 mg/kg cohort. Further pharmacodynamic analysis of this study suggested that the success correlates with the free-drug time above the MIC (43).
Oritavancin has been evaluated in Phase II and Phase III trials in the treatment of complicated skin and skin structures infections caused by Gram-positive organisms, including MRSA. Two randomized, doubled-blind, multicenter trials were published as abstracts. In the first study, 517 adult patients received 1.5 and 3.0 mg/kg/day of oritavancin intravenously for 3-7 days or vancomycin 10-15mg/kg twice/day intravenously for 3-7 days followed by oral cephalexin 500-1000mg twice/day to complete 10-14 days of therapy. In the first follow-up visit (day 28 + 7) respective success rates were 76%, 76% and 80% in 384 clinically and 256 bacteriologically evaluable patients. The overall adverse events were not significantly different among the three groups but tremors, pulmonary embolism or thrombosis were seen in the oritavancin group (364). In the second trial, patients were randomized (2:1) to receive intravenous oritavancin 200mg/day for 3-7 days followed by oral placebo, or i.v. vancomycin 15mg/kg twice/day for 3-7 days followed by oral cephalexin 1000mg twice/day. Both regimens were given for a total of 10-14 days. A total of 1246 patients received study drugs of which 1000 were clinically evaluable. The clinical cure rates were 79% and 76% for oritavancin and vancomycin-cephalexin group respectively and in the bacteriologically evaluable (686) patients the bacteriologic eradication rates were 75% and 73% for oritavancin and vancomycin-cephalexin respectively (126, 364).
Ongoing trials are also examining oral oritavancin in the treatment of Clostridium difficile associated diseases.
Telavancin
Telavancin has been approved in November 2008 by the FDA for the treatment of complicated skin and soft tissues infections, on the basis of the data collected in phase II (FAST 1 and 2) and phase III (ATLAS 1 and 2) studies.
In FAST 1 study (337), telavancin 7.5 mg/kg once daily was compared to standard therapy (1 g vancomycin twice daily, 0.5-1 g cloxacillin or 2 g nafcillin or oxacillin 4 times a day) in 167 patients suffering from skin and skin structure infections and treated for 7 to 14 days. Cure rates and microbiological eradication were similar in both arms (~ 80%) but higher for MRSA in the telavancin group (82 vs 69%). This difference was not significant because of the small number of patients infected by MRSA (about 25 in each arm). FAST 2 enrolled 195 patients to evaluate an increased dose of telavancin (10 mg/kg) vs the same comparators, with however 93% of patients receiving vancomycin (335). Cure rate were similar in both arms (96 % vs 90%, respectively), including in patients infected by MRSA, but bacterial eradication was significantly better in the telavancin group (92 % vs 78 % in the microbiologically evaluable population and 89% vs 68% in the MRSA subgroups).
In ATLAS studies, telavancin 10 mg/kg once daily was compared to vancomycin 1g twice daily in 1867 patients (336), Similar clinical success (88% vs 87% in the global population and 91% vs 86% in MRSA subgroups) and microbiologic eradication (90% vs 85% for MRSA) were obtained. The trend to higher success in the MRSA subgroup was not significant because of the small number of patients (953) (74). A pharmacoeconomic evaluation of the ATLAS study also suggests that telavancin may be more cost-effective than vancomycin, especially in the MRSA subpopulation (185).
Still ongoing phase III studies are evaluating telavancin in the treatment of hospital acquired pneumonia including patients with ventilator-associated pneumonia caused by S. aureus (ATTAIN 1 and 2 studies). In both studies, telavancin achieved the non-inferiority objective, with cure rates of 82.7% for telavancin versus 80.9% for vancomycin in the clinically-evaluable population, and of 82% versus 74% in the microbiologically evaluable patients infected with MRSA. Moreover, in clinically evaluable patients with ventilator-associated pneumonia, the cure rate was 80% for telavancin compared with 68% for vancomycin (Preliminary results were presented at the 48th ICAAC/56th IDSA meeting) (77, 296, 297). A new drug application was submitted to the FDA for this indication in January 2009.
Dalbavancin
Dalbavancin has been studied in skin and skin structure as well as in bloodstream infections (see for review 40), but the application submitted for the first indication was withdrawn in 2008 from both the FDA and the EMEA.
One 2 Phase II and one Phase III trials evaluating the efficacy of dalbavancin in skin and skin structure infections have been published so far. In the Phase II trial, dalbavancin 1100 mg as a single dose or 1000 mg at day 1 followed by 500 mg at day 8 was compared to standard treatments (vancomcyin, cephalosporins, clindamycin, piperacillin-tazobactam, linezolid); additional anti-Gram-negative or anti-anaerobic coverage was added is judged necessary (317). Each group counted about 20 patients Gram-positive bacteria were isolated in 66% of the patients, among which 83% of staphylococci. Success rates were 91% for the 2-dose group vs 75% for the one-dose group and 81% for the comparators. A same trend was observed for microbiological success in MRSA infections (80%, 50% and 50%, respectively), but with only 2 to 5 patients in each subgroup. In the Phase III trial enrolling 854 patients (2:1 randomization), dalbavancin (1000 mg at day 1 and 500 mg at day 8) was compared to linezolid 600 mg for 14 days, with at least 24 h of intravenous therapy (162). 90% of bacterial isolates were S. aureus, among which 51% were MRSA. Similar clinical success (88.9% vs 91.2%) and bacterial eradication (91% vs 89%) were obtained in both treatment groups.
Another Phase II study compared dalbavancin (1000 mg at day 1 and 500 mg at day 8) to vancomycin (1000 mg twice daily for 14 days) in 75 patients with catheter-related bloodstream infections (277). MRSA accounted for 19.2% and 32.1% of isolates in the dalbavancin and vancomycin groups. MICs of all bacterial isolated in this study were < 0.25 mg/L for both drugs (132). Overall success rate and microbiological eradication were obtained in 87 and 96% of the patients in the dalbavancin group, and 50% in the vancomycin group. This study suffers however from severe limitations (40) that include (a) the choice a second line comparator for MSSA infections, (b) the disparity of the number of MRSA in the two arms, and (c) the fact that the study was designed to demonstrate efficacy and not superiority.
REFERENCES
1. Abadia PL, Courvalin P, Perichon B. vanE gene cluster of vancomycin-resistant Enterococcus faecalis BM4405. J Bacteriol 2002;184(23):6457-6464. [PubMed]
2. Ackerman BH, Vannier AM, Eudy EB. Analysis of vancomycin time-kill studies with Staphylococcus species by using a curve stripping program to describe the relationship between concentration and pharmacodynamic response. Antimicrob Agents Chemother 1992; 36(8):1766-1769.[PubMed]
3. Aeschlimann JR, Hershberger E, Rybak MJ. Analysis of vancomycin population susceptibility profiles, killing activity, and postantibiotic effect against vancomycin-intermediate Staphylococcus aureus. Antimicrob Agents Chemother 1999; 43(8):1914-1918. [PubMed]
4. Aldridge KE, Schiro DD, Varner LM. In vitro antistaphylococcal activity and testing of RP 59500, a new streptogramin, by two methods. Antimicrob Agents Chemother 1992; 36(4):854-855.[PubMed]
5. Alexander MR. A review of vancomcyin after 15 years of use. Drug Intell Clin Pharm 1974; 8:520.
6. Aligholi M, Emaneini M, Jabalameli F, Shahsavan S, Dabiri H, Sedaght H. Emergence of high-level vancomycin-resistant Staphylococcus aureus in the Imam Khomeini Hospital in Tehran. Med Princ Pract 2008; 17(5):432-434.[PubMed]
7. Allen NE, Nicas TI. Mechanism of action of oritavancin and related glycopeptide antibiotics. FEMS Microbiol Rev 2003; 26(5):511-532.[PubMed]
8. Allen GP, Cha R, Rybak MJ. In vitro activities of quinupristin-dalfopristin and cefepime, alone and in combination with various antimicrobials, against multidrug-resistant staphylococci and enterococci in an in vitro pharmacodynamic model. Antimicrob Agents Chemother 2002; 46(8):2606-2612.[PubMed]
9. Alos JI, Garcia-Canas A, Garcia-Hierro P, Rodriguez-Salvanes F. Vancomycin MICs did not creep in Staphylococcus aureus isolates from 2002 to 2006 in a setting with low vancomycin usage. J Antimicrob Chemother 2008; 62(4):773-775.[PubMed]
10. Andes D, Craig WA. In vivo pharmacodynamic activity of the glycopeptide dalbavancin. Antimicrob Agents Chemother 2007; 51(5):1633-1642.[PubMed]
11. Anderson RC, Griffith RS, Higgins HMJ, Pattinga CD. Symposium: how a drug is born. Cincinnati Jounal of Medicine 1961; 42:49-60. [PubMed]
12. Anderson VR, Keating GM. Dalbavancin. Drugs 2008; 68(5):639-648. [PubMed]
13. Antony KK, Lewis EW, Kenny MT, Dulworth JK, Brackman MB, Kuzma R et al. Pharmacokinetics and bioavailability of a new formulation of teicoplanin following intravenous and intramuscular administration to humans. J Pharm Sci 1991; 80(6):605-607. [PubMed]
14. Appelbaum PC. Reduced glycopeptide susceptibility in methicillin-resistant Staphylococcus aureus (MRSA). Int J Antimicrob Agents 2007; 30(5):398-408. [PubMed]
15. Arhin FF, Sarmiento I, Belley A, McKay GA, Draghi DC, Grover P et al. Effect of polysorbate 80 on oritavancin binding to plastic surfaces: implications for susceptibility testing. Antimicrob Agents Chemother 2008;52(5):1597-1603.[PubMed]
16. Arias CA, Courvalin P, Reynolds PE. vanC cluster of vancomycin-resistant Enterococcus gallinarum BM4174. Antimicrob Agents Chemother 2000; 44(6):1660-1666. [PubMed]
17. Arias CA, Martin-Martinez M, Blundell TL, Arthur M, Courvalin P, Reynolds PE. Characterization and modelling of VanT: a novel, membrane-bound, serine racemase from vancomycin-resistant Enterococcus gallinarum BM4174. Mol Microbiol 1999;31(6):1653-1664. [PubMed]
17a. Arthur M, Molinas C, Courvalin P. The VanS-VanR two-component regulatory system controls synthesis of depsipeptide peptidoglycan precursors in Enterococcus faecium BM4147. J Bacteriol 1992;174(8):2582-91. [PubMed]
18. Arthur M, Molinas C, Depardieu F, Courvalin P. Characterization of Tn1546, a Tn3-related transposon conferring glycopeptide resistance by synthesis of depsipeptide peptidoglycan precursors in Enterococcus faecium BM4147. J Bacteriol 1993; 175(1):117-127. [PubMed]
19. Arthur M, Reynolds P, Courvalin P. Glycopeptide resistance in enterococci. Trends Microbiol 1996;4(10):401-407.[PubMed]
20. Arthur M, Depardieu F, Snaith HA, Reynolds PE, Courvalin P. Contribution of VanY D,D-carboxypeptidase to glycopeptide resistance in Enterococcus faecalis by hydrolysis of peptidoglycan precursors. Antimicrob Agents Chemother 1994; 38(9):1899-1903. [PubMed]
21. Arthur M, Depardieu F, Molinas C, Reynolds P, Courvalin P. The vanZ gene of Tn1546 from Enterococcus faecium BM4147 confers resistance to teicoplanin. Gene 1995; 154(1):87-92. [PubMed]
22. Attwood RJ, LaPlante KL. Telavancin: a novel lipoglycopeptide antimicrobial agent. Am J Health Syst Pharm 2007; 64(22):2335-2348. [PubMed]
23. Bailey EM, Rybak MJ, Kaatz GW. Comparative effect of protein binding on the killing activities of teicoplanin and vancomycin. Antimicrob Agents Chemother 1991; 35(6):1089-1092. [PubMed]
24. Bailey J, Summers KM. Dalbavancin: a new lipoglycopeptide antibiotic. Am J Health Syst Pharm 2008; 65(7):599-610.[PubMed]
25. Baines SD, O'Connor R, Saxton K, Freeman J, Wilcox MH. Comparison of oritavancin versus vancomycin as treatments for clindamycin-induced Clostridium difficile PCR ribotype 027 infection in a human gut model. J Antimicrob Chemother 2008; 62(5):1078-1085. [PubMed]
26. Baldassarre JS, Ingerman MJ, Nansteel J, Santoro J. Development of Listeria meningitis during vancomycin therapy: a case report. J Infect Dis 1991; 164(1):221-222. [PubMed]
27. Baltch AL, Smith RP, Ritz WJ, Bopp LH. Comparison of inhibitory and bactericidal activities and postantibiotic effects of LY333328 and ampicillin used singly and in combination against vancomycin-resistant Enterococcus faecium. Antimicrob Agents Chemother 1998; 42(10):2564-2568. [PubMed]
28. Bannerman TL, Wadiak DL, Kloos WE. Susceptibility of Staphylococcus species and subspecies to teicoplanin. Antimicrob Agents Chemother 1991; 35(9):1919-1922. [PubMed]
29. Barcia-Macay M, Seral C, Mingeot-Leclercq MP, Tulkens PM, Van Bambeke F. Pharmacodynamic evaluation of the intracellular activities of antibiotics against Staphylococcus aureus in a model of THP-1 macrophages. Antimicrob Agents Chemother 2006; 50(3):841-851. [PubMed]
30. Barcia-Macay M, Lemaire S, Mingeot-Leclercq MP, Tulkens PM, Van Bambeke F. Evaluation of the extracellular and intracellular activities (human THP-1 macrophages) of telavancin versus vancomycin against methicillin-susceptible, methicillin-resistant, vancomycin-intermediate and vancomycin-resistant Staphylococcus aureus. J Antimicrob Chemother 2006; 58(6):1177-1184. [PubMed]
31. Barcia-Macay M, Mouaden F, Mingeot-Leclercq MP, Tulkens PM, Van Bambeke F. Cellular pharmacokinetics of telavancin, a novel lipoglycopeptide antibiotic, and analysis of lysosomal changes in cultured eukaryotic cells (J774 mouse macrophages and rat embryonic fibroblasts). J Antimicrob Chemother 2008; 61(6):1288-1294.[PubMed]
32. Bayer AS, Lam K. Efficacy of vancomycin plus rifampin in experimental aortic-valve endocarditis due to methicillin-resistant Staphylococcus aureus: in vitro-in vivo correlations. J Infect Dis 1985; 151(1):157-165. [PubMed]
33. Beauchamp D, Gourde P, Simard M, Bergeron MG. Subcellular localization of tobramycin and vancomycin given alone and in combination in proximal tubular cells, determined by immunogold labeling. Antimicrob Agents Chemother 1992;36(10):2204-2210. [PubMed]
34. Beauregard DA, Williams DH, Gwynn MN, Knowles DJ. Dimerization and membrane anchors in extracellular targeting of vancomycin group antibiotics. Antimicrob Agents Chemother 1995; 39(3):781-785. [PubMed]
35. Beckers B, Brodersen HP, Stolpmann RM, Jansen G, Larbig D. Efficacy and pharmacokinetics of teicoplanin in hemodialysis patients. Infection 1993; 21(1):71-74.[PubMed]
36. Belley A, Neesham-Grenon E, Arhin FF, McKay GA, Parr TR, Jr., Moeck G. Assessment by time-kill methodology of the synergistic effects of oritavancin in combination with other antimicrobial agents against Staphylococcus aureus. Antimicrob Agents Chemother 2008; 52(10):3820-3822. [PubMed]
37. Belley A, Neesham-Grenon E, McKay G, Arhin FF, Harris R, Beveridge T et al. Oritavancin kills stationary-phase and biofilm Staphylococcus aureus in vitro. Antimicrob Agents Chemother 2008. [PubMed]
38. Belley A, Harris R, Beveridge T, Parr T, Jr., Moeck G. Ultrastructural effects of oritavancin on methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus. Antimicrob Agents Chemother 2009; 53(2):800-804. [PubMed]
39. Bellomo R, Ernest D, Parkin G, Boyce N. Clearance of vancomycin during continuous arteriovenous hemodiafiltration. Crit Care Med 1990; 18(2):181-183. [PubMed]
40. Bennett JW, Lewis JS, Ellis MW. Dalbavancin in the treatment of complicated skin and soft-tissue infections: a review. Ther Clin Risk Manag 2008; 4(1):31-40. [PubMed]
41. Benton B, Breukink E, Visscher I, Debabov BLC, Janc J, Mammen M et al. Telavancin inhibits peptidoglycan biosynthesis through preferential targeting of transglycosylation: evidence for a multivalent interaction between telavancin and lipid II. 17th European Congress of Clinical Microbiology and Infectious Diseases, Munich, Germany 2007;O160.
