Polymyxins
Authors: Matthew E. Falagas, M.D., M.Sc. and Konstantinos Z. Vardakas, M.D., Ph.D.
Previous authors: Matthew E. Falagas, M.D., M.Sc., Argyris Michalopoulos, M.D., Nicolaos Choulis, Ph.D.
CLASS
Chemical Structure
Polymyxins are a group of polypeptide antibiotics which were discovered in 1947 (185). They are produced by fermentation in different species of Paenibacillus polymyxa (previously Bacillus polymyxa) by nonribosomal peptide synthetase enzyme complexes (26, 82). They are pentacationic polypeptides consisting of a cyclic heptapeptide, a linear tripeptide and a fatty acid tail linked to the N-terminal of the tripeptide (86). They are formed by both cationic and hydrophobic amino acids (82). The five L-diaminobutyric acid (L-Dab) molecules are positively charged in positions 1,3,5,8 and 9.
Polymyxins included historically 5 main different chemical compounds (polymyxin A, B, C, D, and E). The main differences between these compounds are found in amino acid sequences and fatty acid side chains. The major representatives of polymyxins that have been used in clinical practice are polymyxin B and polymyxin E (colistin). The main difference between these two molecules is that polymyxin B contains phenylalanine in position 6 (starting from the N-terminal), while colistin contains D-leucine (Figure 1a and 1b) (100, 185,136). Polymyxin B preparations consist mainly of two major components, polymyxin B1 and B2 in addition to a small amount of other polymyxins B. Similarly, colistin consists mainly of polymyxin E1 (colistin A) and polymyxin E2 (colistin B) and fewer amounts of other polymyxins E (82). The proportion of these components may vary between different pharmaceutical preparations. At least 30 different components have been detected in colistin (36, 144). These components differ only in the fatty acid side chain (106).
Polymyxin B was developed from P. polymyxa. Its tripeptide side chain is linked to the fatty acid residue, which has been identified as 6-methyl-octan-oic acid (polymyxin B1) or 6-methyl-eptanoic acid (polymyxin B2). Colistin was developed from P. polymyxa subsp. Colistinus (95, 96). Its tripeptide side chain is linked to the fatty acid residue, which has been identified as 6-methyl-octan-oic acid (colistin A) or 6-methyl-eptanoic acid (colistin B) (185). Besides the 5 main compounds, several other naturally produced polymyxins have been discovered. The main representatives are polymyxin M (179) polymyxin S, and polymyxin T (122, 177, 178). They are produced by different species and differ from polymyxin B and colistin in the amino acids included in their molecule; in addition, polymyxin M and T are also active against Gram positive bacteria.
Novel polymyxins have been developed by enzymatic cleavage of the fatty acid and the first L-α-γ-diaminobutyric acid (nonapeptides) or the entire linear tripeptide (heptapeptides), introduction of benzyl groups, or reduction of the positively charged Dab molecules from five to three (207, 209). In derivatives by enzymatic cleavage, although binding to lipopolysacharides (LPS) was preserved, the loss of the fatty acid resulted in lesser toxicity, suggesting a key role of the fatty acid for the antibacterial activity of polymyxins (143, 169). Derivatives with longer (nine or more carbons) or shorter (six or less carbons) fatty acid chains had in general lesser activity against Gram negative bacteria in additional experiments. Nonapeptides were also associated with fewer adverse events than their parent molecules. The introduction of benzyl groups enhanced antimicrobial activity against Gram positive bacteria, but reduced the activity against Escherichia coli(210). The reduction of the cationic Dab components resulted in molecules with different or no antibacterial activity, which was correlated with the location of the positive charges (200, 201, 202,203).
ANTIMICROBIAL ACTIVITY
Spectrum
Most GNB are susceptible to polymyxins, including multi-drug resistant (MDR) Acinetobacter baumannii and Pseudomonas aeruginosa, Klebsiella spp.>, Enterobacterspp., Escherichia coli, Salmonella spp., Shigella spp., Citrobacter spp., Yersinia pseudotuberculosis, and Haemophilus influenzae (55, 77, 139). Increase in inoculum size decreases the antibacterial activity of colistin; its effects are not altered in the presence of plasma. Using colistin sulfate, Wright and Welch (1959) demonstrated a greater degree of activity of this compound than of polymyxin B against Pseudomonas, Salmonella, Shigella, and members of the coli-aerogenes group (211). Colistin has also been shown to possess a considerable in vitro activity againstStenotrophomonas maltophilia strains (83-88% of the tested isolates were sensitive to colistin in two studies) (55, 77, 139). It has also been reported that colistin is potentially active against several mycobacterial species including M. xenopi, M. intracellulare, M. tuberculosis, M. fortuitum, M. phlei, and M. smegmatis (159, 130, 161). Several pathogens such as Proteus spp.,Providencia spp., Morganella spp., and most isolates of Serratia spp., isolates of Brucella spp., Neisseria spp., Chromobacterium spp., and Burkholderia spp. exhibit intrinsic resistance to the polymyxins. Finally, polymyxin B was found to be active against Cryptococcus neoformans (215).
In Vitro Colistin Susceptibility
The disk diffusion method (DDM) using 10 micrograms of colistin sulfate disk (Oxoid, Basingstoke, Hants, England) or 30 micrograms polymyxin B disk, has been a common method for susceptibility testing of colistin and polymyxin B, respectively. Isolates are considered sensitive if the inhibition zone is ≥ 11 mm. However, recent studies showed that the Etest, broth microdilution method, agar dilution and VITEK 2 are preferable tests compared to the DDM for the determination of in vitro colistin susceptibility (54, 166, 181). Two studies reported that there is general agreement in the results obtained from agar dilution and broth microdilution methods regarding testing of polymyxins (189, 205). Reports showing an 87% agreement between agar dilution and Etest and 82% between agar dilution and Vitek 2 suggest that both methods require at least confirmation by a standard MIC susceptibility testing method.
When DDM was compared with six other methods of colistin susceptibility testing (agar dilution on Mueller-Hinton [MH] and Isosensitest agar, Etest on MH and Isosensitest agar, broth microdilution, and VITEK 2) on clinical isolates from intensive care unit patients, it was found to be an unreliable method to measure susceptibility to colistin (114). High error rates and low levels of reproducibility were observed in the DDM as well. On the contrary, the colistin Etest, agar dilution, and the VITEK 2 showed a high level of agreement with the broth microdilution reference method. Heteroresistant to colistin populations of Enterobacter cloacae and A. baumannii were reported in this study; all methods besides VITEK 2 were able to detect heteroresistant subpopulations of E. cloacae (114).
Both the Clinical and Laboratory Standards Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) provide breakpoints for colistin susceptibility for P. aeruginosa, A. baumannii, and Enterobacteriaceae. Susceptibility break points refer to colistin sulfate. Only CLSI provides breakpoints for polymyxin B, which are the same as for colistin. Several reports has shown nearly complete agreement of susceptibility results for colistin and polymyxin B (55, 205). The general MIC breakpoint to identify bacteria susceptible to polymyxins is≤ 2 μg/ml (27, 46). The main difference is that CLSI defines as susceptible those P. aeruginosa strains with an MIC ≤2 μg/ml, while EUCAST those with an MIC ≤ 4μg/ml. Table 1 shows the current breakpoints provided from the CLSI and EUCAST for the aforementioned bacteria.
Currently, A. baumannii, P. aeruginosa and Enterobacteriaceae (those not inherently resistant to polymyxins) remain susceptible to polymyxins. K. pneumoniae may be an exception in a few areas of the globe (Asia-Pacific, South America, Mediterranean countries).
Mechanisms of Action and Structure-Activity Relationship
Polymyxin B and colistin share the same mechanism of action. The L-Dab molecules contained in polymyxins are positively charged, while the lipopolysaccharides (LPS) present in the cell wall of Gram-negative bacteria, are negatively charged. Polymyxins possess a higher affinity for LPS molecules of the outer cell membrane of Gram-negative bacteria than do divalent cations such as magnesium (Mg+2) and calcium (Ca+2) which normally stabilize the LPS molecules (35, 136, 175). Polymyxins bind to the Gram-negative bacterial cell membrane phospholipids, producing a disruptive physicochemical effect, which leads to cell-membrane permeability changes and ultimately cell death (47). The initial association of polymyxins with the bacterial membrane occurs through electrostatic interactions between the cationic polypeptide and anionic LPS molecules in the outer membrane of the Gram-negative bacteria. Polymyxins displace magnesium (Mg+2) and calcium (Ca+2), from the negatively charged LPS, leading to a local disturbance of the outer membrane. The result of this process causes an increase in the permeability of the cell envelope consisting of the cell wall and the cytoplasmic membrane, leakage of cell contents, and subsequently cell death (35, 136). However, high concentrations of calcium and/or magnesium can antagonize the binding of polymyxins with LPS molecules (35, 68). It has been suggested that this detergent effect may render Gram negative bacteria more susceptible to other antimicrobials following exposure to polymyxins.
A secondary target site for polymyxins is the type II NADH-quinone oxydoreductase respiratory enzyme of the inner bacterial membrane. These enzymes are an integral part of the bacterial electron transport pathway. Newer studies show that polymyxins inhibit these enzymes in both Gram positive and Gram negative bacteria (39).
In addition, polymyxins exert anti-endotoxin activity by binding to and neutralizing endotoxins of Gram negative bacteria. The significance of this mechanism (namely the prevention of the endotoxin's ability to induce shock through the release of cytokines) for in vivo antimicrobial action is not clear, since plasma endotoxin is immediately bound by LPS-binding protein and the complex quickly bound to cell-surface CD14 (61).
MecHANISMS OF RESISTANCE
Organisms Inherently Resistant
Proteus spp., Neisseria spp., Serratia spp., Morganella spp., and Providencia spp. are resistant to polymyxins. Gram-positive bacteria are, in the main, also unaffected. Pseudomonas mallei,Burkholderia cepacia, Edwardsiella spp., and Brucella spp. are all resistant to polymyxins. In addition, polymyxins are not active against all anaerobes, fungi, and parasites (25, 185). Most of the data regarding intrinsic resistance come from studies in Proteus spp, which possess a cell envelope that prevents polymyxins from accessing the susceptible lipid target sites or changes in lipid A that account for reduced binding (100, 101, 142, 186). Ιncreased polymyxin susceptibility in B. cepacia and P. mirabilis, has been associated with defects in UDP-glucose dehydrogenase and UDP-glucose phosphorylase, enzymes involved in the biosynthesis of the LPS precursor, UDP-glucose (85).
In addition, sterol-like compounds called hopanoids in the outer membrane of Burkholderia multivorans create a barrier, and they have been associated with intrinsic polymyxin resistance (117). Finally, the periplasmic proteases found in B. cenocepacia and the multidrug efflux pump NorM in Burkholderia vietnamien have been shown to contribute to polymyxin resistance (51, 115,116).
Mechanisms of Acquired Resistance
De novo resistance to polymyxins in Gram-negative bacteria can develop through either mutation or adaptation mechanisms. Mutation is inherited, low-level, and independent of the continuous presence of the antibiotic, whereas adaptation is the opposite (non-inherited, high level, and requires the continuous presence of the antibiotic). Almost complete cross-resistance exists between colistin and polymyxin B (62, 65,133).
Modifications of the LPS moiety, in addition to further alterations of the outer bacterial membrane, are probably the most important mechanism conferring resistance to polymyxins (22, 89, 133). These modifications are a result of a gene mediated transcription pathway leading to the production of 4-amino-4-deoxy-l-arabinose (LAra4N) and its addition to lipid A. This causes an increase of the absolute charge of lipid A that lowers the affinity for polymyxins (89). The major gene implicated in this pathway is called arn (formerly known as pmr – polymyxin resistance operon) and the whole process is mediated by the PmrA/PmrB and PhoP/PhoQ regulatory systems with two components: a sensor cytoplasmic membrane kinase (PmrB or PhoQ) which upon activation phosphorylates the regulator component (PmrB or PhoP) (163). These in turn promote arn transcription (49, 66, 124, 134). These genes were first studied in S. enterica serotype Typhimuriumand E. coli (66). However, similar pathways, that may implicate more regulatory genes, exist in other gram-negative bacteria, such as P. aeruginosa, B. cepacia, Salmonella spp., Yersinia pestis and K. pneumonia (21, 23). These mechanisms, although common in gram-negative bacteria, exhibit interspecies variation.
It has been suggested that another mechanism of resistance in P. aeruginosa is that the outer membrane protein OprH blocks the self-promoted uptake pathway of polymyxins by replacing Mg+2on the LPS molecule. Thus, the overexpression of OprH caused by mutation or as a result of adaptation to a Mg+2-deficient medium can be associated with resistance to polymyxins (137). Furthermore, increased levels of the outer membrane protein H1 inhibits the action of polymyxins by replacing Mg2+ or Ca2+ at the binding sites on LPS (11, 138). Cell surface changes were also related with the development of resistance. These include alterations of the outer membrane of the bacteria cell (reduction of LPS, reduced levels of specific outer membrane proteins, reduction in cell envelope Mg+2 and Ca+2 contents, and lipid alterations). Specifically, the absence of 2-hydroxylaurate or an increase in the palmitate content of lipid A has been associated with resistance of P. aeruginosa to polymyxins (38). In biofilms of P. aeruginosa, colistin resistance coincided with the over expression of the mexAB–oprM efflux pump system (148).
In A. baumannii, resistance mechanisms are not well understood. However, mutations in the PmrA/PmrB encoding genes are linked to polymyxin resistance (1). Another surprising polymyxin resistance mechanism reported in A. baumannii involves complete loss of LPS production (130). In order to compensate for the decreased outer membrane integrity due to the LPS loss, polymyxin-resistant A. baumannii strains upregulated the expression of genes responsible for phospholipid, lipoprotein and poly-β-1,6-N-acetylglucosamine production (75).
In K. pneumoniae strains the increased production of capsule polysaccharide (CPS) limits the interaction of polymyxins with their target sites. Thus, upregulation of CPS production contributes to increased polymyxin resistance (22). In K. pneumoniae, a deficiency in the outer membrane protein OmpA, which mediates adhesion to eukaryotic cells, has been also associated with an increased susceptibility to polymyxin B (113). Moreover, the AcrAB–TolC efflux pump has been associated with polymyxin resistance in both K. pneumoniae and E. coli (147, 208).
An efflux pump/potassium system has been found to be associated with the mediation of resistance to cationic antimicrobial peptides in general, including polymyxin B in Yersinia spp.
Methods to Overcome or Prevent Resistance
Treatment with a combination of antibiotics has been long proposed as a mechanism to reduce resistance. A few in vitro studies on the synergistic activity of carbapenems and polymyxins provided evidence that this can be achieved (218). However, this data require confirmation in clinical studies. To date, no studies have focused on the effect of the dosage and/or duration of treatment with polymyxins on the development of resistance. However, studies had shown that low concentrations of colistin have been associated with amplification of the resistant subpopulations in hetero-resistant strains (13, 41, 157).
Ecological Considerations
Colistin resistance among Gram-negative microorganisms has been reported in several in vitro studies, as well as in surveillance studies for antimicrobial resistance (58, 154). In most of these studies the isolated strains of P. aeruginosa, K. pneumoniae, and A. baumannii exhibited a greater than 98% susceptibility to colistin or polymyxin B (168). There are only few clinical reports in the current literature regarding infections due to P. aeruginosa with in vitro resistance to colistin (38, 188). Colistin resistant P. aeruginosa was found in 5 of 150 children with cystic fibrosis over a 5-year period of follow up (188). However, reports of polymyxin-resistant K. pneumoniae infections are increasingly published (24, 45,123).
Isolation of colistin-resistant gram negative bacteria has been associated with colistin use in several studies (92, 123). Recently, reports have been published regarding the potential selection of strains inherently resistant to polymyxins following the increasing consumption of polymyxins worldwide. These included strains of B. cepacia in Czech Republic, P. stuartii in the USA, and Enterobacteriaceae inherently resistant to colistin in Greece (69, 72, 92, 172).Breakthrough infections due to such organisms were also reported (40).
PHARMACOKINETICS AND PHARMACODYNAMICS
Following the worldwide spread of extensively drug resistant (XDR) Gram negative bacteria in the early years of the 21st century, for which limited treatment options were available, the pharmacokinetic (PK) and pharmacodynamic (PD) properties of polymyxins were studied using more rigorous scientific methods. It was only recently that their PK properties were properly delineated. The following data refer to parenteral administration, since polymyxins are not absorbed through the gastrointestinal tract or the intact skin. Since colistin is the most widely used antibiotic, most data refer to it. The known differences between polymyxin B and colistin will be then shortly described (Table 2).
Distribution, Protein Binding, and Elimination
It is important to note that colistin is administered in its inactive prodrug form, colistimethate sodium (CMS). Following intravenous administration, CMS displays linear dose dependent PK properties, and approximately 60% of the dose is cleared through the kidneys. Colistin on the other hand is mainly (>99%) non-renally excreted by means of mechanisms not yet fully understood (110) while the evidence suggests extensive renal tubular re-absorption (107). Despite that, colistin urinary concentration are high due to conversion of CMS to colistin in the urine. Both CMS and colistin are eliminated by venovenous hemofiltration and hemodialysis (29, 118).
In vitro 31.2% of CMS was hydrolyzed to colistin in 4 hours at 37oC in human plasma (108) while in vivo a smaller proportion of CMS is hydrolyzed to colistin (109, 119). This arises mainly from the fact that CMS renal clearance is higher than conversion clearance to colistin, especially in patients with normal renal function. Thus it is estimated that in such patients only up to 25% of CMS is converted to active colistin (135). Furthermore, there is inter-individual variability in conversion of CMS to colistin at a given creatinine clearance (a ten-fold range in the steady state of plasma colistin concentration has been observed), that arises from the inefficiency of CMS as a pro-drug and the probability of batch-to-batch variability in the composition of the administered formulation (135). In renal failure, the renal excretion of CMS is decreased resulting in a greater conversion to colistin (67).
Thus, following intravenous administration, colistin plasma concentration increases slowly due to the slow conversion of CMS to colistin. In ICU patients with variable renal function treated with colistin 3x106 IU or 240mg every 8 hours, approximately 7 hours were required to reach peak plasma concentrations after the first dose. The steady state mean plasma concentration was 2μg/ml, however at least 3 doses were required to achieve these values. Fluctuation of mean plasma concentrations was limited as a result of the long half-life and the selected dosing interval. The elimination half-life was 14.4 hours (2.3 hours for CMS) (155). The administration of a loading dose (6x106 IU or 480 mg) resulted in faster achievement of bactericidal concentrations in critically ill patients compared to patients who did not receive a loading dose (131). Still, this took several hours (4 hours). It is suggested that a CMS loading dose according to body weight (the lower of either actual or ideal body weight) should be considered but should not exceed 10x106 IU or 800mg. Similarly, maintenance daily dose should not exceed 10x106 IU (29).