42. Berger-Bachi B, Strassle A, Kayser FH. Characterization of an isogenic set of methicillin-resistant and susceptible mutants of Staphylococcus aureus. Eur J Clin Microbiol 1986; 5(6):697-701. [PubMed]
43. Bhavnani SM, Passarell JA, Owen JS, Loutit JS, Porter SB, Ambrose PG. Pharmacokinetic-pharmacodynamic relationships describing the efficacy of oritavancin in patients with Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 2006; 50(3):994-1000. [PubMed]
44. Bhavnani SM, Rubino CA, Forrest A, Okusania OO, Craig WA, Moeck G et al. Pharmacokinetic-Pharmacodynamic Target Attainment as Decision Support for Oritavancin Susceptibility Breakpoints for Staphylococcus aureus. 48th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC , A994. 2008.
45. Bhavnani SM, Iyer V, Hammel JP, Forrest A, Rubino CA, Van Wart A et al. Pharmacokinetics-Pharmacodynamics (PK-PD) of Oritavancin (ORI) Efficacy in Patients with Complicated Skin and Skin Structure Infections (cSSSI). 48th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC , A1893. 2008.
46. Biavasco F, Manso E, Varaldo PE. In vitro activities of ramoplanin and four glycopeptide antibiotics against clinical isolates of Clostridium difficile. Antimicrob Agents Chemother 1991; 35(1):195-197. [PubMed]
47. Biavasco F, Vignaroli C, Lupidi R, Manso E, Facinelli B, Varaldo PE. In vitro antibacterial activity of LY333328, a new semisynthetic glycopeptide. Antimicrob Agents Chemother 1997; 41(10):2165-2172. [PubMed]
48. Bibler MR, Frame PT, Hagler DN, Bode RB, Staneck JL, Thamlikitkul V et al. Clinical evaluation of efficacy, pharmacokinetics, and safety of teicoplanin for serious gram-positive infections. Antimicrob Agents Chemother 1987; 31(2):207-212. [PubMed]
49. Billeter M, Zervos MJ, Chen AY, Dalovisio JR, Kurukularatne C. Dalbavancin: a novel once-weekly lipoglycopeptide antibiotic. Clin Infect Dis 2008; 46(4):577-583. [PubMed]
50. Bonten MJ, Willems R, Weinstein RA. Vancomycin-resistant enterococci: why are they here, and where do they come from? Lancet Infect Dis 2001;1(5):314-325. [PubMed]
51. Borghi A, Coronelli C, Faniuolo L, Allievi G, Pallanza R, Gallo GG. Teichomycins, new antibiotics from Actinoplanes teichomyceticus nov. sp. IV. Separation and characterization of the components of teichomycin (teicoplanin). J Antibiot (Tokyo) 1984; 37(6):615-620. [PubMed]
52. Borland CD, Farrar WE. Reversible neutropenia from vancomycin. JAMA 1979; 242(22):2392-2393.[PubMed]
53. Boylan CJ, Campanale K, Iversen PW, Phillips DL, Zeckel ML, Parr TR, Jr. Pharmacodynamics of oritavancin (LY333328) in a neutropenic-mouse thigh model of Staphylococcus aureus infection. Antimicrob Agents Chemother 2003;47(5):1700-1706. [PubMed]
54. Brink AJ, Richards GA, Cummins RR, Lambson J. Recommendations to achieve rapid therapeutic teicoplanin plasma concentrations in adult hospitalised patients treated for sepsis. Int J Antimicrob Agents 2008; 32(5):455-458.[PubMed]
55. Brummett RE, Fox KE. Vancomycin- and erythromycin-induced hearing loss in humans. Antimicrob Agents Chemother 1989; 33(6):791-796. [PubMed]
56. Bryan CS, White WL. Safety of oral vancomycin in functionally anephric patients. Antimicrob Agents Chemother 1978; 14(4):634-635. [PubMed]
57. Buckwalter M, Dowell JA. Population pharmacokinetic analysis of dalbavancin, a novel lipoglycopeptide. J Clin Pharmacol 2005; 45(11):1279-1287. [PubMed]
58. Bugg TD, Wright GD, Dutka-Malen S, Arthur M, Courvalin P, Walsh CT. Molecular basis for vancomycin resistance in Enterococcus faecium BM4147: biosynthesis of a depsipeptide peptidoglycan precursor by vancomycin resistance proteins VanH and VanA. Biochemistry 1991; 30(43):10408-10415. [PubMed]
59. Bugg TD, Dutka-Malen S, Arthur M, Courvalin P, Walsh CT. Identification of vancomycin resistance protein VanA as a D-alanine:D-alanine ligase of altered substrate specificity. Biochemistry 1991; 30(8):2017-2021. [PubMed]
60. Buniva G, Del Favero A, Bernareggi A, Patoia L, Palumbo R. Pharmacokinetics of 14C-teicoplanin in healthy volunteers. J Antimicrob Chemother 1988; 21 Suppl A:23-28. [PubMed]
61. Bunke CM, Aronoff GR, Brier ME, Sloan RS, Luft FC. Vancomycin kinetics during continuous ambulatory peritoneal dialysis. Clin Pharmacol Ther 1983; 34(5):631-637. [PubMed]
62. Cabellos C, Fernandez A, Maiques JM, Tubau F, Ardanuy C, Viladrich PF et al. Experimental study of LY333328 (oritavancin), alone and in combination, in therapy of cephalosporin-resistant pneumococcal meningitis. Antimicrob Agents Chemother 2003; 47(6):1907-1911. [PubMed]
63. Campbell KC, Kelly E, Targovnik N, Hughes L, Van Saders C, Gottlieb AB et al. Audiologic monitoring for potential ototoxicity in a phase I clinical trial of a new glycopeptide antibiotic. J Am Acad Audiol 2003;14(3):157-168. [PubMed]
64. Candiani GP, Abbondi M, Borgonovi M, Romano G, Parenti F. In-vitro and in-vivo antibacterial activity of BI 397, a new semi- synthetic glycopeptide antibiotic. J Antimicrob Chemother 1999;44(2):179-192. [PubMed]
65. Cantu TG, Yamanaka-Yuen NA, Lietman PS. Serum vancomycin concentrations: reappraisal of their clinical value. Clin Infect Dis 1994; 18(4):533-543. [PubMed]
66. Cavaleri M, Riva S, Valagussa A, Guanci M, Colombo L, Dowell J et al. Pharmacokinetics and excretion of dalbavancin in the rat. J Antimicrob Chemother 2005; 55 Suppl 2:ii31-ii35. [PubMed]
67. Centers for Disease Control and Prevention. Nosocomial enterococci resistant to vancomycin--United States, 1989-1993. MMWR Morb Mortal Wkly Rep 1993; 42(30):597-599. [PubMed]
68. Centers for Disease Control and Prevention. Staphylococcus aureus resistant to vancomycin--United States, 2002. MMWR Morb Mortal Wkly Rep 2002; 51(26):565-567. [PubMed]
69. Centers for Disease Control and Prevention. Vancomycin-resistant Staphylococcus aureus--Pennsylvania, 2002. MMWR Morb Mortal Wkly Rep 2002; 51(40):902. [PubMed]
70. Chien J, Allerheiligen S, Cerimele B, Thomasson HR. Safety and pharmacokinetics of single intravenous doses of LY333328 diphosphate (glycopeptide) in healty men. 38th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, Cal. A-55. 1998.
71. Cherubin CE, Corrado ML, Sierra MF, Gombert ME, Shulman M. Susceptibility of gram-positive cocci to various antibiotics, including cefotaxime, moxalactam, and N-formimidoyl thienamycin. Antimicrob Agents Chemother 1981; 20(4):553-555. [PubMed]
72. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16(1):31-41.[PubMed]
73. Cony-Makhoul P, Brossard G, Marit G, Pellegrin JL, Texier-Maugein J, Reiffers J. A prospective study comparing vancomycin and teicoplanin as second-line empiric therapy for infection in neutropenic patients. Br J Haematol 1990;76 Suppl 2:35-40. [PubMed]
74. Cooper MA, Jin YF, Ashby JP, Andrews JM, Wise R. In-vitro comparison of the post-antibiotic effect of vancomycin and teicoplanin. J Antimicrob Chemother 1990; 26(2):203-207. [PubMed]
75. Cooper RD, Snyder NJ, Zweifel MJ, Staszak MA, Wilkie SC, Nicas TI et al. Reductive alkylation of glycopeptide antibiotics: synthesis and antibacterial activity. J Antibiot (Tokyo) 1996; 49(6):575-581. [PubMed]
76. Cooper GL, Given BD. Vancomycin: a comprehensive review of 30 years of clinical experience. Park Row Publishers, San Diego, CA, 1986.
77. Corey GR, Rubinstein E, Lalani T, Kollef MH, Shorr AF, Genter F et al. Telavancin for hospital-acquired pneumonia caused by Staphylococcus auresu: efficacy analysis according to the in vitro susceptibility to vancomycin. 48th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC , K258. 2008.