Tissue binding is a dominant factor in the distribution and elimination of polymyxins. Old experimental studies have shown that polymyxins accumulate in liver, lung, kidney, heart, and muscles (97). At 24h, less than 50 % of the doses of polymyxins were bound to tissues; the largest amount was in the skeletal muscle (31). A plasma protein binding of 55% was reported for colistin in experimental animal studies following intravenous administration (3, 107). Additionally, higher protein binding for colistin A compared with colistin B was observed, probably associated with the different fatty acid residue (107).
Intravenous colistin achieves low concentrations to the cerebrospinal fluid and in general, meningeal or systemic inflammation does not enhance their central nervous system (CNS) penetration to adequate therapeutic levels (76, 196). In both experimental and human studies in patients with CNS infections, the cerebrospinal fluid (CSF) to plasma ratio was very low (around 5%), although steady in time, suggesting probably inadequate bactericidal concentrations in the CSF (8, 80, 120). The reasons for that could be the higher than optimal molecular weight of colistin to cross the blood-brain barrier, chemical properties of colistin that do not favor passive permeability, and the presence of active efflux pumps (P-glycoproteins) (80, 81). When CMS was administered intrathecally/intraventricularly, the colistin trough levels were consistently above 2μg/ml if the administered daily dose was over 65000 IU, but there was still great inter-patient variability (8, 78). If CMS was administered both intravenously and intraventricularly, mean CSF colistin concentrations were higher than intravenous or intraventricular alone (217).
Polymyxin B is administered as its sulfate salt which is the active antibiotic. Thus conversion is not required. It is eliminated mainly by non-renal pathways. As a result, polymyxin B concentration in the urine is low (214). The total body clearance appears to be relatively insensitive to renal function (173). Inter-individual variability in plasma concentration is low (173). Its serum half-life depends on the age of the patient and ranges from 3.1 (in neonates) to 13.6 hours (47, 98, 99, 170, 214). The unbound fraction in plasma is approximately 40%. A loading dose seems to improve significantly its PK parameters (173). Recently, the liposomal form of polymyxin B has been developed, which was found to achieve higher concentrations in the epithelial lining fluid and better effectiveness than aqueous formulations in neutropenic mice models (73).
Pharmacodynamic Effects
Colistin shows concentration dependent, rapid bactericidal activity against P. aeruginosa, K. pneumoniae and A. baumannii in in vitro static kill studies (17, 145, 157). However, regrowth occurs even at the highest concentrations (64μg/ml), most likely due to heteroresistance. In addition, bactericidal activity diminishes at higher inocula (15, 16, 17). Dynamic kill studies in P. aeruginosa simulating once, twice and three times daily administration of the same colistin dose showed that, all regimens rapidly killed bacteria, regrowth occurred in all, but the thrice daily regimen delayed the development of resistance (17, 187). Similar findings were observed for polymyxin B (13, 17). However, in A. baumannii models, no difference was observed between the colistin regimens in development of resistance (17, 190).
Colistin produces a moderate post-antibiotic effect (PAE) against P. aeruginosa and K. pneumoniae strains at high concentrations (2-3 hours) (106, 157). In A. baumannii studies, both negative and significant (up to 8h) PAE has been reported based upon the exposure time to colistin (145, 146, 156). The clinical utility of the colistin PAE in is unclear, since colistin has a relatively long half-life compared to the current dosing schedules and the fluctuation of its plasma concentrations is low (17).
The PK/PD parameter that best predicts the activity of colistin is the free AUC/MIC. In an in vitro model against P. aeruginosa colistin activity was best correlated with the AUC/MIC ratio of total and unbound colistin. This index seemed to be superior to Cmax/MIC ratio. Following this finding, it has been suggested that time-averaged exposure to colistin is a more important target than the achievement of high peak concentrations (14). Accordingly, in vivo lung and murine models showed that colistin activity best correlated with free AUC/MIC against A. baumannii and P. aeruginosa (41, 42).
In Vitro Synergistic Activity
As already stated, polymyxins act by increasing the permeability of the bacterial cell membrane, thus they could be synergistic with other antimicrobial agents by facilitating their entrance into the bacterial cell. Therefore, the possibility of synergistic activity of polymyxins with other antimicrobial agents against Gram-negative bacteria has been explored in several studies. Although the synergy testing methods are not standardized and their results are not readily reproducible or comparable (191), several studies showed that synergism seems to exist but not in all isolates; this depends on bacterial species, resistance pattern and the combination of studied antibiotics. In general, time-kill studies showed the higher synergistic activity, followed by Etest and microdilution methods (218). Combinations showed synergistic activity against resistant strains to at least one of the studied antibiotics or the antibiotic with the higher MICs, but not in susceptible strains. In a systematic review regarding synergy of carbapenems with polymyxins, synergy was higher for A. baumannii (77%), followed by P. aeruginosa (50%) and K. pneumoniae (44%). In addition, doripenem showed high synergy for all bacteria, while meropenem was more synergistic for A. baumannii and imipenem for P. aeruginosa (218).
Synergistic activity has been shown for KPC producing K. pneumoniae strains with rifampicin, tigecycline, fosfomycin, vancomycin and carbapenems for polymyxin resistant strains, and with carbapenems and fosfomycin for polymyxin susceptible strains (53, 103, 149, 171, 182, 206). On the contrary, in VIM producing strains antagonism with carbapenems was shown in colistin-resistant strains and synergism for colistin susceptible strains (183). Finally, in a study with NDM Enterobacteriaceae colistin showed minimal or no synergistic activity with fosfomycin and tigecycline for colistin susceptible and colistin resistant strains, respectively (10).
Synergistic activity of colistin with ceftazidime was also noted in an in vitro study performed in two MDR P. aeruginosa strains (64). Carbapenems, fosfomycin and rifampin have also shown in vitro synergistic activity with polymyxins to a greater or lesser extent for colistin susceptible or resistant strains of P. aeruginosa (74, 171, 150, 192). The combination of colistin, rifampin, and amikacin was found to be synergistic in vitro (20) and led to treatment success in an immunosuppressed patient with multiple abscesses of the lungs, perineum, and gluteus due to MDRPseudomonas aeruginosa (193). On the other hand, vancomycin and trimethoprim-sulfamethoxazole did not show synergy with colistin for P. aeruginosa (206).
The addition of rifampin to colistin was also found to have in vitro synergistic bactericidal activity against MDR strains A. baumannii (59, 191, 151). In addition, synergy was reported between polymyxin B and imipenem, polymyxin B and rifampin, and polymyxin B, imipenem, and rifampin against MDR A. baumannii strains (213). Synergistic effectiveness of colistin with meropenem and sulbactam was also shown (104). In this study bacterial regrowth at 24 hours was observed when colistin was combined with meropenem, but not when it was combined with sulbactam or both of them.
Regarding MDR S. maltophilia strains, in vitro synergy of colistin with rifampin and to a lesser extent of colistin with trimethoprim/sulfamethoxazole was documented (60). The combination of polymyxin B with neomycin demonstrated, in vitro, a synergistic effect against a Salmonella enteritidis strain (63).
Overall, these studies provided valuable data rearding the polymyxin containing combination regimens. First, regrowth consistently seen in both colistin and polymyxin B monotherapy was eliminated by combination. This could reduce the possibility for selection of resistant subpopulations or de novo development of resistance. Second, synergy was more evident in polymyxin-resistant strains. Third, bactericidal activity was observed even at subinhibitory colistin concentrations in susceptible strains (100).
DosAGE
Adults
Two different forms of colistin are available for clinical use: 1) colistin sulfate which is administered orally for bowel decontamination and topically as a powder for the treatment of bacterial skin infections, and 2) colistimethate sodium (CMS) (also called colistin methanesulfate, pentasodium colistimethanesulfate, and colistin sulfonyl methate) for parenteral (intravenous, intramuscular, aerosolized and intrathecal/intraventricular) therapy. CMS has been less toxic and with fewer adverse events than colistin sulfate (7, 18). Commercially polymyxin B is available as the sulfate salt. Polymyxin B sulfate is available for parenteral (intravenous and intramuscular), topical (ophthalmic and otic instillation), and intrathecal use.
CMS is available commercially in several formulations that contain different amounts of colistin A, colistin B and other components. In general, 1 mg of CMS equals to 12.500 IU CMS; this means that 80 mg is equal to 1 million IU CMS. Furthermore, 1 mg of colistin base is contained in 2.4 mg or 30.000 IU of CMS. The intravenous preparations of colistin contain only the dry antibiotic powder, which is dissolved in 0.9% sodium chloride 5% or dextrose in water solution or water for injection before injection. The solution can be administered slowly over 3-5 minutes, 15-30 minutes or continuously over 24 hours (126).
The currently recommended intravenous doses for CMS in adult patients with normal renal function differ in Europe and USA. The manufacturers of European CMS products recommend in general 4-6mg/kg or 50.000-75.000 IU/kg daily of CMS in 2-3 divided doses, i.e. for a 70 kg adult patient a dose of 90-140 mg or 1.2-1.75 million IU every 8 hours. Greece, France and Austria are the exceptions. In Greece the manufacturer recommends 6-9 million IU daily, divided in 3 doses (9 million IU recommended for ICU patients). In France and Austria up to 12mg/kg (150000 IU/kg) daily is recommended (195). In the USA, colistin products contain 150 mg colistin base (equivalent to 360 mg or 4.5 million IU of CMS) and the recommended dose is 2.5-5 mg/kg colistin base daily divided in 2-4 doses, depending on the severity of the infection (equivalent to 6-12 mg/kg or 75000-150000 IU/kg of CMS, i.e. for a 70 kg adult patient a dose of 140-280mg or 1.75-3.5 million IU every 8 hours).
It is evident that at least on PK/PD grounds discussed previously, most of the currently recommended doses are not sufficient for the treatment of patients. In the same grounds, sustainable concentrations would be achieved even with once daily administration. However, other issues including the higher possibility of development of resistance in studies simulating once or twice daily administration and lack of comparative data from clinical studies preclude such proposals. Currently, the most accurate method to calculate the dosing scheme of colistin base (in mg) in order to achieve adequate concentrations for an MIC target is by using the formula (57):
Loading dose: colistin Css,avg target x 2.0 x body weight
Maintenance daily dose: colistin Css,avg targetx (1.50 x CrCL + 30),
where colistin Css,avg target is the desirable mean colistin concentration at steady state expressed in mg/l, which depends on MIC of the pathogen, site, and severity of infection. The lower of ideal or actual body weight is used, expressed in kg.
In addition, colistin can be administered intramuscularly at doses similar to the recommended intravenous ones. However, intramuscular administration is not commonly used in clinical practice due to the severe pain caused at the injection site. The combination of CMS with 0.5% lidocaine may be used to treat eye infections. Colistin sulfate could be administered orally to infants and children with diarrhea caused by bacteria susceptible to the drug; the dose is 3-5 mg/kg daily, in three divided doses. Colistin sulfate is also used as part of selective bowel decontamination regimens, however in an era of advanced bacterial resistance and lack of definitive data regarding the usefulness of bowel decontamination in institutions with MDR bacteria, this approach should be discouraged.
When CMS is given by inhalation (nebulized), the dosage recommended by the manufacturers in the United Kingdom is 40 mg (500.000 IU) every 12 hours for patients with body-weight ≤ 40 kg and 80 mg (1 million IU) every 12 hours for patients with body-weight > 40 kg. For recurrent pulmonary infections and for the treatment of ventilator-associated pneumonia in critically ill patients the dosage of aerosolized CMS can be increased to 160 mg (2 million IU) every 8-12 hours (94, 128). In spontaneously breathing patients CMS can be administered as follows: 1 million IU (80mg) of CMS is added to 4ml of normal saline, swirled slowly to mix, and the solution is nebulized with 8 liters/min oxygen flow and inhaled via a face mask. In patients undergoing mechanical ventilation aerosolized CMS can be delivered by means of most ventilators. In addition, inhaled colistin can be administered through jet or ultrasonic nebulizers. More recently, CMS has been formulated as a dry powder to be administered via a hand-held inhaler in cystic fibrosis patients ≥6 years of age with chronic P. aeruginosa infections. Compared with nebulized CMS, the CMS dry powder for inhalation (DPI) formulation reduces treatment time and improves patient convenience (28).
As previously described, penetration of colistin into the CSF is inadequate and therefore intravenous colistin alone is not recommended for the treatment of post-neurosurgical meningitis or ventriculitis due to MDR or XDR Gram negative bacilli. Intrathecal or intraventricular administration of CMS has been described. The IDSA guidelines proposed a daily dose of 10mg (125000 IU) for intrathecal administration (198). A systematic review of patients with MDR A. baumannii (mainly), P. aeruginosa, and K. pneumoniae CNS infections reported that the dosing of CMS in case reports was mainly empirical and ranged from 1.6-40mg (20000-500000 IU) (8).
Commercial formulations of polymyxin B are not currently available in most areas of the globe. In general, 1 mg of polymyxin B is equal to 10.000 IU. The dosage of intravenous polymyxin B recommended by the manufacturer is 1.5-2.5 mg/kg/day (15.000-25.000 IU/kg/day), divided to 2 equal doses for adults and children older than 2 years, with normal renal function. Polymyxin B may also be administered intramuscularly, although this mode is not routinely recommended due to the severe pain caused at the injection site. The recommended dosage for intramuscular administration is 2.5-3 mg/kg/day (25.000-30.000 IU/kg/day) divided to 4 or 6 equal doses for adults and children older than 2 years (9).
Polymyxin B has been used intrathecally in cases of MDR Gram-negative meningitis. The dosage recommended for intrathecal use for adults and children under 2 years of age is 5 mg (50.000 IU) once daily for 3 to 4 days and then 5 mg (50.000 IU) once every other day for at least 2 weeks after the cultures of the cerebrospinal fluid are negative and sugar levels of the cerebrospinal fluid are normal. For children under 2 years of age the dosage of intrathecal polymyxin B should be 2 mg (20.000 IU) once daily for 3 to 4 days, then 2.5 mg (25.000 IU) once every other day until the previous mentioned conditions are met (9).
When polymyxin B is used to treat eye infections due to P. aeruginosa a concentration of 0.1-0.25% (10.000 IU to 25.000 IU/ml) is administered in 1 to 3 drops every hour. Subconjuctival injection of up to 10 mg/day (100.000 IU/day) may also be used for the treatment of Pseudomonas aeruginosa infections of the cornea and conjunctiva (9). In addition, polymyxin B with a caine-type local anesthetic is an FDA permitted combination, which is used in intramuscular administration of the medication, in ear drops, and ointments.
Children
The currently available data for intravenous dosing recommendations in children outside the cystic fibrosis population are limited, untraceable or non-existing. Most dosing schemes in case reports or case series are empirical. Therefore, the definite dose for children has not been defined (195). For colistin, the adult dosing is recommended for children in the manufacturers’ recommendations. For polymyxin B, it is suggested that infants with normal renal function may receive up to 4 mg/kg/day (40.000 IU/kg/day) in cases of life-threatening infections.
Renal Failure
According to the manufacturers, the dose of polymyxins must be reduced in patients with impaired renal function. However, the recommended doses differ substantially between USA and several European countries (Table 3) (195). In general, the data from PK studies show that for polymyxin B dose adjustment is not required for patients with any degree of renal impairment, including patients in dialysis (173, 174). However, manufacturers and the FDA recommend dose adjustment with declining renal function, without specification of the dosages. For colistin, the situation is far more complicated. As already mentioned, colistin is not removed by the kidneys, but CMS is. In USA, dose adjustment is recommended even for mild renal impairment. In Europe, recommendations differ between countries, from adjustment even in mild renal impairment to adjustment only for severe renal impairment. Table 3 provides a summary of recommendations for colistin adjustment. Since both CMS and colistin are removed in dialysis, the dose should be adjusted accordingly.
Recent PK studies showed that an independent of renal function loading colistin dose followed by dose adjustment according to renal function is required. The aforementioned formulas for patients with normal renal function apply also for ICU patients with renal impairment (57). Recommended dosage intervals based on creatinine clearance (CrCl) are as follows (colistin base activity, CBA):
CrCl 10-70 ml/min/1.73 m2: every 12 (or 8) hours
CrCl <10 ml/min/1.73 m2: every 12 hours
Receiving intermittent hemodialysis: Daily dose of CBA on a non-hemodialysis day to achieve each 1.0-mg/liter colistin Css,avg target is 30 mg. On a hemodialysis day, a supplemental dose of colistin should be added. If the supplemental dose is administered during the last hour of the session, 50% of the daily dose should be added. If it is administered after the session, 30% of the daily dose should be added. Twice-daily dosing is suggested.
Receiving continuous renal replacement therapy: Daily dose of CBA to achieve each 1 mg/liter colistin Css,avg target is 192 mg. Doses may be given every 8-12 hours.
For patients in continuous ambulatory peritoneal dialysis no formal recommendations exist from the manufacturers. PK studies showed that the dose of CSM should not be increased since clearance for both CMS and colistin was minimal. A loading dose of 300mg colistin base followed by 150-200mg of colistin base every day was sufficient to achieve steady-state plasma concentration of 2.5μg/ml (93).
Hepatic Failure
There is no need for dosage modification of polymyxins in patients with liver failure.
Body Composition (Obesity, Wasting, Various Body Builds)
In obese patients dosage of colistin should be based on ideal body weight (132).
Pregnancy
Currently, polymyxins are listed in the FDA’s pregnancy category C. Subsequently, they should be used only for cases where the potential benefit is higher than the potential risk. There are no adequate and well-controlled studies assessing the safety of polymyxin B and colistin in women during pregnancy. In a small study, the authors reported that the risk for congenital abnormalities in newborns whose mothers were treated with polymyxin B during pregnancy was minimal but could not be ruled out (87).
ADVERSE EVENTS
Nephrotoxicity
The mechanism of polymyxins associated neprotoxicity at the molecular level remains unknown. Recent studies showed that it involves the mitochondrial, death receptor, and endoplasmic reticulum pathways (33, 44). The final result is induction of apoptosis of renal cells and acute tubular necrosis manifested as elevation in serum creatinine and urea levels. It seems that nephrotoxicity depends on both the concentration and length of exposure to polymyxins (6, 48).
The reported frequency of nephrotoxicity of polymyxins in studies published in the last 15 years varied from as low as 0% up to 55% (50, 70, 121, 125, 127). The definitions of nephrotoxicity, the characteristics and number of enrolled patients and the doses of administered polymyxins were variable. Polymyxins were administered mainly to patients with severe infections and co-morbidity and concurrent use of other potentially toxic drugs, including antibiotics might have also contributed to nephrotoxicity. Adjustment for these factors was not performed in the majority of the studies. Although definitive conclusions cannot be made, it seems that the frequency of nephrotoxicity was higher in patients with previous renal impairment, in older individuals and those who received higher doses (125, 176).