78. Courvalin P. Vancomycin resistance in gram-positive cocci. Clin Infect Dis 2006; 42 Suppl 1:S25-S34. [PubMed]
79. Coyle EA, Rybak MJ. Activity of oritavancin (LY333328), an investigational glycopeptide, compared to that of vancomycin against multidrug-resistant Streptococcus pneumoniae in an in vitro pharmacodynamic model. Antimicrob Agents Chemother 2001; 45(3):706-709. [PubMed]
80. Craig WA. Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect Dis Clin North Am 2003; 17(3):479-501. [PubMed]
81. Crandon J, Nicolau DP. Oritavancin: a potential weapon in the battle against serious Gram-positive pathogens. Future Microbiol 2008; 3:251-263. [PubMed]
82. Craven PC. Treating bone and joint infections with teicoplanin: hospitalization vs outpatient cost issues. Hosp Formul 1993; 28 Suppl 1:41-45. [PubMed]
83. Cruciani M, Gatti G, Lazzarini L, Furlan G, Broccali G, Malena M et al. Penetration of vancomycin into human lung tissue. J Antimicrob Chemother 1996; 38(5):865-869. [PubMed]
84. Cruciani M, Navarra A, Di Perri G, Andreoni M, Danzi MC, Concia E et al. Evaluation of intraventricular teicoplanin for the treatment of neurosurgical shunt infections. Clin Infect Dis 1992; 15(2):285-289. [PubMed]
85. Crumby T, Rinehart E, Carby MC, Kuhl D, Talati AJ. Pharmacokinetic comparison of nomogram-based and individualized vancomycin regimens in neonates. Am J Health Syst Pharm 2009; 66(2):149-153. [PubMed]
86. Cui L, Tominaga E, Neoh HM, Hiramatsu K. Correlation between Reduced Daptomycin Susceptibility and Vancomycin Resistance in Vancomycin-Intermediate Staphylococcus aureus. Antimicrob Agents Chemother 2006; 50(3):1079-1082. [PubMed]
87. Cunha BA. Vancomycin. Med Clin North Am 1995; 79(4):817-831. [PubMed]
88. Dagan R, Einhorn M, Howard CB, William AH. Outpatient and inpatient teicoplanin treatment for serious infections in children. Pediatr Infect Dis J 1993; 12:S17-S20. [PubMed]
89. Das B, Sarkar C, Biswas R, Pandey S. Review: dalbavancin--a novel lipoglycopeptide antimicrobial for gram positive pathogens. Pak J Pharm Sci 2008; 21(1):78-87. [PubMed]
90. Davey PG, Williams AH. A review of the safety profile of teicoplanin. J Antimicrob Chemother 1991; 27 Suppl B:69-73.[PubMed]
90a. Debbia E, Pesce A, Schito GC. In vitro interactions between teicoplanin and other antibiotics against enterococci and staphylococci. J Hospital Infection 1986;7: 73-77. [PubMed]
91. Decousser JW, Bourgeois-Nicolaos N, Doucet-Populaire F. Dalbavancin, a long-acting lipoglycopeptide for the treatment of multidrug-resistant Gram-positive bacteria. Expert Rev Anti Infect Ther 2007;5(4):557-571. [PubMed]
92. Depardieu F, Kolbert M, Pruul H, Bell J, Courvalin P. VanD-type vancomycin-resistant Enterococcus faecium and Enterococcus faecalis. Antimicrob Agents Chemother 2004; 48(10):3892-3904. [PubMed]
93. Depardieu F, Bonora MG, Reynolds PE, Courvalin P. The vanG glycopeptide resistance operon from Enterococcus faecalis revisited. Mol Microbiol 2003; 50(3):931-948. [PubMed]
94. de Lalla F, Privitera G, Rinaldi E, Ortisi G, Santoro D, Rizzardini G. Treatment of Clostridium difficile-associated disease with teicoplanin. Antimicrob Agents Chemother 1989; 33(7):1125-1127. [PubMed]
95. del Mar Fernandez de Gatta Garcia, Revilla N, Calvo MV, Dominguez-Gil A, Sanchez NA. Pharmacokinetic/ pharmacodynamic analysis of vancomycin in ICU patients. Intensive Care Med 2007; 33(2):279-285. [PubMed]
96. Dieterich C, Puey A, Lyn S, Swezey R, Furimsky A, Fairchild D et al. Gene expression analysis reveals new possible mechanisms of vancomycin-induced nephrotoxicity and identifies gene markers candidates. Toxicol Sci 2009; 107(1):258-269. [PubMed]
97. Dorr MB, Jabes D, Cavaleri M, Dowell J, Mosconi G, Malabarba A et al. Human pharmacokinetics and rationale for once-weekly dosing of dalbavancin, a semi-synthetic glycopeptide. J Antimicrob Chemother 2005; 55 Suppl 2:ii25-ii30.[PubMed]
98. Dowell JA, Goldstein BP, Buckwalter M, Stogniew M, Damle B. Pharmacokinetic-pharmacodynamic modeling of dalbavancin, a novel glycopeptide antibiotic. J Clin Pharmacol 2008; 48(9):1063-1068. [PubMed]
99. Dowell JA, Gottlieb AB, Van Saders C, Dorr MB, Leighton A, Cavaleri M et al. The pharmacokinetics and renal excretion of dalbavancin in healthy subjects. 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, CA 2002;A-1386.
100. Dowell JA, Seltzer E, Stogniew M, Dorr MB, Faocavitz S, Krause D et al. Dalbavancin pharmacokinetics in subjects with mild, moderate or severe hepatic impairment. 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Ill 2003;A-19.
101. Dryden MS, Jones NF, Phillips I. Vancomycin therapy failure in Listeria monocytogenes peritonitis in a patient on continuous ambulatory peritoneal dialysis. J Infect Dis 1991;164(6):1239. [PubMed]
102. Duchin K. Single dose pharmacokinetics (PK) of telavancin (TLV) in healthy elderly subjects. 14th European Congress of Clinical Microbiology and Infectious Diseases, Prague, Czech Republic 2004;P1027.
103. Duffull SB, Begg EJ, Chambers ST, Barclay ML. Efficacies of different vancomycin dosing regimens against Staphylococcus aureus determined with a dynamic in vitro model. Antimicrob Agents Chemother 1994;38(10):2480-2482. [PubMed]
104. Dunbar LM, Tang DM, Manausa RM. A review of telavancin in the treatment of complicated skin and skin structure infections (cSSSI). Ther Clin Risk Manag 2008;4(1):235-244. [PubMed]
105. Dykhuizen RS, Harvey G, Stephenson N, Nathwani D, Gould IM. Protein binding and serum bactericidal activities of vancomycin and teicoplanin. Antimicrob Agents Chemother 1995; 39(8):1842-1847. [PubMed]
106. Dzink J, Bartlett JG. In vitro susceptibility of Clostridium difficile isolates from patients with antibiotic-associated diarrhea or colitis. Antimicrob Agents Chemother 1980;17(4):695-698. [PubMed]
107. Ein ME, Smith NJ, Aruffo JF, Heerema MS, Bradshaw MW, Williams TW, Jr. Susceptibility and synergy studies of methicillin-resistant Staphylococcus epidermidis. Antimicrob Agents Chemother 1979;16(5):655-659. [PubMed]
108. Eng RH, Wynn L, Smith SM, Tecson-Tumang F. Effect of intravenous vancomycin on renal function. Chemotherapy 1989; 35(5):320-325. [PubMed]
109. European Organization for Research and Treatment of Cancer (EORTC) International Antimicrobial Therapy Cooperative Group and the National Cancer Institute of Canada-Clinical Trials Group. Vancomycin added to empirical combination antibiotic therapy for fever in granulocytopenic cancer patients. J Infect Dis 1991; 163(5):951-958.[PubMed]
110. Evers S, Reynolds PE, Courvalin P. Sequence of the vanB and ddl genes encoding D-alanine:D-lactate and D-alanine:D-alanine ligases in vancomycin-resistant Enterococcus faecalis V583. Gene 1994;140(1):97-102. [PubMed]
111. Falcoz C, Ferry N, Pozet N, Cuisinaud G, Zech PY, Sassard J. Pharmacokinetics of teicoplanin in renal failure. Antimicrob Agents Chemother 1987; 31(8):1255-1262. [PubMed]
112. Farber BB. Vancomycin: renewed interest in an old drug. Eur J Clin Microbiol 1984; 3(1):1-3. [PubMed]
113. Farber BF, Moellering RC, Jr. Retrospective study of the toxicity of preparations of vancomycin from 1974 to 1981. Antimicrob Agents Chemother 1983;23(1):138-141. [PubMed]
114. Feketi R. Vancomycin, teicoplanin, and the streptogramins: quinupristin and dalfopristin. In: Mandell GE, Bennett JE, Dolin R, editors. Principles and practice of infectious diseases. Churchill Livingstone, Philadelphia, Pen, 2000: 382-392.
115. Felmingham D. Glycopeptides: vancomcyin and teicoplanin. In: Reeves DS, Wise R, Andrews JM, White LO, editors. Clinical antimicrobial assays. Oxford, UK: Oxford University press, 1999: 137-148.
116. Fernandez De Gatta, Calvo MV, Hernandez JM, Caballero D, San Miguel JF, Dominguez-Gil A. Cost-effectiveness analysis of serum vancomycin concentration monitoring in patients with hematologic malignancies. Clin Pharmacol Ther 1996; 60(3):332-340. [PubMed]
117. Fetterly GJ, Ong CM, Bhavnani SM, Loutit JS, Porter SB, Morello LG et al. Pharmacokinetics of oritavancin in plasma and skin blister fluid following administration of a 200-milligram dose for 3 days or a single 800-milligram dose. Antimicrob Agents Chemother 2005; 49(1):148-152. [PubMed]
118. Finan JE, Archer GL, Pucci MJ, Climo MW. Role of penicillin-binding protein 4 in expression of vancomycin resistance among clinical isolates of oxacillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2001;45(11):3070-3075. [PubMed]
119. Forouzesh A, Moise PA, Sakoulas G. Vancomycin ototoxicity: a reevaluation in an era of increasing doses. Antimicrob Agents Chemother 2009; 53(2):483-486. [PubMed]
120. Friedman HL, Moriarty SR, Hund ME, Lee SK, Mason JW, Moon TE et al. A Phase I, Double-Blind, Randomized, Placebo- and Positive-Controlled, Single Dose, Parallel Design Trial to Assess the Potential Electrocardiographic Effects of Oritavancin in Healthy Adults. 48th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC , A1893. 2008.
121. Froggatt JW, Johnston JL, Galetto DW, Archer GL. Antimicrobial resistance in nosocomial isolates of Staphylococcus haemolyticus. Antimicrob Agents Chemother 1989;33(4):460-466. [PubMed]
122. Galetto DW, Boscia JA, Kobasa WD, Kaye D. Teicoplanin compared with vancomycin for treatment of experimental endocarditis due to methicillin-resistant Staphylococcus epidermidis. J Infect Dis 1986; 154(1):69-75.[PubMed]
123. Geraci JE, Heilman FR, Nichols DR, Wellman WE. Antibiotic therapy of bacterial endocarditis. VII. Vancomycin for acute micrococcal endocarditis; preliminary report. Proc Staff Meet Mayo Clin 1958; 33(7):172-181. [PubMed]
124. Geraci JE, Hermans PE. Vancomycin. Mayo Clin Proc 1983; 58(2):88-91. [PubMed]
125. Gholizadeh Y, Courvalin P. Acquired and intrinsic glycopeptide resistance in enterococci. Int J Antimicrob Agents 2000; 16 Suppl 1:S11-S17. [PubMed]
126. Giamarellou H, O'Riordan W, Harris H, Owen S, Porter S, Loutit JS. Phase 3 trial comparing 3-7 days of oritavancin vs. 10-14 days of vancomycin/cephalexin in the treatment of pateints with complicated skin and skin structure infections (CSSI). 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Ill , L-739a. 2003.
127. Glicklich D, Figura I. Vancomycin and cardiac arrest. Ann Intern Med 1984; 101(6):880. [PubMed]
128. Goetz MB, Sayers J. Nephrotoxicity of vancomycin and aminoglycoside therapy separately and in combination. J Antimicrob Chemother 1993; 32(2):325-334. [PubMed]
129. Goldstein BP, Draghi DC, Sheehan DJ, Hogan P, Sahm DF. Bactericidal activity and resistance development profiling of dalbavancin. Antimicrob Agents Chemother 2007; 51(4):1150-1154. [PubMed]
130. Goldstein EJ, Citron DM, Warren YA, Tyrrell KL, Merriam CV, Fernandez HT. In vitro activities of dalbavancin and 12 other agents against 329 aerobic and anaerobic gram-positive isolates recovered from diabetic foot infections. Antimicrob Agents Chemother 2006; 50(8):2875-2879. [PubMed]
131. Goldstein FW, Geslin P, Acar JF. Comparative activity of teicoplanin and vancomycin against 400 penicillin susceptible and resistant Streptococcus pneumoniae. The French Study Group. Eur J Clin Microbiol Infect Dis 1994; 13(1):33-34. [PubMed]
132. Goldstein BP, Jones RN, Fritsche TR, Biedenbach DJ. Microbiologic characterization of isolates from a dalbavancin clinical trial for catheter-related bloodstream infections. Diagn Microbiol Infect Dis 2006;54(2):83-87.[PubMed]
133. Gombert ME, Landesman SH, Corrado ML, Stein SC, Melvin ET, Cummings M. Vancomycin and rifampin therapy for Staphylococcus epidermidis meningitis associated with CSF shunts: report of three cases. J Neurosurg 1981; 55(4):633-636. [PubMed]
134. Gopal V, Bisno AL, Silverblatt FJ. Failure of vancomycin treatment in Staphylococcus aureus endocarditis. In vivo and in vitro observations. JAMA 1976; 236(14):1604-1606. [PubMed]
135. Gotfried MH, Shaw JP, Benton BM, Krause KM, Goldberg MR, Kitt MM et al. Intrapulmonary distribution of intravenous telavancin in healthy subjects and effect of pulmonary surfactant on in vitro activities of telavancin and other antibiotics. Antimicrob Agents Chemother 2008; 52(1):92-97. [PubMed]
136. Graziani AL, Lawson LA, Gibson GA, Steinberg MA, MacGregor RR. Vancomycin concentrations in infected and noninfected human bone. Antimicrob Agents Chemother 1988; 32(9):1320-1322 [PubMed]
137. Grek V, Andrien F, Collignon J, Fillet G. Allergic cross-reaction of teicoplanin and vancomycin. J Antimicrob Chemother 1991; 28(3):476-477. [PubMed]
138. Griffith RS. Vancomycin: continous clinical studies. In: Medical Encyclopedia, editor. Antibiotic Annual. New York, NY: 1956: 118-122.
139. Gump DW. Vancomycin for treatment of bacterial meningitis. Rev Infect Dis 1981; 3 suppl:S289-S292.[PubMed]
140. Hall RG, Payne KD, Bain AM, Rahman AP, Nguyen ST, Eaton SA et al. Multicenter evaluation of vancomycin dosing: emphasis on obesity. Am J Med 2008; 121(6):515-518. [PubMed]
141. Harding I, MacGowan AP, White LO, Darley ES, Reed V. Teicoplanin therapy for Staphylococcus aureus septicaemia: relationship between pre-dose serum concentrations and outcome. J Antimicrob Chemother 2000; 45(6):835-841. [PubMed]
142. Harwick HJ, Kalmanson GM, Guze LB. In vitro activity of ampicillin or vancomycin combined with gentamicin or streptomycin against enterococci. Antimicrob Agents Chemother 1973; 4(4):383-387. [PubMed]
143. Hayden MK, Trenholme GM, Schultz JE, Sahm DF. In vivo development of teicoplanin resistance in a VanB Enterococcus faecium isolate. J Infect Dis 1993; 167(5):1224-1227. [PubMed]
144. Hegde SS, Reyes N, Skinner R, Difuntorum S. Efficacy of telavancin in a murine model of pneumonia induced by methicillin-susceptible Staphylococcus aureus. J Antimicrob Chemother 2008; 61(1):169-172. [PubMed]
145. Hegde SS, Reyes N, Wiens T, Vanasse N, Skinner R, McCullough J et al. Pharmacodynamics of telavancin (TD-6424), a novel bactericidal agent, against gram-positive bacteria. Antimicrob Agents Chemother 2004; 48(8):3043-3050. [PubMed]
146. Heine HS, Bassett J, Miller L, Bassett A, Ivins BE, Lehoux D et al. Efficacy of oritavancin in a murine model of Bacillus anthracis spore inhalation anthrax. Antimicrob Agents Chemother 2008; 52(9):3350-3357. [PubMed]
147. Henry NK, Rouse MS, Whitesell AL, McConnell ME, Wilson WR. Treatment of methicillin-resistant Staphylococcus aureus experimental osteomyelitis with ciprofloxacin or vancomycin alone or in combination with rifampin. Am J Med 1987; 82(4A):73-75. [PubMed]
148. Hermsen ED, Ross GH, Rotschafer JC. Glycopeptide Pharmacodynamics. In: Nightingale CH, Ambrose PG, Drusano GL, Murakawa T, editors. Antimicrobial Pharmacodynamics in Theory and Clinical Practice. New York, NY: Informa Heathcare, 2007: 189-216.
149. Higgins DL, Chang R, Debabov DV, Leung J, Wu T, Krause KM et al. Telavancin, a multifunctional lipoglycopeptide, disrupts both cell wall synthesis and cell membrane integrity in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2005; 49(3):1127-1134. [PubMed]
150. Hiramatsu K, Aritaka N, Hanaki H, Kawasaki S, Hosoda Y, Hori S et al. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet 1997; 350(9092):1670-1673.[PubMed]
151. Hiramatsu K, Hanaki H. Glycopeptide resistance in staphylococci. Curr Opin Infect Dis 1998; 11(6):653-658. [PubMed]
152. Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, Tenover FC. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother 1997; 40(1):135-136. [PubMed]
153. Hook EW, III, Johnson WD, Jr. Vancomycin therapy of bacterial endocarditis. Am J Med 1978; 65(3):411-415.[PubMed]
154. Hope R, Livermore DM, Brick G, Lillie M, Reynolds R. Non-susceptibility trends among staphylococci from bacteraemias in the UK and Ireland, 2001-06. J Antimicrob Chemother 2008; 62 Suppl 2:ii65-ii74. [PubMed]
155. Hsiao SH, Chang CM, Tsai JC, Lin CY, Liao LH, Lin WL et al. Glycopeptide-induced neutropenia: cross-reactivity between vancomycin and teicoplanin. Ann Pharmacother 2007; 41(5):891-894. [PubMed]
156. Ingram PR, Lye DC, Tambyah PA, Goh WP, Tam VH, Fisher DA. Risk factors for nephrotoxicity associated with continuous vancomycin infusion in outpatient parenteral antibiotic therapy. J Antimicrob Chemother 2008; 62(1):168-171. [PubMed]
157. Jabes D, Candiani G, Romano G, Brunati C, Riva S, Cavaleri M. Efficacy of dalbavancin against methicillin-resistant Staphylococcus aureus in the rat granuloma pouch infection model. Antimicrob Agents Chemother 2004; 48(4):1118-1123. [PubMed]
158. Jadeja L, Fainstein V, LeBlanc B, Bodey GP. Comparative in vitro activities of teichomycin and other antibiotics against JK diphtheroids. Antimicrob Agents Chemother 1983; 24(2):145-146. [PubMed]
159. James A, Koren G, Milliken J, Soldin S, Prober C. Vancomycin pharmacokinetics and dose recommendations for preterm infants. Antimicrob Agents Chemother 1987; 31(1):52-54. [PubMed]
160. James JK, Palmer SM, Levine DP, Rybak MJ. Comparison of conventional dosing versus continuous-infusion vancomycin therapy for patients with suspected or documented gram-positive infections. Antimicrob Agents Chemother 1996; 40(3):696-700. [PubMed]
161. Jansen WT, Verel A, Verhoef J, Milatovic D. In vitro activity of telavancin against gram-positive clinical isolates recently obtained in Europe. Antimicrob Agents Chemother 2007;51(9):3420-3424. [PubMed]
162. Jauregui LE, Babazadeh S, Seltzer E, Goldberg L, Krievins D, Frederick M et al. Randomized, double-blind comparison of once-weekly dalbavancin versus twice-daily linezolid therapy for the treatment of complicated skin and skin structure infections. Clin Infect Dis 2005; 41(10):1407-1415. [PubMed]
163. Johnson AP, Uttley AH, Woodford N, George RC. Resistance to vancomycin and teicoplanin: an emerging clinical problem. Clin Microbiol Rev 1990; 3(3):280-291. [PubMed]
164. Johnson DM, Fritsche TR, Sader HS, Jones RN. Evaluation of dalbavancin in combination with nine antimicrobial agents to detect enhanced or antagonistic interactions. Int J Antimicrob Agents 2006;27(6):557-560. [PubMed]
165. Jones RN, Barrett MS, Erwin ME. In vitro activity and spectrum of LY333328, a novel glycopeptide derivative. Antimicrob Agents Chemother 1997;41(2):488-493. [PubMed]
166. Jones RN, Stilwell MG, Sader HS, Fritsche TR, Goldstein BP. Spectrum and potency of dalbavancin tested against 3322 Gram-positive cocci isolated in the United States Surveillance Program (2004). Diagn Microbiol Infect Dis 2006; 54(2):149-153. [PubMed]
167. Jordan DC, Inniss WE. Selective inhibition of ribonucleic acid synthesis in Staphylococcus aureus by vancomycin. Nature 1959; 184(Suppl 24):1894-1895. [PubMed]
168. Jordan DC, Mallory HD. Site of action of vancomycin on Staphylococcus aureus. Antimicrob Agents Chemother (Bethesda ) 1964; 10:489-494. [PubMed]
169. Jorgenson L, Reiter PD, Freeman JE, Winston KR, Fish D, McBride LA et al. Vancomycin disposition and penetration into ventricular fluid of the central nervous system following intravenous therapy in patients with cerebrospinal devices. Pediatr Neurosurg 2007; 43(6):449-455. [PubMed]
170. Kaatz GW, Seo SM, Dorman NJ, Lerner SA. Emergence of teicoplanin resistance during therapy of Staphylococcus aureus endocarditis. J Infect Dis 1990; 162(1):103-108. [PubMed]
171. Kahne D, Leimkuhler C, Lu W, Walsh C. Glycopeptide and lipoglycopeptide antibiotics. Chem Rev 2005; 105(2):425-448. [PubMed]
172. Kanafani ZA. Telavancin: a new lipoglycopeptide with multiple mechanisms of action. Expert Rev Anti Infect Ther 2006; 4(5):743-749. [PubMed]
173. Kang SL, Rybak MJ. Pharmacodynamics of RP 59500 alone and in combination with vancomycin against Staphylococcus aureus in an in vitro-infected fibrin clot model. Antimicrob Agents Chemother 1995; 39(7):1505-1511.[PubMed]
174. Karchmer AW, Archer GL, Dismukes WE. Staphylococcus epidermidis causing prosthetic valve endocarditis: microbiologic and clinical observations as guides to therapy. Ann Intern Med 1983; 98(4):447-455. [PubMed]
175. Kim SJ, Cegelski L, Stueber D, Singh M, Dietrich E, Tanaka KS et al. Oritavancin exhibits dual mode of action to inhibit cell-wall biosynthesis in Staphylococcus aureus. J Mol Biol 2008; 377(1):281-293. [PubMed]
176. Kim A, Kuti JL, Nicolau DP. Review of dalbavancin, a novel semisynthetic lipoglycopeptide. Expert Opin Investig Drugs 2007; 16(5):717-733. [PubMed]
177. Klugman KP, Friedland IR, Bradley JS. Bactericidal activity against cephalosporin-resistant Streptococcus pneumoniae in cerebrospinal fluid of children with acute bacterial meningitis. Antimicrob Agents Chemother 1995; 39(9):1988-1992. [PubMed]
178. Knudsen JD, Fuursted K, Espersen F, Frimodt-Moller N. Activities of vancomycin and teicoplanin against penicillin-resistant pneumococci in vitro and in vivo and correlation to pharmacokinetic parameters in the mouse peritonitis model. Antimicrob Agents Chemother 1997; 41(9):1910-1915. [PubMed]
179. Kosowska K, Hoellman DB, Lin G, Clark C, Credito K, McGhee P et al. Antipneumococcal activity of ceftobiprole, a novel broad-spectrum cephalosporin. Antimicrob Agents Chemother 2005; 49(5):1932-1942. [PubMed]
180. Kralovicova K, Spanik S, Halko J, Netriova J, Studena-Mrazova M, Novotny J et al. Do vancomycin serum levels predict failures of vancomycin therapy or nephrotoxicity in cancer patients? J Chemother 1997; 9(6):420-426. [PubMed]
181. Kureishi A, Jewesson PJ, Rubinger M, Cole CD, Reece DE, Phillips GL et al. Double-blind comparison of teicoplanin versus vancomycin in febrile neutropenic patients receiving concomitant tobramycin and piperacillin: effect on cyclosporin A-associated nephrotoxicity. Antimicrob Agents Chemother 1991; 35(11):2246-2252. [PubMed]
182. Lake KD, Peterson CD. A simplified dosing method for initiating vancomycin therapy. Pharmacotherapy 1985; 5(6):340-344. [PubMed]
183. Lam YW, Kapusnik-Uner JE, Sachdeva M, Hackbarth C, Gambertoglio JG, Sande MA. The pharmacokinetics of teicoplanin in varying degrees of renal function. Clin Pharmacol Ther 1990; 47(5):655-661. [PubMed]
184. Lang E, Schafer V, Schaaf B, Dennhardt R. Comparison of efficacy and safety of teicoplanin in gram-positive infections: a multicentre study. Scand J Infect Dis Suppl 1990; 72:54-60. [PubMed]
185. Laohavaleeson S, Barriere SL, Nicolau DP, Kuti JL. Cost-effectiveness of telavancin versus vancomycin for treatment of complicated skin and skin structure infections. Pharmacotherapy 2008; 28(12):1471-1482. [PubMed]
186. Laohavaleeson S, Kuti JL, Nicolau DP. Telavancin: a novel lipoglycopeptide for serious gram-positive infections. Expert Opin Investig Drugs 2007; 16(3):347-357. [PubMed]
187. Larsson AJ, Walker KJ, Raddatz JK, Rotschafer JC. The concentration-independent effect of monoexponential and biexponential decay in vancomycin concentrations on the killing of Staphylococcus aureus under aerobic and anaerobic conditions. J Antimicrob Chemother 1996; 38(4):589-597. [PubMed]
188. Leadbetter MR, Adams SM, Bazzini B, Fatheree PR, Karr DE, Krause KM et al. Hydrophobic vancomycin derivatives with improved ADME properties: discovery of telavancin (TD-6424). Journal Antibiotics (Tokyo) 2004; 57(5):326-336. [PubMed]
189. Leclercq R, Derlot E, Duval J, Courvalin P. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N Engl J Med 1988; 319(3):157-161. [PubMed]
190. Lefort A, Pavie J, Garry L, Chau F, Fantin B. Activities of dalbavancin in vitro and in a rabbit model of experimental endocarditis due to Staphylococcus aureus with or without reduced susceptibility to vancomycin and teicoplanin. Antimicrob Agents Chemother 2004; 48(3):1061-1064. [PubMed]
191. Lefort A, Saleh-Mghir A, Garry L, Carbon C, Fantin B. Activity of LY333328 combined with gentamicin in vitro and in rabbit experimental endocarditis due to vancomycin-susceptible or -resistant Enterococcus faecalis. Antimicrob Agents Chemother 2000; 44(11):3017-3021. [PubMed]
192. LeFrock JL, Ristuccia AM, Ristuccia PA, Quenzer RW, Haggerty PG, Allen JE et al. Teicoplanin in the treatment of bone and joint infections. Teicoplanin Bone and Joint Cooperative Study Group, USA. Eur J Surg Suppl 1992;(567):9-13.[PubMed]
193. Leighton A, Gottlieb AB, Dorr MB, Jabes D, Mosconi G, VanSaders C et al. Tolerability, pharmacokinetics, and serum bactericidal activity of intravenous dalbavancin in healthy volunteers. Antimicrob Agents Chemother 2004; 48(3):940-945. [PubMed]
194. Lemaire S, Kosowska-Shick K, Julian K, Tulkens PM, Van Bambeke F, Appelbaum PC. Activities of antistaphylococcal antibiotics towards the extracellular and intraphagocytic forms of Staphylococcus aureus isolates from a patient with persistent bacteraemia and endocarditis. Clin Microbiol Infect 2008; 14(8):766-777. [PubMed]
195. Lemerle S, de La RF, Lamy R, Fremaux A, Bernaudin F, Lobut JB et al. Teicoplanin in combination therapy for febrile episodes in neutropenic and non-neutropenic paediatric patients. J Antimicrob Chemother 1988; 21 Suppl A:113-116. [PubMed]
196. Leonard SN, Rybak MJ. Telavancin: an antimicrobial with a multifunctional mechanism of action for the treatment of serious gram-positive infections. Pharmacotherapy 2008; 28(4):458-468. [PubMed]
197. Lerner PI. Susceptibility of pathogenic actinomycetes to antimicrobial compounds. Antimicrob Agents Chemother 1974; 5(3):302-309. [PubMed]
198. Leuthner KD, Cheung CM, Rybak MJ. Comparative activity of the new lipoglycopeptide telavancin in the presence and absence of serum against 50 glycopeptide non-susceptible staphylococci and three vancomycin-resistant Staphylococcus aureus. J Antimicrob Chemother 2006; 58(2):338-343. [PubMed]
199. Levine DP, Cushing RD, Jui J, Brown WJ. Community-acquired methicillin-resistant Staphylococcus aureus endocarditis in the Detroit Medical Center. Ann Intern Med 1982; 97(3):330-338. [PubMed]
200. Levine DP, Fromm BS, Reddy BR. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med 1991; 115(9):674-680. [PubMed]
201. Lewis P, Garaud JJ, Parenti F. A multicentre open clinical trial of teicoplanin in infections caused by gram-positive bacteria. J Antimicrob Chemother 1988; 21 Suppl A:61-67. [PubMed]
202. Lindholm DD, Murray JS. Persistence of vancomycin in the blood during renal failure and its treatment by hemodialysis. N Engl J Med 1966; 274(19):1047-1051. [PubMed]
203. Liu C, Chambers HF. Staphylococcus aureus with heterogeneous resistance to vancomycin: epidemiology, clinical significance, and critical assessment of diagnostic methods. Antimicrob Agents Chemother 2003; 47(10):3040-3045.[PubMed]
204. Lodise TP, Jr., Gotfried M, Barriere S, Drusano GL. Telavancin penetration into human epithelial lining fluid determined by population pharmacokinetic modeling and Monte Carlo simulation. Antimicrob Agents Chemother 2008; 52(7):2300-2304. [PubMed]
205. Lodise TP, Lomaestro B, Graves J, Drusano GL. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Chemother 2008; 52(4):1330-1336.[PubMed]
206. Loll PJ, Axelsen PH. The structural biology of molecular recognition by vancomycin. Annu Rev Biophys Biomol Struct 2000; 29:265-289. [PubMed]
207. Lopez S, Hackbarth C, Romano G, Trias J, Jabes D, Goldstein BP. In vitro antistaphylococcal activity of dalbavancin, a novel glycopeptide. J Antimicrob Chemother 2005; 55 Suppl 2:ii21-ii24. [PubMed]
208. Lopez S, Hackbarth CJ, White R, Trias J. In vitro susceptibility and population analysis of staphylococci after serial passage at sub-MIC levels of dalbavancin and other glycopeptides. 13th European Congress of Clinical Microbiology and Infectious Diseases, Glasgow, UK , P1539. 2003.