One study reported that although nephrotoxicity was observed during colistin administration, a decrease in the mean (±SD)/median serum creatinine level of 0.2 (±1.3)/0.1 mg/dl was noticed at the end of CMS treatment compared to the baseline levels (84). Interestingly, in patients who received intravenous CMS for more than 4 weeks for the treatment of MDR Gram-negative infections, the median creatinine value increased by 0.25 mg/dl during treatment compared to the baseline, and returned to baseline levels at the end of treatment (48). Another study in cystic fibrosis patients showed that renal dysfunction was potentiated by the co-administration of aminoglycosides; however, colistin alone or in combination with other antibiotics did not appear to be highly nephrotoxic (4).
Recent experimental studies also showed that polymyxin B is 3 times more cytotoxic to human kidney proximal tubular cells than colistin (203). In contrast to that and to older clinical studies (102,141), recent ones using the more strict RIFLE criteria for definition of kidney injury reported a lower frequency of nephrotoxicity among polymyxin B than colistin treated patients (2, 152, 199).
Neurotoxicity
Neurological toxicity may be manifested with dizziness, weakness, facial and peripheral paresthesia, vertigo, confusion, ataxia, visual and speech disturbances, and neuromuscular blockade, which can lead to respiratory failure or apnea. Partial deafness and severe ataxia have been reported in patients with excessively high plasma levels of colistin. The incidence of colistin-associated neurotoxicity reported in earlier literature was approximately 7%, with paresthesias comprising the main manifestation (90, 180). The neurotoxic events related to colistin therapy appears to occur more frequently in cystic fibrosis (19, 162) than in non-cystic fibrosis patients (56, 105,121). Intraventricular administration of polymyxins may lead to convulsions (88).
Other Adverse Events
Pruritus, dermatoses, drug fever, gastrointestinal disturbances and superinfections may develop infrequently during the course of therapy with polymyxins. Leukopenia and granulocytopenia may be possibly associated with the use of colistin. Pain at the site of intramuscular injection or superficial thrombophlebitis may occur. Furthermore, the development of Clostridium difficile infection represents an additional, although rare, potential adverse event of polymyxin treatment (90).
Treatment with aerosolized colistin may further be complicated with bronchoconstriction and chest tightness. Treatment with inhaled β2-agonists before the initiation of aerosolized colistin could prevent the development of bronchoconstriction (32).
Treatment and Avoidance
Both, renal and neurological toxicity associated with the use of polymyxins, are considered to be dose-dependent and reversible after early discontinuation of the drugs. However, there are scarce published reports of irreversible nephrotoxicity after the cessation of colistin treatment (90). Supportive treatment to patients treated with polymyxins, close monitoring of renal function, restriction of factors and drugs that may precipitate renal impairment when polymyxins are administered may prevent the development of adverse events.
Overdoses
A fatal case of a previously healthy child who received colistin therapy for intra-abdominal infection due to P. aeruginosa and E. coli strains has been reported. This patient developed both acute tubular necrosis, confirmed by autopsy and apnea requiring mechanical ventilation. However, the dose of CMS used was 120 mg/kg/day, which is 10 times above the currently upper recommended dose (167). Although colistin and CMS are removed at a higher extent than polymyxin B with dialysis, there are no data regarding the effectiveness of hemodialysis or other treatment strategies for the management of patients with polymyxin overdose.
MoniTORING REQUIREMENTS
Therapeutic Drug Monitoring
There are no conclusive data about the usefulness of therapeutic drug monitoring for adjustment of CMS dose. Recent studies on CMS PK properties suggest that even though the concentration of active colistin at steady state does not fluctuate, the 2 μg/ml target is not achieved in many patients, mainly due to insufficient dosing, especially in patients with mild to moderate renal dysfunction. In addition, some of them will have plasma concentrations up to 10μg/ml due to unknown enzymatic activities in the tissues or drug to drug interactions. If such an approach was to be applied, it would be advisable to collect blood samples before the administration of the next dose, in order to minimize overestimation of the results due to the conversion of CMS to colistin after collection (29).
Other Laboratory Monitoring
Clinicians should be alert for the possibility of development of polymyxins-related adverse reactions, mainly nephrotoxicity and neurotoxicity. Subsequently, monitoring of renal function by measuring serum creatinine every two days is advised, until more data on the value of other tests for patient monitoring become available.
DRUG INTERACTIONS
Other antibiotics, such as aminoglycosides, can increase the potential of polymyxins for nephrotoxicity and neurotoxicity and they should be co-administered with the greatest caution with polymyxins. The concurrent use of polymyxins with a curariform muscle relaxant and other neurotoxic drugs such as ether, tubocurarine, succinylcholine, gallamine, decamethonium, and sodium citrate should be used with great caution also, since these agents may potentiate the development of neuromuscular blockade (37). Co-administration of sodium cephalothin and colistin may enhance the development of nephrotoxicity, so this medication should be avoided (90).
CLINICAL INDICATIONS
The intravenous formulations of polymyxins were used therapeutically against Gram negative infections from the early 1950s for approximately two decades (164). They were gradually abandoned worldwide in the early 1970s because of the reported severe toxicities, mainly nephrotoxicity (52, 129, 165). However, the emergence of MDR and XDR Gram-negative bacteria in combination with the lack of development of new antimicrobial agents led to the revival of polymyxins as “salvage” therapy for the treatment of nosocomial infections due to MDR Gram negative bacteria. Nowadays, polymyxins should be considered for the treatment of infections caused by Gram negative bacteria resistant to other available antimicrobial agents, confirmed by appropriate in vitro susceptibility testing. In addition, polymyxins appear a valuable option in patients with infections due to Gram-negative bacteria, which are in vitro susceptible to other antimicrobial agents, when the treatment with these agents has been clinically ineffective.
Several, mainly retrospective studies have been published regarding the effectiveness of intravenous polymyxins, alone or in combination, in several dosing regimens, for the treatment of ICU or hospitalized patients with MDR or XDR A. baumannii, P. aeruginosa and lately K. pneumoniae infections. Most of them concluded that polymyxins are an adequate treatment option for such infections based on the acceptable mortality and clinical cure rates, given the critical condition and co-morbidity of the enrolled patients (212). Polymyxins were mostly administered with other active or inactive antibiotics. In studies that compared a polymyxin alone against the combination of a polymyxin with one or more antibiotics, there was no difference in mortality or clinical cure in multivariate analyses between the compared groups. Only one non-comparative preliminary study regarding the application of a 9 million IU loading dose has been published. The cure rate was similar to the cure rates reported in other studies (34). Small, inconclusive studies have been also published regarding the continuous administration of polymyxins (114).
Following the publication of data regarding the synergism of rifampin with colistin against A. baumannii, two RCTs examined the effectiveness of the combination against colistin monotherapy. No difference was observed in all cause or infection-related mortality and the duration of hospitalization between the compared groups. Combination therapy was associated with faster and higher microbiological eradication (5, 43).
There is extensive experience with the use of aerosolized colistin in patients with cystic fibrosis (140). Intravenous and aerosolized colistin is provided in these patients in an attempt to eradicate P. aeruginosa colonization of the respiratory tract and to treat exacerbations mainly caused by the same microorganism. A 3-week course of CMS inhalation in combination with oral ciprofloxacin led to promising results in eradicating early P. aeruginosa colonization. Concerns about rapid development of P. aeruginosa strains resistant to colistin or the emergence of lung infections due to microorganisms with inherited resistance to colistin were not confirmed after more than 10 years of published experience in patients with cystic fibrosis. Compared with tobramycin, the rate of development of resistance to colistin was slower (112).
Besides cystic fibrosis, inhaled polymyxins have been increasingly used for the treatment of patients with pneumonia, and especially ventilator-associated pneumonia (56, 111, 128). Thus far the data support a higher clinical and microbiological cure rate and fewer days under mechanical ventilation in patients receiving intravenous and nebulised colistin compared to intravenous colistin alone. A difference in mortality was not observed (9, 197). Aerosolized polymyxins has been used also to reduce the exacerbations in patients with non-cystic fibrosis bronchiectasis, but the results are not as promising as in patients with cystic fibrosis (71).
Since penetration of polymyxins in the CSF is inadequate, intraventricular and intrathecal administration has been used for neurosurgical patients with meningitis or ventriculitis due to MDR Gram negative bacteria. Most of the published evidence comes from case reports due to A. baumannii infections. Reviews on the issue reported successful treatment in the majority of patients (8, 83,153). Polymyxins were administered alone or in combination with other antibiotics, including rifampin. Comparative data regarding monotherapy and combination regimens are not available.
Reports published in the old literature suggested that the penetration of colistin into bones is not adequate, thus it was used only as an irrigation solution (166). However, there is limited promising experience with the use of intravenous colistin for the treatment of bone infections (related to prosthetic materials or not) (184, 204).
Finally, due to its adsorptive properties, polymyxin B has been used as a substrate for endotoxin removal in patients with septic shock. Most of the data come from small trials in Japan. A systematic review and meta-analysis concluded that blood purification was associated with lower mortality in patients with septic shock. Trials studying hemoperfusion with polymyxin B were the ones that primarily influenced the results of the meta-analysis (216).