209. Loutit JS, O'Riordan W, San Juan J, Mensa J, Hanning R, Huang S et al. Phase 2 trial comparing four regimens of oritavancin vs comparator in the treatment of patients with S. aureus bacteraemia. Clin.Microbiol.Infect. 10[suppl. 3], P541. 2004.
210. Lowy FD, Walsh JA, Mayers MM, Klein RS, Steigbigel NH. Antibiotic activity in vitro against methicillin-resistant Staphylococcus epidermidis and therapy of an experimental infection. Antimicrob Agents Chemother 1979; 16(3):314-321. [PubMed]
211. Lubenko IY, Strukova EV, Smirnova MV, Vostrov SN, Portnoy YA, Zinner SH et al. Telavancin and vancomycin pharmacodynamics with Staphylococcus aureus in an in vitro dynamic model. J Antimicrob Chemother 2008; 62(5):1065-1069. [PubMed]
212. MacGowan AP, Holt HA, Bywater MJ, Reeves DS. In vitro antimicrobial susceptibility of Listeria monocytogenes isolated in the UK and other Listeria species. Eur J Clin Microbiol Infect Dis 1990; 9(10):767-770.[PubMed]
213. MacGowan AP, McMullin CM, White LO, Reeves DS, Davis E, Speller DC. Serum monitoring of teicoplanin. J Antimicrob Chemother 1992; 30(3):399-402. [PubMed]
214. MacGowan AP, Reeves DS, Wise R. Interpretation of antimicrobial assays. In: Reeves DS, Wise R, Andrews JM, White LO, editors. Clinical antimicrobial assays. Oxford, UK: Oxford University Press, 1999.
215. Madrigal AG, Basuino L, Chambers HF. Efficacy of Telavancin in a rabbit model of aortic valve endocarditis due to methicillin-resistant Staphylococcus aureus or vancomycin-intermediate Staphylococcus aureus. Antimicrob Agents Chemother 2005; 49(8):3163-3165. [PubMed]
216. Magera BE, Arroyo JC, Rosansky SJ, Postic B. Vancomycin pharmacokinetics in patients with peritonitis on peritoneal dialysis. Antimicrob Agents Chemother 1983; 23(5):710-714. [PubMed]
217. Malabarba A, Ciabatti R. Glycopeptide derivatives. Curr Med Chem 2001; 8(14):1759-1773. [PubMed]
218. Malabarba A, Goldstein BP. Origin, structure, and activity in vitro and in vivo of dalbavancin. J Antimicrob Chemother 2005; 55 Suppl 2:ii15-ii20.[PubMed]
219. Malabarba A, Ciabatti R, Scotti R, Goldstein BP, Ferrari P, Kurz M et al. New semisynthetic glycopeptides MDL 63,246 and MDL 63,042, and other amide derivatives of antibiotic A-40,926 active against highly glycopeptide-resistant VanA enterococci. J Antibiot (Tokyo) 1995; 48(8):869-883. [PubMed]
220. (283) Mariani-Kurkdjian P, Nebbad H, Aujard Y, Bingen E. [Monitoring serum vancomycin concentrations in the treatment of Staphylococcus infections in children]. Arch Pediatr 2008; 15(11):1625-1629. [PubMed]
221. Marion Merrel Dow Pharmaceuticals. Targocid. In: Datapharm Publications, editor. ABPI Data Sheet Compendium. London, UK: 1993: 936-938.
222. Matthews PC, Taylor A, Byren I, Atkins BL. Teicoplanin levels in bone and joint infections: are standard doses subtherapeutic? J Infect 2007; 55(5):408-413. [PubMed]
223. Matzke GR, Zhanel GG, Guay DR. Clinical pharmacokinetics of vancomycin. Clin Pharmacokinet 1986; 11(4):257-282. [PubMed]
224. Matzke GR, McGory RW, Halstenson CE, Keane WF. Pharmacokinetics of vancomycin in patients with various degrees of renal function. Antimicrob Agents Chemother 1984; 25(4):433-437. [PubMed]
225. Mayhew JF, Deutsch S. Cardiac arrest following administration of vancomycin. Can Anaesth Soc J 1985;32(1):65-66. [PubMed]
226. McCormick MH, McGuire JM, Pittenger GE, Pittenger RC, Stark WM. Vancomycin, a new antibiotic. I. Chemical and biologic properties. Antibiot Annu 1955; 3:606-611.
227. McHenry MC, Gavan TL. Vancomycin. Pediatr Clin North Am 1983; 30(1):31-47. [PubMed]
228. Mendez-Alvarez S, Perez-Hernandez X, Claverie-Martin F. Glycopeptide resistance in enterococci. Int Microbiol 2000; 3(2):71-80. [PubMed]
229. Mercier RC, Hrebickova L. Oritavancin: a new avenue for resistant Gram-positive bacteria. Expert Rev Anti Infect Ther 2005; 3(3):325-332. [PubMed]
230. Mercier RC, Houlihan HH, Rybak MJ. Pharmacodynamic evaluation of a new glycopeptide, LY333328, and in vitro activity against Staphylococcus aureus and Enterococcus faecium. Antimicrob Agents Chemother 1997; 41(6):1307-1312. [PubMed]
231. Mercier RC, Stumpo C, Rybak MJ. Effect of growth phase and pH on the in vitro activity of a new glycopeptide, oritavancin (LY333328), against Staphylococcus aureus and Enterococcus faecium. J Antimicrob Chemother 2002; 50(1):19-24.[PubMed]
232. Miller MA, Anderson P, Parker JW, Rohrer HH. Inhibition of Neisseria gonorrhoeae isolates by Martin-Lewis medium. Epidemiology, susceptibility profile, and plasma analysis. Br J Vener Dis 1982; 58(2):96-100. [PubMed]
233. Mimoz O, Rolland D, Adoun M, Marchand S, Breilh D, Brumpt I et al. Steady-state trough serum and epithelial lining fluid concentrations of teicoplanin 12 mg/kg per day in patients with ventilator-associated pneumonia. Intensive Care Med 2006; 32(5):775-779. [PubMed]
234. Miro JM, Garcia-de-la-Maria C, Armero Y, de Lazzari E, Soy D, Moreno A et al. Efficacy of telavancin in the treatment of experimental endocarditis due to glycopeptide-intermediate Staphylococcus aureus. Antimicrob Agents Chemother 2007; 51(7):2373-2377.[PubMed]
235. Moellering RC, Jr. Pharmacokinetics of vancomycin. J Antimicrob Chemother 1984; 14 Suppl D:43-52. [PubMed]
236. Moellering RC, Jr., Krogstad DJ, Greenblatt DJ. Vancomycin therapy in patients with impaired renal function: a nomogram for dosage. Ann Intern Med 1981; 94(3):343-346. [PubMed]
237. (156) Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet 2004; 43(13):925-942. [PubMed]
238. Moise-Broder PA, Sakoulas G, Eliopoulos GM, Schentag JJ, Forrest A, Moellering RC, Jr. Accessory gene regulator group II polymorphism in methicillin-resistant Staphylococcus aureus is predictive of failure of vancomycin therapy. Clin Infect Dis 2004; 38(12):1700-1705. [PubMed]
239. Montecalvo MA. Ramoplanin: a novel antimicrobial agent with the potential to prevent vancomycin-resistant enterococcal infection in high-risk patients. J Antimicrob Chemother 2003; 51 Suppl 3:iii31-iii35. [PubMed]
240. Nakamura T, Kokuryo T, Hashimoto Y, Inui KI. Effects of fosfomycin and imipenem-cilastatin on the nephrotoxicity of vancomycin and cisplatin in rats. J Pharm Pharmacol 1999; 51(2):227-232. [PubMed]
241. Nannini EC, Stryjewski ME. A new lipoglycopeptide: telavancin. Expert Opin Pharmacother 2008;9(12):2197-2207. [PubMed]
242. Nelson JL, Rice KC, Slater SR, Fox PM, Archer GL, Bayles KW et al. Vancomycin-intermediate Staphylococcus aureus strains have impaired acetate catabolism: implications for polysaccharide intercellular adhesin synthesis and autolysis. Antimicrob Agents Chemother 2007; 51(2):616-622. [PubMed]
243. Neu HC, Labthavikul P. In vitro activity of teichomycin compared with those of other antibiotics. Antimicrob Agents Chemother 1983; 24(3):425-428. [PubMed]
244. Neu HC, Chin NX, Niu WW. In vitro activity of the new glycopeptide decaplanin. Eur J Clin Microbiol Infect Dis 1992; 11(5):458-462. [PubMed]
245. Nguyen HA, Denis O, Vergison A, Tulkens PM, Struelens MJ, Van Bambeke F. Intracellular activity of antibiotics in a model of human THP-1 macrophages infected by a Staphylococcus aureus Small Colony Variant isolated from a cystic fibrosis patient : 2. Study of antibiotic combinations. Antimicrob Agents Chemother 2009; 53(4):1443-1449.[PubMed]
246. Nguyen HA, Denis O, Vergison A, Theunis A, Tulkens PM, Struelens MJ et al. Intracellular activity of antibiotics in a model of human THP-1 macrophages infected by a Staphylococcus aureus Small Colony Variant isolated from a cystic fibrosis patient : 1. Pharmacodynamic evaluation and comparison with isogenic normal phenotype and revertant strains. Antimicrob Agents Chemother 2009; 53(4):1434-1442. [PubMed]
247. Nicolau DP, Sun HK, Seltzer E, Buckwalter M, Dowell JA. Pharmacokinetics of dalbavancin in plasma and skin blister fluid. J Antimicrob Chemother 2007; 60(3):681-684. [PubMed]
248. Nishino Y, Takemura S, Minamiyama Y, Hirohashi K, Ogino T, Inoue M et al. Targeting superoxide dismutase to renal proximal tubule cells attenuates vancomycin-induced nephrotoxicity in rats. Free Radic Res 2003; 37(4):373-379.[PubMed]
249. Nitanai Y, Kikuchi T, Kakoi K, Hanamaki S, Fujisawa I, Aoki K. Crystal structures of the complexes between vancomycin and cell-wall precursor analogs. J Mol Biol 2009;385(5):1422-1432. [PubMed]
250. Niu WW, Neu HC. Activity of mersacidin, a novel peptide, compared with that of vancomycin, teicoplanin, and daptomycin. Antimicrob Agents Chemother 1991;35(5):998-1000. [PubMed]
251. Nord C, Rasmanis G, Wahlund E. Effect of dalbavancin on normal interstinal flora. 45th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC 2005;A13.
252. Odenholt I, Lowdin E, Cars O. Pharmacodynamic effects of telavancin against methicillin-resistant and methicillin-susceptible Staphylococcus aureus strains in the presence of human albumin or serum and in an in vitro kinetic model. Antimicrob Agents Chemother 2007; 51(9):3311-3316. [PubMed]
253. Pace JL, Judice JK. Telavancin (Theravance). Curr Opin Investig Drugs 2005; 6(2):216-225. [PubMed]
254. Pace JL, Yang G. Glycopeptides: Update on an old successful antibiotic class. Biochem Pharmacol 2006; 71(7):968-980. [PubMed]
255. Pallanza R, Berti M, Goldstein BP, Mapelli E, Randisi E, Scotti R et al. Teichomycin: in-vitro and in-vivo evaluation in comparison with other antibiotics. J Antimicrob Chemother 1983; 11(5):419-425. [PubMed]
256 Pancorbo S, Comty C. Peritoneal transport of vancomycin in 4 patients undergoing continuous ambulatory peritoneal dialysis. Nephron 1982; 31(1):37-39. [PubMed]
257. Pankuch GA, Appelbaum PC. Post-Antibiotic Effect Of Telavancin Against 16 Gram-Positive Organisms. Antimicrob Agents Chemother 2009;53(3):1275-7. [PubMed]
258. Parenti F, Beretta G, Berti M, Arioli V. Teichomycins, new antibiotics from Actinoplanes teichomyceticus Nov. Sp. I. Description of the producer strain, fermentation studies and biological properties. J Antibiot (Tokyo) 1978;31(4):276-283. [PubMed]
259. Parenti F. Structure and mechanism of action of teicoplanin. J Hosp Infect 1986; 7 Suppl A:79-83. [PubMed]
260. Pavie J, Lefort A, Zarrouk V, Chau F, Garry L, Leclercq R et al. Efficacies of quinupristin-dalfopristin combined with vancomycin in vitro and in experimental endocarditis due to methicillin-resistant Staphylococcus aureus in relation to cross-resistance to macrolides, lincosamides, and streptogramin B- type antibiotics. Antimicrob Agents Chemother 2002; 46(9):3061-3064. [PubMed]
261. Peleg AY, Monga D, Pillai S, Mylonakis E, Moellering Jr RC, Eliopoulos GM. Reduced Susceptibility to Vancomycin Influences Pathogenicity in Staphylococcus aureus Infection. J Infect Dis 2009;199(4):532-6. [PubMed]
262. Peller P, Aichholzer B, Fell JJ, Dieterich HA. Safety and efficacy of teicoplanin in the treatment of Gram-positive infections in paediatric patients in Germany. Pediatr Infect Dis J 1993; 12:S7-S9.
263. Pea F, Viale P. Should the currently recommended twice-daily dosing still be considered the most appropriate regimen for treating MRSA ventilator-associated pneumonia with vancomycin? Clin Pharmacokinet 2008;47(3):147-152.[PubMed]
264. Perichon B, Courvalin P. Heterologous expression of the enterococcal vanA operon in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2004; 48(11):4281-4285. [PubMed]
265. Perkins HR. Specificity of combination between mucopeptide precursors and vancomycin or ristocetin. Biochem J 1969; 111(2):195-205. [PubMed]
266. Philippon A, Bimet F. In vitro susceptibility of Corynebacterium group D2 and Corynebacterium jeikeium to twelve antibiotics. Eur J Clin Microbiol Infect Dis 1990; 9(12):892-895. [PubMed]
267. Podmore AH, Reynolds PE. Purification and characterization of VanXY(C), a D,D-dipeptidase/D,D-carboxypeptidase in vancomycin-resistant Enterococcus gallinarum BM4174. Eur J Biochem 2002;269(11):2740-2746.[PubMed]
268. Pohlod DJ, Saravolatz LD, Somerville MM. In-vitro susceptibility of gram-positive cocci to LY146032 teicoplanin, sodium fusidate, vancomycin, and rifampicin. J Antimicrob Chemother 1987; 20(2):197-202. [PubMed]
269. Polk RE, Healy DP, Schwartz LB, Rock DT, Garson ML, Roller K. Vancomycin and the red-man syndrome: pharmacodynamics of histamine release. J Infect Dis 1988;157:502-507. [PubMed]
270. Polk RE. Anaphylactoid reactions to glycopeptide antibiotics. J Antimicrob Chemother 1991; 27 Suppl B:17-29.[PubMed]
271. Pollard TA, Lampasona V, Akkerman S, Tom K, Hooks MA, Mullins RE et al. Vancomycin redistribution: dosing recommendations following high-flux hemodialysis. Kidney Int 1994;45(1):232-237. [PubMed]
272. Potel G, Moutet J, Bernareggi A, Le Normand Y, Meigner M, Baron D. Pharmacokinetics of teicoplanin in burn patients. Scand J Infect Dis Suppl 1990; 72:29-34. [PubMed]
273. Poulakou G, Giamarellou H. Oritavancin: a new promising agent in the treatment of infections due to Gram-positive pathogens. Expert Opin Investig Drugs 2008; 17(2):225-243. [PubMed]
274. Prakash V, Lewis JS, Jorgensen JH. Vancomycin MICs for methicillin-resistant Staphylococcus aureus isolates differ based upon the susceptibility test method used. Antimicrob Agents Chemother 2008; 52(12):4528. [PubMed]
275. Presterl E, Graninger W, Georgopoulos A. The efficacy of teicoplanin in the treatment of endocarditis caused by gram-positive bacteria. J Antimicrob Chemother 1993; 31(5):755-766. [PubMed]
276. Pryka RD, Rodvold KA, Erdman SM. An updated comparison of drug dosing methods. Part IV: Vancomycin. Clin Pharmacokinet 1991; 20(6):463-476. [PubMed]
277. Raad I, Darouiche R, Vazquez J, Lentnek A, Hachem R, Hanna H et al. Efficacy and safety of weekly dalbavancin therapy for catheter-related bloodstream infection caused by gram-positive pathogens. Clin Infect Dis 2005;40:374-380.[PubMed]
278. Raimondi A, Moosdeen F, Williams JD. Antibiotic resistance pattern of Flavobacterium meningosepticum. Eur J Clin Microbiol 1986;5(4):461-463. [PubMed]
279. Ramphal R, Bolger M, Oblon DJ, Sherertz RJ, Malone JD, Rand KH et al. Vancomycin is not an essential component of the initial empiric treatment regimen for febrile neutropenic patients receiving ceftazidime: a randomized prospective study. Antimicrob Agents Chemother 1992; 36(5):1062-1067. [PubMed]
280. Ramsey AM, Zilberberg MD. Secular trends of hospitalization with vancomycin-resistant enterococcus infection in the United States, 2000-2006. Infect Control Hosp Epidemiol 2009;30(2):184-186. [PubMed]
281. Rennie RP, Koeth L, Jones RN, Fritsche TR, Knapp CC, Killian SB et al. Factors influencing broth microdilution antimicrobial susceptibility test results for dalbavancin, a new glycopeptide agent. J Clin Microbiol 2007; 45(10):3151-3154. [PubMed]
282. Reyes N, Skinner R, Kaniga K, Krause KM, Shelton J, Obedencio GP et al. Efficacy of telavancin (TD-6424), a rapidly bactericidal lipoglycopeptide with multiple mechanisms of action, in a murine model of pneumonia induced by methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2005; 49(10):4344-4346. [PubMed]
283. Reynolds PE. Structure, biochemistry and mechanism of action of glycopeptide antibiotics. Eur J Clin Microbiol Infect Dis 1989; 8(11):943-950. [PubMed]
284. Reynolds PE, Depardieu F, Dutka-Malen S, Arthur M, Courvalin P. Glycopeptide resistance mediated by enterococcal transposon Tn1546 requires production of VanX for hydrolysis of D-alanyl-D-alanine. Mol Microbiol 1994; 13(6):1065-1070. [PubMed]
285. Reynolds PE, Snaith HA, Maguire AJ, Dutka-Malen S, Courvalin P. Analysis of peptidoglycan precursors in vancomycin-resistant Enterococcus gallinarum BM4174. Biochem J 1994;301(Pt 1):5-8. [PubMed]
286. Ricard JD, Wolff M, Lacherade JC, Mourvillier B, Hidri N, Barnaud G et al. Levels of vancomycin in cerebrospinal fluid of adult patients receiving adjunctive corticosteroids to treat pneumococcal meningitis: a prospective multicenter observational study. Clin Infect Dis 2007;44(2):250-255. [PubMed]
287. Rodvold KA, Gotfried MH, Loutit JS, Porter SB. Plasma and intrapulmonary concentrations of oritavancin and vancomycin in normal healthy adults. Clin Microbiol Infect. 2004;10[suppl 3].
288. Roecker AM, Pope SD. Dalbavancin: a lipoglycopeptide antibacterial for Gram-positive infections. Expert Opin Pharmacother 2008; 9(10):1745-1754. [PubMed]
289. Rolston KV, Nguyen H, Messer M. In vitro activity of LY264826, a new glycopeptide antibiotic, against gram-positive bacteria isolated from patients with cancer. Antimicrob Agents Chemother 1990; 34(11):2137-2141. [PubMed]
290. Rose WE, Leonard SN, Rossi KL, Kaatz GW, Rybak MJ. Impact of inoculum size and heterogeneous vancomycin-intermediate Staphylococcus aureus (hVISA) on vancomycin activity and emergence of VISA in an in vitro pharmacodynamic model. Antimicrob Agents Chemother 2009; 53(2):805-807. [PubMed]
291. Rose WE, Kaatz GW, Sakoulas G, Rybak MJ. Teicoplanin pharmacodynamics in reference to the accessory gene regulator (agr) in Staphylococcus aureus using an in vitro pharmacodynamic model. J Antimicrob Chemother 2008; 61(5):1099-1102. [PubMed]
292. Rossolini GM, Mantengoli E. Antimicrobial resistance in Europe and its potential impact on empirical therapy. Clin Microbiol Infect 2008; 14 Suppl 6:2-8. [PubMed]
293. Rotschafer JC, Crossley K, Zaske DE, Mead K, Sawchuk RJ, Solem LD. Pharmacokinetics of vancomycin: observations in 28 patients and dosage recommendations. Antimicrob Agents Chemother 1982;22(3):391-394. [PubMed]
294. Rowland M. Clinical pharmacokinetics of teicoplanin. Clin Pharmacokinet 1990;18(3):184-209. [PubMed]
295. Rubin M, Hathorn JW, Marshall D, Gress J, Steinberg SM, Pizzo PA. Gram-positive infections and the use of vancomycin in 550 episodes of fever and neutropenia. Ann Intern Med 1988;108(1):30-35. [PubMed]
296. Rubinstein E, Corey GR, Boucher HW, Niederman MS, Shorr AF, Torres A et al. Telavancin for the treatment of hospital-acquired pneumonia in severelly-ill and older patients: the ATTAIN studies. 48th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC , K529. 2008.
297. Rubinstein E, Corey GR, Stryjewski ME, Vincent JL, Fagon JY, Kollef MH et al. Telavancin for the treatment of hospital-acquired pneumonia caused by MRSA and MSSA: the ATTAIN study. 48th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC , K530. 2008.
298. Ruoff KL, Kuritzkes DR, Wolfson JS, Ferraro MJ. Vancomycin-resistant gram-positive bacteria isolated from human sources. J Clin Microbiol 1988; 26(10):2064-2068. [PubMed]
299. Rupp ME, Fey PD, Longo GM. Effect of LY333328 against vancomycin-resistant Enterococcus faecium in a rat central venous catheter-associated infection model. J Antimicrob Chemother 2001; 47(5):705-707. [PubMed]
300. Rybak MJ. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis 2006;42 Suppl 1:S35-S39. [PubMed]
301. Rybak MJ, Leonard SN, Rossi KL, Cheung CM, Sadar HS, Jones RN. Characterization of vancomycin-heteroresistant Staphylococcus aureus from the metropolitan area of Detroit, Michigan, over a 22-year period (1986 to 2007). J Clin Microbiol 2008; 46(9):2950-2954. [PubMed]
302. Rybak M, Lomaestro B, Rotschafer JC, Moellering R, Jr., Craig W, Billeter M et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 2009; 66(1):82-98. [PubMed]
303. Rybak MJ, Albrecht LM, Berman JR, Warbasse LH, Svensson CK. Vancomycin pharmacokinetics in burn patients and intravenous drug abusers. Antimicrob Agents Chemother 1990; 34(5):792-795. [PubMed]
304. Rybak MJ, Lerner SA, Levine DP, Albrecht LM, McNeil PL, Thompson GA et al. Teicoplanin pharmacokinetics in intravenous drug abusers being treated for bacterial endocarditis. Antimicrob Agents Chemother 1991;35(4):696-700.[PubMed]
305. Rybak MJ, Albrecht LM, Boike SC, Chandrasekar PH. Nephrotoxicity of vancomycin, alone and with an aminoglycoside. J Antimicrob Chemother 1990; 25(4):679-687. [PubMed]
306. Rybak MJ, Boike SC. Monitoring vancomycin therapy. Drug Intell Clin Pharm 1986; 20(10):757-761. [PubMed]
307. Sabath LD, Wheeler N, Laverdiere M, Blazevic D, Wilkinson BJ. A new type of penicillin resistance of Staphylococcus aureus. Lancet 1977;1(8009):443-447. [PubMed]
308. Saha B, Singh AK, Ghosh A, Bal M. Identification and characterization of a vancomycin-resistant Staphylococcus aureus isolated from Kolkata (South Asia). J Med Microbiol 2008;57(Pt 1):72-79. [PubMed]
309. Sakoulas G, Eliopoulos GM, Moellering RC, Jr., Wennersten C, Venkataraman L, Novick RP et al. Accessory gene regulator (agr) locus in geographically diverse Staphylococcus aureus isolates with reduced susceptibility to vancomycin. Antimicrob Agents Chemother 2002;46(5):1492-1502. [PubMed]
310. Sakoulas G, Eliopoulos GM, Moellering RC, Novick RP, Venkataraman L, Wennersten C et al. Staphylococcus aureus accessory gene regulator (agr) group II: is there a relationship to the development of intermediate-level glycopeptide resistance? J Infect Dis 2003;187(6):929-938. [PubMed]
311. Sakoulas G, Eliopoulos GM, Fowler VG, Jr., Moellering RC, Jr., Novick RP, Lucindo N et al. Reduced susceptibility of Staphylococcus aureus to vancomycin and platelet microbicidal protein correlates with defective autolysis and loss of accessory gene regulator (agr) function. Antimicrob Agents Chemother 2005;49(7):2687-2692. [PubMed]
312. Saunders NJ. Why monitor peak vancomycin concentrations? Lancet 1994;344:1748-1750. [PubMed]
313. Schaad UB, Nelson JD, McCracken GH, Jr. Pharmacology and efficacy of vancomycin for staphylococcal infections in children. Rev Infect Dis 1981; 3 suppl:S282-S288. [PubMed]
314. Scheinfeld N. Dalbavancin: a review. Drugs Today (Barc) 2007; 43(5):305-316. [PubMed]
315. Scherl A, Francois P, Charbonnier Y, Deshusses JM, Koessler T, Huyghe A et al. Exploring glycopeptide-resistance in Staphylococcus aureus: a combined proteomics and transcriptomics approach for the identification of resistance-related markers. BMC Genomics 2006; 7:296. [PubMed]
316. Schwalbe RS, Stapleton JT, Gilligan PH. Emergence of vancomycin resistance in coagulase-negative staphylococci. N Engl J Med 1987;316(15):927-931. [PubMed]
317. Seltzer E, Dorr MB, Goldstein BP, Perry M, Dowell JA, Henkel T. Once-weekly dalbavancin versus standard-of-care antimicrobial regimens for treatment of skin and soft-tissue infections. Clin Infect Dis 2003; 37(10):1298-1303. [PubMed]
318. Shaw JP, Seroogy J, Kaniga K, Higgins DL, Kitt M, Barriere S. Pharmacokinetics, serum inhibitory and bactericidal activity, and safety of telavancin in healthy subjects. Antimicrob Agents Chemother 2005; 49(1):195-201.[PubMed]
319. Shea KW, Cunha BA. Teicoplanin. Med Clin North Am 1995;79(4):833-844. [PubMed]
320. Sheldrick GM, Jones PG, Kennard O, Williams DH, Smith GA. Structure of vancomycin and its complex with acetyl-D-alanyl-D-alanine. Nature 1978; 271(5642):223-225. [PubMed]
321. Sievert DM, Rudrik JT, Patel JB, McDonald LC, Wilkins MJ, Hageman JC. Vancomycin-resistant Staphylococcus aureus in the United States, 2002-2006. Clin Infect Dis 2008; 46(5):668-674. [PubMed]
322. Simmons NA, Ball AP, Cawson RA, Eykyn SJ, Feldman R, Littler WA et al. Dental prophylaxis for endocarditis. Lancet 1992; 340(8831):1353. [PubMed]
323. Smith TL, Pearson ML, Wilcox KR, Cruz C, Lancaster MV, Robinson-Dunn B et al. Emergence of vancomycin resistance in Staphylococcus aureus. Glycopeptide-Intermediate Staphylococcus aureus Working Group. N Engl J Med 1999; 340(7):493-501. [PubMed]
324. (340) Smith SR, Cheesbrough JS, Makris M, Davies JM. Teicoplanin administration in patients experiencing reactions to vancomycin. J Antimicrob Chemother 1989; 23(5):810-812. [PubMed]