REFERENCES
1. Adams MD, Nickel GC, Bajaksouzian S, Lavender H, Murthy AR, Jacobs MR, Bonomo RA. Resistance to colistin in Acinetobacter baumannii associated with mutations in the PmrAB two-component system. Antimicrob Agents Chemother, 2009;53(9): 3628-34. [PubMed]
2. Akajagbor DS, Wilson SL, Shere-Wolfe KD, Dakum P, Charurat ME, Gilliam BL. Higher incidence of acute kidney injury with intravenous colistimethate sodium compared with polymyxin B in critically ill patients at a tertiary care medical center. Clin Infect Dis, 2013;57(9):1300-3. [PubMed]
3. al Khayyat AA, Aronson AL. Pharmacologic and toxicologic studies with the polymyxins. II. Comparative pharmnacologic studies of the sulfate and methanesulfonate salts of polymyxin B and colistin in dogs. Chemotherapy. 1973;19:82-97. [PubMed]
4. Al Aloul M, Miller H, Alapati S, Stockton PA, Ledson MJ, Walshaw MJ. Renal impairment in cystic fibrosis patients due to repeated intravenous aminoglycoside use. Pediatr Pulmonol. 2005;39:15-20. [PubMed]
5. Aydemir H, Akduman D, Piskin N, Comert F, Horuz E, Terzi A, Kokturk F, Ornek T, Celebi G. Colistin vs. the combination of colistin and rifampicin for the treatment of carbapenem-resistant Acinetobacter baumannii ventilator-associated pneumonia. Epidemiol Infect, 2013;141(6):1214-22. [PubMed]
6. Azad MA, Finnin BA, Poudyal A, Davis K, Li J, Hill PA, Nation RL, Velkov T, Li J. Polymyxin B induces apoptosis in kidney proximal tubular cells. Antimicrob Agents Chemother, 2013.[PubMed]
7. Barnett M, Bushby SR, Wilkinson S. Sodium sulphomethyl derivatives of polymyxins. Br J Pharmacol. 1964;23:552-74. [PubMed]
8. Bargiacchi O, Rossati A, Car P, Brustia D, Brondolo R, Rosa F, Garavelli PL, De Rosa FG. Intrathecal/intraventricular colistin in external ventricular device-related infections by multi-drug resistant Gram negative bacteria: case reports and review. Infection, 2014. [PubMed]
9. Bedford Laboratories. Polymyxin B for injection (package insert). Bedford Laboratoties, Bedford, OH 44146. 1999.
10. Berçot B, Poirel L, Dortet L, Nordmann P. In vitro evaluation of antibiotic synergy for NDM-1-producing Enterobacteriaceae. J Antimicrob Chemother, 2011:66:2295-7. [PubMed]
11. Bell A, Hancock RE. Outer membrane protein H1 of Pseudomonas aeruginosa: purification of the protein and cloning and nucleotide sequence of the gene. J Bacteriol, 1989;171:3211-7.[PubMed]
12. Bengoechea JA, Skurnik M. Temperature-regulated efflux pump/potassium antiporter system mediates resistance to cationic antimicrobial peptides in Yersinia. Mol Microbiol. 2000;37:67-80. [PubMed]
13. Bergen PJ, Li J, Nation RL, Turnidge JD, Coulthard K, Milne RW. Comparison of once-, twice- and thrice-daily dosing of colistin on antibacterial effect and emergence of resistance: studies with Pseudomonas aeruginosa in an in vitro pharmacodynamic model. J Antimicrob Chemother, 2008;61:636-42. [PubMed]
14. Bergen PJ, Bulitta JB, Forrest A, Tsuji BT, Li J, Nation RL. Pharmacokinetic/pharmacodynamic investigation of colistin against Pseudomonas aeruginosa using an in vitro model. Antimicrob Agents Chemother, 2010;(9):3783-9. [PubMed]
15. Bergen PJ, Forrest A, Bulitta JB, Tsuji BT, Sidjabat HE, Paterson DL, Li J, Nation RL. Clinically relevant plasma concentrations of colistin in combination with imipenem enhance pharmacodynamic activity against multidrug-resistant Pseudomonas aeruginosa at multiple inocula. Antimicrob Agents Chemother, 2011;55:5134-42. [PubMed]
16. Bergen PJ, Tsuji BT, Bulitta JB, Forrest A, Jacob J, Sidjabat HE, Paterson DL, Nation RL, Li J. Synergistic killing of multidrug-resistant Pseudomonas aeruginosa at multiple inocula by colistin combined with doripenem in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrob Agents Chemother, 2011;55:685-95. [PubMed]
17. Bergen PJ, Landersdorfer CB, Zhang J, Zhao M, Lee HJ, Nation RL, Li J. Pharmacokinetics and pharmacodynamics of 'old' polymyxins: what is new? Diagn Microbiol Infect Dis, 2012;74:213-23. [PubMed]
18. Beveridge EG, Martin AJ. Sodium sulphomethyl derivatives of polymyxins. Br J Pharmacol. 1967;29:125-35. [PubMed]
19. Bosso JA, Liptak CA, Seilheimer DK, Harrison GM. Toxicity of colistin in cystic fibrosis patients. DICP. 1991;25:1168-70. [PubMed]
20. Bozkurt-Guzel, C. Gerceker AA. In vitro pharmacodynamic properties of colistin methanesulfonate and amikacin against Pseudomonas aeruginosa. Indian J Med Microbiol, 2012;30:34-38. [PubMed]
21. Breazeale SD, Ribeiro AA, McClerren AL, Raetz CR. A formyltransferase required for polymyxin resistance in Escherichia coli and the modification of lipid A with 4-Amino-4-deoxy-L-arabinose. Identification and function oF UDP-4-deoxy-4-formamido-L-arabinose. J Biol Chem, 2005;280:14154-67. [PubMed]
22. Campos MA, Vargas MA, Regueiro V, Llompart CM, Albertí S, Bengoechea JA. Capsule polysaccharide mediates bacterial resistance to antimicrobial peptides. Infect Immun, 2004;72:7107-14. [PubMed]
23. Cannatelli A, D'Andrea MM, Giani T, Di Pilato V, Arena F, Ambretti S, Gaibani P, Rossolini GM. In vivo emergence of colistin resistance in Klebsiella pneumoniae producing KPC-type carbapenemases mediated by insertional inactivation of the PhoQ/PhoP mgrB regulator. Antimicrob Agents Chemother, 2013;57:5521-26. [PubMed]
24. Capone A, Giannella M, Fortini D, Giordano A, Meledandri M, Ballardini M, Venditti M, Bordi E, Capozzi D, Balice MP, Tarasi A, Parisi G, Lappa A, Carattoli A, Petrosillo N;SEERBIO-GRAB network. High rate of colistin resistance among patients with carbapenem-resistant Klebsiella pneumoniae infection accounts for an excess of mortality. Clin Microbiol Infect, 2013;19:e23-30. [PubMed]
25. Catchpole CR, Andrews JM, Brenwald N, Wise R. A reassessment of the in-vitro activity of colistin sulphomethate sodium. J Antimicrob Chemother. 1997;39:255-60. [PubMed]
26. Choi SK, Park SY, Kim R, Kim SB, Lee CH, Kim JF, Park SH. Identification of a polymyxin synthetase gene cluster of Paenibacillus polymyxa and heterologous expression of the gene in Bacillus subtilis. J Bacteriol 2009;191:3350-3358. [PubMed]
27. CLSI, Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. 24th informational supplement. M100-S24. Wayne, PA. 2014.