325. Smith SR, Cheesbrough J, Spearing R, Davies JM. Randomized prospective study comparing vancomycin with teicoplanin in the treatment of infections associated with Hickman catheters. Antimicrob Agents Chemother 1989; 33(8):1193-1197. [PubMed]
326. Somma S, Gastaldo L, Corti A. Teicoplanin, a new antibiotic from Actinoplanes teichomyceticus nov. sp. Antimicrob Agents Chemother 1984; 26(6):917-923. [PubMed]
327. Sorrell TC, Collignon PJ. A prospective study of adverse reactions associated with vancomycin therapy. J Antimicrob Chemother 1985; 16(2):235-241. [PubMed]
328. Spencer RC, Goering R. A critical review of the in-vitro activity of teicoplanin. Int J Antimicrob Agents 1995; 5(3):169-177. [PubMed]
329. Spitzer PG, Eliopoulos GM. Systemic absorption of enteral vancomycin in a patient with pseudomembranous colitis. Ann Intern Med 1984; 100(4):533-534. [PubMed]
330. Stahl JP, Croize J, Wolff M, Garaud JJ, Leclercq P, Vachon F et al. Poor penetration of teicoplanin into cerebrospinal fluid in patients with bacterial meningitis. J Antimicrob Chemother 1987; 20(1):141-142. [PubMed]
331. Steiert M, Schmitz FJ. Dalbavancin (Biosearch Italia/Versicor). Curr Opin Investig Drugs 2002; 3(2):229-233.[PubMed]
332. Steinkraus G, White R, Friedrich L. Vancomycin MIC creep in non-vancomycin-intermediate Staphylococcus aureus (VISA), vancomycin-susceptible clinical methicillin-resistant S. aureus (MRSA) blood isolates from 2001-05. J Antimicrob Chemother 2007; 60(4):788-794. [PubMed]
333. Streit JM, Fritsche TR, Sader HS, Jones RN. Worldwide assessment of dalbavancin activity and spectrum against over 6,000 clinical isolates. Diagn Microbiol Infect Dis 2004; 48(2):137-143. [PubMed]
334. Streit JM, Sader HS, Fritsche TR, Jones RN. Dalbavancin activity against selected populations of antimicrobial-resistant Gram-positive pathogens. Diagn Microbiol Infect Dis 2005; 53(4):307-310. [PubMed]
335. Stryjewski ME, Chu VH, O'Riordan WD, Warren BL, Dunbar LM, Young DM et al. Telavancin versus standard therapy for treatment of complicated skin and skin structure infections caused by gram-positive bacteria: FAST 2 study. Antimicrob Agents Chemother 2006; 50(3):862-867. [PubMed]
336. Stryjewski ME, Graham DR, Wilson SE, O'Riordan W, Young D, Lentnek A et al. Telavancin versus vancomycin for the treatment of complicated skin and skin-structure infections caused by gram-positive organisms. Clin Infect Dis 2008; 46(11):1683-1693. [PubMed]
337. Stryjewski ME, O'Riordan WD, Lau WK, Pien FD, Dunbar LM, Vallee M et al. Telavancin versus standard therapy for treatment of complicated skin and soft-tissue infections due to gram-positive bacteria. Clin Infect Dis 2005; 40(11):1601-1607. [PubMed]
338. Stucki A, Gerber P, Acosta F, Cottagnoud M, Cottagnoud P. Efficacy of telavancin against penicillin-resistant pneumococci and Staphylococcus aureus in a rabbit meningitis model and determination of kinetic parameters. Antimicrob Agents Chemother 2006; 50(2):770-773. [PubMed]
339. Sun HK, Duchin K, Nightingale CH, Shaw JP, Seroogy J, Nicolau DP. Tissue penetration of telavancin after intravenous administration in healthy subjects. Antimicrob Agents Chemother 2006; 50(2):788-790. [PubMed]
340. Swenson JM, Facklam RR, Thornsberry C. Antimicrobial susceptibility of vancomycin-resistant Leuconostoc, Pediococcus, and Lactobacillus species. Antimicrob Agents Chemother 1990; 34(4):543-549. [PubMed]
341. Tarral E, Jehl F, Tarral A, Simeoni U, Monteil H, Willard D et al. Pharmacokinetics of teicoplanin in children. J Antimicrob Chemother 1988; 21 Suppl A:47-51. [PubMed]
342. Terol MJ, Sierra J, Gatell JM, Rozman C. Thrombocytopenia due to use of teicoplanin. Clin Infect Dis 1993; 17(5):927. [PubMed]
343. Tenover FC, Moellering RC, Jr. The rationale for revising the Clinical and Laboratory Standards Institute vancomycin minimal inhibitory concentration interpretive criteria for Staphylococcus aureus. Clin Infect Dis 2007; 44(9):1208-1215. [PubMed]
344. Tenover FC, Lancaster MV, Hill BC, Steward CD, Stocker SA, Hancock GA et al. Characterization of staphylococci with reduced susceptibilities to vancomycin and other glycopeptides. J Clin Microbiol 1998; 36(4):1020-1027.[PubMed]
345. Tenover FC, Biddle JW, Lancaster MV. Increasing resistance to vancomycin and other glycopeptides in Staphylococcus aureus. Emerg Infect Dis 2001; 7(2):327-332. [PubMed]
346. Traber PG, Levine DP. Vancomycin Ototoxicity in patient with normal renal function. Ann Intern Med 1981;95(4):458-460. [PubMed]
347. Tsuji BT, Rybak MJ, Lau KL, Sakoulas G. Evaluation of accessory gene regulator (agr) group and function in the proclivity towards vancomycin intermediate resistance in Staphylococcus aureus. Antimicrob Agents Chemother 2007; 51(3):1089-1091. [PubMed]
348. Uttley AH, Collins CH, Naidoo J, George RC. Vancomycin-resistant enterococci. Lancet 1988; 1(8575-6):57-58.[PubMed]
349. Van Bambeke F, Van Laethem Y, Courvalin P, Tulkens PM. Glycopeptide antibiotics: from conventional molecules to new derivatives. Drugs 2004; 64(9):913-936. [PubMed]
350. Van Bambeke F. Glycopeptides in clinical development: pharmacological profile and clinical perspectives. Curr Opin Pharmacol 2004; 4(5):471-478. [PubMed]
351. Van Bambeke F. Glycopeptides and glycodepsipeptides in clinical development: a comparative review of their antibacterial spectrum, pharmacokinetics and clinical efficacy. Curr Opin Investig Drugs 2006;7(8):740-749. [PubMed]
352. Van Bambeke F, Mingeot-Leclercq MP, Struelens MJ, Tulkens PM. The bacterial envelope as a target for novel anti-MRSA antibiotics. Trends Pharmacol Sci 2008; 29(3):124-134. [PubMed]
353. Van Bambeke F, Carryn S, Seral C, Chanteux H, Tyteca D, Mingeot-Leclercq MP et al. Cellular pharmacokinetics and pharmacodynamics of the glycopeptide antibiotic oritavancin (LY333328) in a model of J774 mouse macrophages. Antimicrob Agents Chemother 2004; 48(8):2853-2860. [PubMed]
354. Van der Auwera P, Aoun M, Meunier F. Randomized study of vancomycin versus teicoplanin for the treatment of gram-positive bacterial infections in immunocompromised hosts. Antimicrob Agents Chemother 1991; 35(3):451-457.[PubMed]
355. van Heijenoort J. Lipid intermediates in the biosynthesis of bacterial peptidoglycan. Microbiol Mol Biol Rev 2007; 71(4):620-635 [PubMed]
356. Varaldo PE, Debbia E, Schito GC. In vitro activity of teichomycin and vancomycin alone and in combination with rifampin. Antimicrob Agents Chemother 1983; 23(3):402-406. [PubMed]
357. Verbist L, Tjandramaga B, Hendrickx B, Van Hecken A, Van Melle P, Verbesselt R et al. In vitro activity and human pharmacokinetics of teicoplanin. Antimicrob Agents Chemother 1984; 26(6):881-886. [PubMed]
358. Viladrich PF, Gudiol F, Linares J, Pallares R, Sabate I, Rufi G et al. Evaluation of vancomycin for therapy of adult pneumococcal meningitis. Antimicrob Agents Chemother 1991; 35(12):2467-2472. [PubMed]
359. Walker RW, Heaton A. Thrombocytopenia due to vancomycin. Lancet 1985; 1(8434):932. [PubMed]
360. Walker S, Chen L, Hu Y, Rew Y, Shin D, Boger DL. Chemistry and biology of ramoplanin: a lipoglycodepsipeptide with potent antibiotic activity. Chem Rev 2005; 105(2):449-476. [PubMed]
361. Wall R, Klenerman L, McCullough C, Fyfe I. A comparison of teicoplanin and cefuroxime as prophylaxis for orthopaedic implant surgery: a preliminary report. J Antimicrob Chemother 1988; 21 Suppl A:141-146. [PubMed]
362. Wang G, Hindler JF, Ward KW, Bruckner DA. Increased vancomycin MICs for Staphylococcus aureus clinical isolates from a university hospital during a 5-year period. J Clin Microbiol 2006; 44(11):3883-3886.[PubMed]
363. Ward KE, Mersfelder TL, LaPlante KL. Oritavancin--an investigational glycopeptide antibiotic. Expert Opin Investig Drugs 2006; 15(4):417-429. [PubMed]
364. Wasilewski MM, Disch DP, McGill JM, Harris HW, O'Riordan W, Zeckel ML. Equivalence of shorter course therapy with oritavancin vs. vancomycin/cephalexin in complicated skin/skin structure infections. 41th Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Ill. UL-18. 2001.
365. Watanakunakorn C, Tisone JC. Synergism between vancomycin and gentamicin or tobramycin for methicillin-susceptible and methicillin-resistant Staphylococcus aureus strains. Antimicrob Agents Chemother 1982; 22(5):903-905.[PubMed]
366. Watanakunakorn C, Guerriero JC. Interaction between vancomycin and rifampin against Staphylococcus aureus. Antimicrob Agents Chemother 1981; 19(6):1089-1091. [PubMed]
367. Watanakunakorn C, Bakie C. Synergism of vancomycin-gentamicin and vancomycin-streptomycin against enterococci. Antimicrob Agents Chemother 1973; 4(2):120-124. [PubMed]
368. Watanakunakorn C. Treatment of infections due to methicillin-resistant Staphylococcus aureus. Ann Intern Med 1982; 97(3):376-378. [PubMed]
369. Welty TE, Copa AK. Impact of vancomycin therapeutic drug monitoring on patient care. Ann Pharmacother 1994; 28(12):1335-1339. [PubMed]
370. Werner G, Coque TM, Hammerum AM, Hope R, Hryniewicz W, Johnson A et al. Emergence and spread of vancomycin resistance among enterococci in Europe. Euro Surveill 2008; 13(47). [PubMed]
371. West BC. Vancomycin-induced neutropenia. South Med J 1981; 74(10):1255-1256. [PubMed]
372. Williams DH, Kalman J. Structural and mode of action studies on the antibiotic vancomycin. Evidence from 270-MHz proton magnetic resonance. J Am Chem Soc 1977; 99(8):2768-2774. [PubMed]
373. Wilson AP, Gruneberg RN, Neu H. Dosage recommendations for teicoplanin. J Antimicrob Chemother 1993;32(6):792-796. [PubMed]
374.Wilson AP. Clinical pharmacokinetics of teicoplanin. Clin Pharmacokinet 2000; 39(3):167-183. [PubMed]
375.Wilson AP, Taylor B, Treasure T, Gruneberg RN, Patton K, Felmingham D et al. Antibiotic prophylaxis in cardiac surgery: serum and tissue levels of teicoplanin, flucloxacillin and tobramycin. J Antimicrob Chemother 1988;21:201-212.[PubMed]
376. Wilson SE, O'Riordan W, Hopkins A, Friedland HD, Barriere SL, Kitt MM. Telavancin versus vancomycin for the treatment of complicated skin and skin-structure infections associated with surgical procedures. Am J Surg 2008.[PubMed]
377. Wise R, Donovan IA, McNulty CA, Waldron R, Andrews JM. Teicoplanin, its pharmacokinetics, blister and peritoneal fluid penetration. J Hosp Infect 1986; 7 Suppl A:47-55.[PubMed]
378. Woodley DW, Hall WH. The treatment of severe staphylococcal infections with vancomycin. Ann Intern Med 1961; 55:235-249. [PubMed]
379. Wootton M, Howe RA, Hillman R, Walsh TR, Bennett PM, MacGowan AP. A modified population analysis profile (PAP) method to detect hetero-resistance to vancomycin in Staphylococcus aureus in a UK hospital. J Antimicrob Chemother 2001; 47(4):399-403. [PubMed]
380. Wong SL, Barriere SL, Kitt MM, Goldberg MR. Multiple-dose pharmacokinetics of intravenous telavancin in healthy male and female subjects. J Antimicrob Chemother 2008; 62(4):780-783. [PubMed]
381. Wood MJ. The comparative efficacy and safety of teicoplanin and vancomycin. J Antimicrob Chemother 1996; 37(2):209-222. [PubMed]
382. Wysocki M, Delatour F, Faurisson F, Rauss A, Pean Y, Misset B et al. Continuous versus intermittent infusion of vancomycin in severe Staphylococcal infections: prospective multicenter randomized study. Antimicrob Agents Chemother 2001; 45(9):2460-2467. [PubMed]
383. Yang Z, Vorpagel ER, Laskin J. Influence of the Charge State on the Structures and Interactions of Vancomycin Antibiotics with Cell-Wall Analogue Peptides: Experimental and Theoretical Studies. Chemistry 2009. [PubMed]
384. Yang Z, Vorpagel ER, Laskin J. Experimental and theoretical studies of the structures and interactions of vancomycin antibiotics with cell wall analogues. J Am Chem Soc 2008; 130(39):13013-13022. [PubMed]
385. Yin LY, Calhoun JH, Thomas TS, Wirtz ED. Efficacy of telavancin in the treatment of methicillin-resistant Staphylococcus aureus osteomyelitis: studies with a rabbit model. J Antimicrob Chemother 2009; 63(2):357-360.[PubMed]
386. Zhanel GG, Trapp S, Gin AS, DeCorby M, Lagace-Wiens PR, Rubinstein E et al. Dalbavancin and telavancin: novel lipoglycopeptides for the treatment of Gram-positive infections. Expert Rev Anti Infect Ther 2008; 6(1):67-81. [PubMed]
387. Zhanel GG, DeCorby M, Nichol KA, Wierzbowski A, Baudry PJ, Karlowsky JA et al. Antimicrobial susceptibility of 3931 organisms isolated from intensive care units in Canada: Canadian National Intensive Care Unit Study, 2005/2006. Diagn Microbiol Infect Dis 2008; 62(1):67-80.