28. Conole D, Keating GM, Colistimethate Sodium Dry Powder for Inhalation: A Review of Its Use in the Treatment of Chronic Pseudomonas aeruginosa Infection in Patients with Cystic Fibrosis. Drugs 2014;74:377-87. [PubMed]
29 Couet W, Grégoire N, Gobin P, Saulnier PJ, Frasca D, Marchand S, Mimoz O. Pharmacokinetics of colistin and colistimethate sodium after a single 80-mg intravenous dose of CMS in young healthy volunteers. Clin Pharmacol Ther, 2011;89:875-879. [PubMed]
30. Couet W, Grégoire N, Marchand S, Mimoz O. Colistin pharmacokinetics: the fog is lifting. Clin Microbiol Infect 2012;18:30-39. [PubMed]
31. Craig WA, Kunin CN. Significance of serum protein and tissue binding of antimicrobial agents. Annu Rev Med, 1976;27:287-300. [PubMed]
32. Cunningham S, Prasad A, Collyer L, Carr S, Lynn IB, Wallis C. Bronchoconstriction following nebulised colistin in cystic fibrosis. Arch Dis Child, 2001;84:432-433. [PubMed]
33. Dai C, Li J, Tang S, Li J, Xiao X. Colistin-induced nephrotoxicity in mice involves the mitochondrial, death receptor, and endoplasmic reticulum pathways. Antimicrob Agents Chemother, 2014;58:4075-4085. [PubMed]
34. Dalfino L, Puntillo F, Mosca A, Monno R, Spada ML, Coppolecchia S, Miragliotta G, Bruno F, Brienza N. High-dose, extended-interval colistin administration in critically ill patients: is this the right dosing strategy? A preliminary study. Clin Infect Dis, 2012;54:1720-6. [PubMed]
35. Davis SD, Iannetta A, Wedgwood RJ. Activity of colistin against Pseudomonas aeruginosa: inhibition by calcium. J Infect Dis 1971;124::610-612. [PubMed]
36. Decolin D, Leroy P, Nicolas A, Archimbault P. Hyphenated liquid chromatographic method for the determination of colistin residues in bovine tissues. J Chromatogr Sci. 1997;35:557-64.[PubMed]
37. de Gouw NE, Crul JF, Vandermeersch E, Mulier JP, van Egmond J, Van Aken H. Interaction of antibiotics on pipecuronium-induced neuromuscular blockade. J Clin Anesth. 1993;5:212-15.[PubMed]
38. Denton M, Kerr K, Mooney L, Keer V, Rajgopal A, Brownlee K, Arundel P, Conway S. Transmission of colistin-resistant Pseudomonas aeruginosa between patients attending a pediatric cystic fibrosis center. Pediatr Pulmonol. 2002;34:257-61. [PubMed]
39. Deris ZZ, Akter J, Sivanesan S, Roberts KD, Thompson PE, Nation RL, Li J, Velkov T. A secondary mode of action of polymyxins against Gram-negative bacteria involves the inhibition of NADH-quinone oxidoreductase activity. J Antibiot (Tokyo). 2014;67:147-51. [PubMed]
40. Dubrovskaya Y, Chen TY, Scipione MR, Esaian D, Phillips MS, Papadopoulos J, Mehta SA. Risk factors for treatment failure of polymyxin B monotherapy for carbapenem-resistant Klebsiella pneumoniae infections. Antimicrob Agents Chemother, 2013;57:5394-5397. [PubMed]
41. Dudhani R.V., et al., Elucidation of the pharmacokinetic/pharmacodynamic determinant of colistin activity against Pseudomonas aeruginosa in murine thigh and lung infection models.Antimicrob Agents Chemother, 2010;54:1117-1124. [PubMed]
42. Dudhani RV, Turnidge JD, Nation RL, Li J. fAUC/MIC is the most predictive pharmacokinetic/pharmacodynamic index of colistin against Acinetobacter baumannii in murine thigh and lung infection models. J Antimicrob Chemother, 2010;65:1984-90. [PubMed]
43. Durante-Mangoni, E., et al., Colistin and rifampicin compared with colistin alone for the treatment of serious infections due to extensively drug-resistant Acinetobacter baumannii: a multicenter, randomized clinical trial. Clin Infect Dis, 2013;57:349-58. [PubMed]
44. Eadon MT, Hack BK, Alexander JJ, Xu C, Dolan ME, Cunningham PN. Cell cycle arrest in a model of colistin nephrotoxicity. Physiol Genomics, 2013;45:877-88. [PubMed]
45. Esposito S, Pascale R, Esposito I, Noviello S, Russo E, De Simone G, Vitolo M, Rega MR, Massari A. Epidemiology and antibiotic resistance in a large Italian teaching hospital. J Chemother, 2014. [PubMed]
46. EUCAST. Breakpoint tables for interpretation of MICs and zone diameter. 2014; Version 4.0: [Available from: http://www.eucast.org]. [PubMed]
47. Evans ME, Feola DJ, Rapp RP. Polymyxin B sulfate and colistin: old antibiotics for emerging multiresistant gram-negative bacteria. Ann Pharmacother. 1999;33:960-967. [PubMed]
48. Falagas ME, Kasiakou SK. Toxicity of polymyxins: a systematic review of the evidence from old and recent studies. Crit Care, 2006;10:r27. [PubMed]
49. Falagas ME, Rafailidis PI, Matthaiou DK. Resistance to polymyxins: Mechanisms, frequency and treatment options. Drug Resist Updat, 2010;13:132-138. [PubMed]
50. Falagas ME, Rafailidis PI, Ioannidou E, Alexiou VG, Matthaiou DK, Karageorgopoulos DE, Kapaskelis A, Nikita D, Michalopoulos A. Colistin therapy for microbiologically documented multidrug-resistant Gram-negative bacterial infections: a retrospective cohort study of 258 patients. Int J Antimicrob Agents, 2010;35:194-199. [PubMed]
51. Fehlner-Gardiner CC, Valvano MA. Cloning and characterization of the Burkholderia vietnamiensis norM gene encoding a multi-drug efflux protein. FEMS Microbiol Lett, 2002;215:279-283. [PubMed]
52. Fekety, F.R., Jr., Norman PS, Cluff LS. The treatment of gram-negative bacillary infections with colistin. The toxicity and efficacy of large doses in forty-eight patients. Ann Intern Med, 1962;57:214-29. [PubMed]
53. Gaibani P, Lombardo D, Lewis RE, Mercuri M, Bonora S, Landini MP, Ambretti S. In vitro activity and post-antibiotic effects of colistin in combination with other antimicrobials against colistin-resistant KPC-producing Klebsiella pneumoniae bloodstream isolates. J Antimicrob Chemother, 2014;69:1856-65 [PubMed]
54. Galani I, Kontopidou F, Souli M, Rekatsina PD, Koratzanis E, Deliolanis J, Giamarellou H. Colistin susceptibility testing by Etest and disk diffusion methods. Int J Antimicrob Agents, 2008;31:434-439. [PubMed]
55. Gales AC, Reis AO, Jones RN. Contemporary assessment of antimicrobial susceptibility testing methods for polymyxin B and colistin: review of available interpretative criteria and quality control guidelines. J Clin Microbiol, 2001;39:183-190. [PubMed]
56. Garnacha-Montero J, Ortiz-Leyba C, Jiménez-Jiménez FJ, Barrero-Almodóvar AE, García-Garmendia JL, Bernabeu-WittelI M, Gallego-Lara SL, Madrazo-Osuna J. Treatment of multidrug-resistant Acinetobacter baumannii ventilator-associated pneumonia (VAP) with intravenous colistin: a comparison with imipenem-susceptible VAP. Clin Infect Dis, 2003;36:1111-1118.[PubMed]
57. Garonzik SM, Li J, Thamlikitkul V, Paterson DL, Shoham S, Jacob J, Silveira FP, Forrest A, Nation RL. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother, 2011;55:3284-3294. [PubMed]
58. Giamarellos-Bourboulis EJ, Sambatakou H, Galani I, Giamarellou H. In vitro interaction of colistin and rifampin on multidrug-resistant Pseudomonas aeruginosa. J Chemother, 2003;15:235-238. [PubMed]
59. Giamarellos-Bourboulis EJ, Xirouchaki E, Giamarellou H. Interactions of colistin and rifampin on multidrug-resistant Acinetobacter baumannii. Diagn Microbiol Infect Dis, 2001;40:117-120.[PubMed]
60. Giamarellos-Bourboulis EJ, Karnesis L, Giamarellou H. Synergy of colistin with rifampin and trimethoprim/sulfamethoxazole on multidrug-resistant Stenotrophomonas maltophilia. Diagn Microbiol Infect Dis, 2002;44:259-263. [PubMed]
61. Gough M, Hancock RE, Kelly NM. Antiendotoxin activity of cationic peptide antimicrobial agents. Infect Immun 1996;64:4922-4927. [PubMed]
62. Groisman EA, Kayser J, Soncini FC. Regulation of polymyxin resistance and adaptation to low-Mg2+ environments. J Bacteriol. 1997;179:7040-7045. [PubMed]
63. Grzybowska W, Wojcik A, Tyski S. [Interaction of neomycin and the other antibiotic on selected bacterial strains]. Med Dosw Mikrobiol. 2004;56:187-98. [PubMed]
64. Gunderson BW, Ibrahim KH, Hovde LB, Fromm TL, Reed MD, Rotschafer JC. Synergistic activity of colistin and ceftazidime against multiantibiotic-resistant Pseudomonas aeruginosa in an in vitro pharmacodynamic model. Antimicrob Agents Chemother. 2003;47:905-9. [PubMed]
65. Gunn JS, Lim KB, Krueger J, Kim K, Guo L, Hackett M et al. PmrA-PmrB-regulated genes necessary for 4-aminoarabinose lipid A modification and polymyxin resistance. Mol Microbiol. 1998;27:1171-82. [PubMed]
66. Gunn JS, Miller SI. PhoP-PhoQ activates transcription of pmrAB, encoding a two-component regulatory system involved in Salmonella typhimurium antimicrobial peptide resistance. J Bacteriol 1996;178:6857-64. [PubMed]
67. Gupta S, Govil D, Kakar PN, Prakash O, Arora D, Das S, Govil P, Malhotra A. Colistin and polymyxin B: a re-emergence. Indian J Crit Care Med, 2009;13:49-53. [PubMed]
68. Hancock RE, Chapple DS. Peptide antibiotics. Antimicrob Agents Chemother. 1999;43:1317-23. [PubMed]