388. Zimmermann AE, Katona BG, Plaisance KI. Association of vancomycin serum concentrations with outcomes in patients with gram-positive bacteremia. Pharmacotherapy 1995; 15(1):85-91. [PubMed]
Tables
Figure 1: Chemical Structure and Structure-Activity Relationships.
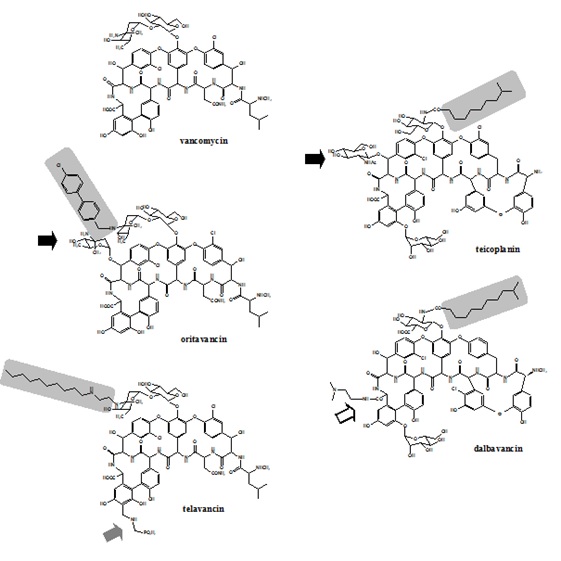
Table 1: List of Common Brand Names and Manufacturers.
| Generic Name | Brand Names | Manufacturers |
|---|---|---|
| Vancomycin | Vancocin Lyphocin, Vancoled | Eli Lilly Generic manufacturers |
| Teicoplanin | Targocid | Sanofi-Aventis |
| Oritavancin | - | Eli Lilly Intermune (2001) Targanta (2005) The Medicines Company (2009) |
| Telavancin | Vibrative | Theravance Inc. in the USA; Astellas in Europe |
| Dalbavancin | - | Biosearch Italia - Versicor Vicuron (2003) Pfizer (2005) |
Table 2: Susceptibility of the Main Target Pathogens to Glycopeptides and Lipoglycopeptides.
| Vancomycin | Teicoplanin | Oritavancin | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
Organism |
Phenotype of resistance | No | MIC50 (mg/L) | MIC90 (mg/L) | MIC range (mg/L) | No | MIC50 (mg/L) | MIC90 (mg/L) | MIC range (mg/L) | No | MIC90 (mg/L) | MIC range (mg/L) |
E. faecalis |
VanS | 345 | 1 | 2 | ≤0.5-4 | 586 | ≤0.12 | 0.5 | ≤0.12-2 | 850 | 0.03 | ≤0.0005-0.5 |
| VanR | 19 | 512 | >512 | 8->512 | 120 | >16 | >16 | ≤0.12->16 | 59 | 1 | 0.015-4 | |
E. faecium |
VanS | 76 | ≤0.5 | 1 | ≤0.5-2 | 77 | 0.5 | 0.5 | ≤0.12-4 | 120 | 0.015 | ≤0.0005 |
| VanR | 184 | 512 | 512 | 8->512 | 51 | >16 | >16 | 0.25->16 | 269 | 0.25 | 0.06 | |
S. aureus |
MSSA | 1077 | 1 | 2 | ≤0.25-2 | 1815 | 0.5 | 1 | ≤0.12-2 | 2518 | 0.12 | ≤0.0005-2 |
| MRSA | 885 | 1 | 1 | 0.5-2 | 1117 | 0.5 | 2 | -- | 2490 | 0.25 | ≤0.004-2 | |
| VISA | 50 | 4 | 8 | 4-8 | -- | -- | -- | -- | 11 | 1 | ≤0.004-4 | |
| VRSA | -- | -- | -- | -- | -- | -- | -- | -- | 5 | -- | 0.5-1 | |
S. coag. Negative |
MS | 30 | 1 | 2 | 0.5-2 | 157 | 1 | 4 | ≤0.12-16 | 213 | 0.25 | 0.06-0.5 |
| MR | 50 | 1 | 2 | 0.5-4 | 617 | 2 | 4 | ≤0.12->16 | 649 | 0.25 | 0.008-1 | |
S. pneumoniae |
PenS | 996 | 0.25 | 0.5 | ≤0.06-1 | 30 | 0.03 | 0.06 | 0.016-0.12 | 646 | 0.004 | ≤0.004-1 |
| PenI | 400 | 0.25 | 0.5 | ≤0.06-2 | 60 | 0.03 | 0.06 | 0.016-.12 | 216 | 0.008 | ≤0.0005-0.25 | |
| PenR | 202 | 0.25 | 0.5 | ≤0.012-0.5 | 209 | 0.06 | 0.12 | 0.016-0.25 | 148 | 0.008 | 0.002-0.015 | |
S. pyogenes |
-- | 49 | 0.5 | 0.5 | ≤0.25-0.5 | 241 | ≤0.12 | ≤0.12 | ≤0.12-0.25 | 287 | 0.25 | 0.008-0.5 |
| S. algalactiae | 39 | 0.5 | 0.5 | ≤0.25-0.5 | ≤0.25-1 | 101 | 0.12 | 0.03-0.5 | ||||
| Telavancin | Dalbavancin | |||||||
|---|---|---|---|---|---|---|---|---|
Organism |
No | MIC50 (mg/L) | MIC90 (mg/L) | MIC range (mg/L) | No | MIC50 (mg/L) | MIC90 (mg/L) | MIC range (mg/L) |
E. faecalis |
345 | 0.5 | 1 | 0.12-2 | 586 | 0.03 | 0.06 | ≤0.015- 4 |
| 19 | 8 | 16 | 0.5-16 | 120 | 4 | 32 | ≤0.015- 32 | |
E. faecium |
76 | 0.12 | 0.025 | ≤0.015-0.5 | 77 | 0.06 | 0.12 | ≤0.015- 4 |
| 184 | 4 | 8 | 0.015-16 | 51 | 8 | 32 | ≤0.03- >32 | |
S. aureus |
1077 | 0.25 | 0.5 | 0.03-1 | 1815 | 0.06 | 0.06 | ≤0.015- 0.25 |
| 885 | 0.25 | 0.5 | 0.06-1 | 1117 | 0.06 | 0.06 | ≤0.015- 0.5 | |
| 50 | 0.5 | 1 | 0.125-1 | -- | -- | -- | -- | |
| -- | -- | - | -- | -- | -- | -- | -- | |
S. coag. Negative |
30 | 0.25 | 0.25 | 0.12-0.25 | 157 | 0.03 | 0.06 | ≤0.015-0.25 |
| 50 | 0.25 | 0.25 | 0.12-0.5 | 617 | 0.03 | 0.06 | ≤0.015- 0.5 | |
S. pneumoniae |
371 | 0.015 | 0.03 | ≤0.001-0.06 | 996 | ≤0.015 | 0.03 | ≤0.015- 0.06 |
| 36 | 0.015 | 0.03 | 0.008-0.06 | 400 | ≤0.015 | 0.03 | ≤0.015- 0.25 | |
| 22 | 0.015 | 0.03 | 0.015-0.03 | 202 | ≤0.016 | 0.016 | ≤0.016- 0.03 | |
S. pyogenes |
60 | 0.03 | 0.06 | 0.03-0.06 | 49 | ≤0.03 | ≤0.03 | ≤0.03 |
| S. algalactiae | 60 | 0.06 | 0.06 | 0.03-0.12 | 39 | ≤0.03 | ≤0.03 | ≤0.03- 0.06 |
Table 3: Types of Resistance to Vancomycin in Enterococci, in Relation with Alternative Peptidoglycan Precursors Produced by Alternative DD-ligases.
| Type | MIC (mg/L) | Bacterial species | Location of genes | Expression | Conjugaison | Alternative dimer | |
|---|---|---|---|---|---|---|---|
| Vancomycin | Teicoplanin | ||||||
| VanA | 64-100 | 16-512 | Enterococci (faecium, faecalis, avium, durans, raffinosus, gallinarum, casseliflavus) ; S. aureus |
Transposon on plasmid or chromosome (Tn1546) | Inducible | Yes | D-ala – D-lac |
| VanB | 4-1000 | 0.5-1 | Enterococci (faecium, faecalis, bovis) |
transposon on plasmid or chromosome (Tn1547 or Tn1549) | Inducible | Yes | D-ala – D-lac |
| VanC | 2-32 | 0.5-1 | Enterococci (gallinarum, casseliflavus, flavescens) |
Chromosome | Constitutive | No | D-ala – D-ser |
| VanD | 64-128 | 4-64 | E. faecium | Chromosome | Constitutive or Inducible | No | D-ala – D-lac |
| VanE | 8-32 | 0.5 | E. faecalis | Chromosome | Inducible | No | D-ala – D-ser |
| VanG | 16 | 0.5 | E. faecalis | Chromosome | Inducible | Yes | D-ala – D-ser |
Table 4: Pharmacokinetic Parameters for Glycopeptides and Lipoglycopeptides at Doses Pertinent of Their Use in Humans.
| Glycopeptide and dosage | |||||
|---|---|---|---|---|---|
| Parameter (units) | Vancomycin (236) (15 mg/kg) | Oritavancin (3) (3 mg/kg) | Telavancin (31) (7.5 mg/kg) |
Teicoplanin (237) (6 mg/kg) | Dalbavancin (238) (15 mg/kg) |
| Cmax (mg/L) | 20-50 | 31 | 89 | 43 | 312 |
| Vd (L/kg) | 0.3 | 0.1 | 0.9-1.6 | 0.11 | |
| Prot. binding (%) | 10-55 | 90 | 90-93 | 90 | 98 |
| Terminal half-life (h) | 4-8 | 360 | 7 | 83-168 | 149 |
| AUC (mg.h/L) | 260 | 152 | 600 | 550 | 27103 |
Table 5: Dosing During Continuous Renal Replacement Therapy.
| CVVH (Continuous venovenous hemofiltration) | 1g IV q48h |
| CVVHD (Continuous venovenous hemodialysis) | 1g IV q24h |
| CVVHDF (Continuous venovenous hemodiafiltration) | 1g IV q24h |
Note: CVVH is mainly for fluid removal alone. Many institutions will employ more CVVHD or CVVHDF which combine dialysis with fluid removal.
Note: Recommended loading dose is 15-20mg/kg.
What's New
Hall RG, et al. Empiric guideline-recommended weight-based vancomycin dosing and nephrotoxicity rates in patients with methicillin-resistant Staphylococcus aureus bacteremia: a retrospective cohort study. BMC Pharmacol Toxicol 2013;14:12.
van Hal SJ, et al. Systematic Review and Meta-Analysis of Vancomycin-Induced Nephrotoxicity Associated with Dosing Schedules that Maintain Troughs Between 15 and 20 Milligrams per Liter. Antimicrob Agents Chemotherapy 2013;57:734-744.
Ito H, et al. Pharmacokinetics of glycopeptides in children. J Infect Chemother 2012.
Bosso JA, et al. Relationship between Vancomycin Trough Concentrations and Nephrotoxicity: a Prospective Multicenter Trial. Antimicrobial Agents Chemother 2011;55:5475-5479.
Chiu CH, et al. Effectiveness of a Guideline to Reduce Vancomycin Use in the Neonatal Intensive Care Unit. Ped Infect Dis J 2011;30:273-275.
GUIDED MEDLINE SEARCH FOR:
Vancomycin
Pharmacokinetics/Pharmacodynamics
Teicoplanin
Pharmacokinetics/Pharmacodynamics
Oritavancin
Pharmacokinetics/Pharmacodynamics
Telavancin
Pharmacokinetics/Pharmacodynamics
Dalbavancin
GUIDED MEDLINE SEARCH FOR RECENT REVIEWS:
Vancomycin
Pharmacokinetics/Pharmacodynamics
Teicoplanin
Pharmacokinetics/Pharmacodynamics
Oritavancin
Pharmacokinetics/Pharmacodynamics
Telavancin
Pharmacokinetics/Pharmacodynamics