69. Hanulik V, Suchánková H, Urbánek K, Imwensi P, Htoutou Sedláková M, Vojtová V, Kolář M., Suchánková H, Urbánek K, Imwensi P, Htoutou Sedláková M, Vojtová V, Kolář M. Effect of colistin consumption and prevalence of colistin-resistant bacteria. Klin Mikrobiol Infekc Lek, 2013;19:52-55. [PubMed]
70. Hartzell JD, Neff R, Ake J, Howard R, Olson S, Paolino K, Vishnepolsky M, Weintrob A, Wortmann G. Nephrotoxicity associated with intravenous colistin (colistimethate sodium) treatment at a tertiary care medical center. Clin Infect Dis, 2009;48:1724-1728. [PubMed]
71. Haworth CS, Foweraker JE, Wilkinson P, Kenyon RF, Bilton D. Inhaled Colistin in Patients with Bronchiectasis and Chronic Pseudomonas aeruginosa Infection. Am J Respir Crit Care Med, 2014;189:975. [PubMed]
72. Hayakawa K, Marchaim D, Divine GW, Pogue JM, Kumar S, Lephart P, Risko K, Sobel JD, Kaye KS. Growing prevalence of Providencia stuartii associated with the increased usage of colistin at a tertiary health care center. Int J Infect Dis, 2012;16:e646-648. [PubMed]
73. He J, Abdelraouf K, Ledesma KR, Chow DS, Tam VH. Pharmacokinetics and efficacy of liposomal polymyxin B in a murine pneumonia model. Int J Antimicrob Agents, 2013;42:559-64. [PubMed]
74. HE J, Kaniga K, Lynch AS, Flamm RK, Davies TA. In vitro Etest synergy of doripenem with amikacin, colistin, and levofloxacin against Pseudomonas aeruginosa with defined carbapenem resistance mechanisms as determined by the Etest method. Diagn Microbiol Infect Dis, 2012;74:417-419. [PubMed]
75. Henry R, Vithanage N, Harrison P, Seemann T, Coutts S, Moffatt JH, Nation RL, Li J, Harper M, Adler B, Boyce JD. Colistin-resistant, lipopolysaccharide-deficient Acinetobacter baumannii responds to lipopolysaccharide loss through increased expression of genes involved in the synthesis and transport of lipoproteins, phospholipids, and poly-beta-1,6-N-acetylglucosamine. Antimicrob Agents Chemother 2012;56:59-69. [PubMed]
76. Hoeprich PD. The polymyxins. Med Clin North Am. 1970;54:1257-65. [PubMed]
77. Hogardt M, Schmoldt S, Gotzfried M, Adler K, Heesemann J. Pitfalls of polymyxin antimicrobial susceptibility testing of Pseudomonas aeruginosa isolated from cystic fibrosis patients. J Antimicrob Chemother. 2004;54:1057-61. [PubMed]
78. Imberti R, Cusato M, Accetta G, Marinò V, Procaccio F, Del Gaudio A, Iotti GA, Regazzi M. Pharmacokinetics of colistin in cerebrospinal fluid after intraventricular administration of colistin methanesulfonate. Antimicrob Agents Chemother, 2012;56:4416-21. [PubMed]
79. Ito-Kagawa M, Koyama Y. Selective cleavage of a peptide antibiotic, colistin by colistinase. J Antibiot (Tokyo). 1980;33:1551-55. [PubMed]
80. Jin L, Li J, Nation RL, Nicolazzo JA. Brain penetration of colistin in mice assessed by a novel high-performance liquid chromatographic technique. Antimicrob Agents Chemother, 2009;53:4247-51. [PubMed]
81. Jin L, Li J, Nation RL, Nicolazzo JA. Impact of p-glycoprotein inhibition and lipopolysaccharide administration on blood-brain barrier transport of colistin in mice. Antimicrob Agents Chemother, 2011;55:502-507. [PubMed]
82. Kadar B, Kocsis B, Nagy K, Szabo D. The renaissance of polymyxins. Curr Med Chem, 2013;20:3759-73. [PubMed]
83. Karaiskos I, Galani L, Baziaka F, Giamarellou H. Intraventricular and intrathecal colistin as the last therapeutic resort for the treatment of multidrug-resistant and extensively drug-resistant Acinetobacter baumannii ventriculitis and meningitis: a literature review. Int J Antimicrob Agents, 2013;14:499-508. [PubMed]
84. Kasiakou SK, Michalopoulos A, Soteriades ES, Samonis G, Sermaides GJ, Falagas ME. Combination therapy with intravenous colistin for management of infections due to multidrug-resistant Gram-negative bacteria in patients without cystic fibrosis. Antimicrob Agents Chemother. 2005;49(8):3136-46. [PubMed]
85. Kasssamali Z, Rotschafer JC, Jones RN, Prince RA, Danziger LH. Polymyxins: wisdom does not always come with age. Clin Infect Dis, 2013;57:877-83. [PubMed]
86. Katz E, Demain AL. The peptide antibiotics of Bacillus: chemistry, biogenesis, and possible functions. Bacteriol Rev. 1977;41:449-74. [PubMed]
87. Kazy Z Puho E, Czeizel AE. Parenteral polymyxin B treatment during pregnancy. Reprod Toxicol, 2005;20:181-182. [PubMed]
88. Khawcharoenporn T, Apisarnthanarak A, Mundy LM. Intrathecal colistin for drug-resistant Acinetobacter baumannii central nervous system infection: a case series and systematic review.Clin Microbiol Infect, 2010;16:888-894. [PubMed]
89. Kline T, Trent MS, Stead CM, Lee MS, Sousa MC, Felise HB, Nguyen HV, Miller SI. Synthesis of and evaluation of lipid A modification by 4-substituted 4-deoxy arabinose analogs as potential inhibitors of bacterial polymyxin resistance. Bioorg Med Chem Lett, 2008;18:1507-10. [PubMed]
90. Koch-Weser J, Sidel VW, Federman EB, Kanarek P, Finer DC, Eaton AE. Adverse effects of sodium colistimethate. Manifestations and specific reaction rates during 317 courses of therapy. Ann Intern Med. 1970;72:857-68. [PubMed]
91. Kofteridis DP, Alexopoulou C, Valachis A, Maraki S, Dimopoulou D, Georgopoulos D, Samonis G. Aerosolized plus intravenous colistin versus intravenous colistin alone for the treatment of ventilator-associated pneumonia: a matched case-control study. Clin Infect Dis, 2010;51:1238-44. [PubMed]
92. Kontopidou F, Plachouras D, Papadomichelakis E, Koukos G, Galani I, Poulakou G, Dimopoulos G, Antoniadou A, Armaganidis A, Giamarellou H. Colonization and infection by colistin-resistant Gram-negative bacteria in a cohort of critically ill patients. Clin Microbiol Infect, 2011;17:e9-11. [PubMed]
93. Koomanachai P, Landersdorfer CB, Chen G, Lee HJ, Jitmuang A, Wasuwattakul S, Sritippayawan S, Li J, Nation RL, Thamlikitkul V. Pharmacokinetics of colistin methanesulfonate and formed colistin in end-stage renal disease patients receiving continuous ambulatory peritoneal dialysis. Antimicrob Agents Chemother, 2014;58:440-6. [PubMed]
94. Korbila IP, Michalopoulos A, Rafailidis PI, Nikita D, Samonis G, Falagas ME. Inhaled colistin as adjunctive therapy to intravenous colistin for the treatment of microbiologically documented ventilator-associated pneumonia: a comparative cohort study. Clin Microbiol Infect, 2010;16:1230-6. [PubMed]
95. Koyama Y, et al. A new antibiotic "colistin" produced by spore-forming soil bacteria. J Antibiot (Tokyo). 1950;30:457-58.
96. Komura S, Kurahashi K.Partial purification and properties of L-2,4-diaminobutyric acid activating enzyme from a polymyxin E producing organism. J Biochem, 1979;86:1013-21. [PubMed]
97. Kunin CM, Bugg A. Binding of polymyxin antibiotics to tissues: the major determinant of distribution and persistence in the body. J Infect Dis. 1971;124:394-400. [PubMed]
98. Kwa AL, Abdelraouf K, Low JG, Tam VH. Pharmacokinetics of polymyxin B in a patient with renal insufficiency: a case report.Clin Infect Dis, 2011;52:1280-81. [PubMed]
99. Kwa AL, Lim TP, Low JG, Hou J, Kurup A, Prince RA, Tam VH. Pharmacokinetics of polymyxin B1 in patients with multidrug-resistant Gram-negative bacterial infections. Diagn Microbiol Infect Dis, 2008;60:163-167. [PubMed]
100. Landman D, Georgescu C, Martin DA, Quale J. Polymyxins revisited. Clin Microbiol Rev, 2008;21:449-65. [PubMed]
101. Leclercq R, Cantón R, Brown DF, Giske CG, Heisig P, MacGowan AP, Mouton JW, Nordmann P, Rodloff AC, Rossolini GM, Soussy CJ, Steinbakk M, Winstanley TG, Kahlmeter G. EUCAST expert rules in antimicrobial susceptibility testing. Clin Microbiol Infect, 2013;19:141-160. [PubMed]
102. Ledson MJ, Gallagher MJ, Cowperthwaite C, Convery RP, Walshaw MJ. Four years' experience of intravenous colomycin in an adult cystic fibrosis unit. Eur Respir J. 1998;12:592-94.[PubMed]
103. Lee GC, Burgess DS. Polymyxins and Doripenem Combination Against KPC-Producing Klebsiella pneumoniae. J Clin Med Res, 2013;5:97-100. [PubMed]
104. Lee CH, Tang YF, Su LH, Chien CC, Liu JW. Antimicrobial effects of varied combinations of meropenem, sulbactam, and colistin on a multidrug-resistant Acinetobacter baumannii isolate that caused meningitis and bacteremia. Microb Drug Resist, 2008;14:233-237. [PubMed]
105. Levin AS, Barone AA, Penco J, Santos MV, Marinho IS, Arruda EA, Manrique EI, Costa SF. Intravenous colistin as therapy for nosocomial infections caused by multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Clin Infect Dis. 1999;28:1008-11. [PubMed]
106. Li J, Turnidge J, Milne R, Nation RL, Coulthard K. In vitro pharmacodynamic properties of colistin and colistin methanesulfonate against Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Antimicrob Agents Chemother. 2001;45:781-85. [PubMed]
107. Li J, Milne RW, Nation RL, Turnidge JD, Smeaton TC, Coulthard K. Use of high-performance liquid chromatography to study the pharmacokinetics of colistin sulfate in rats following intravenous administration. Antimicrob Agents Chemother. 2003;47:1766-70. [PubMed]
108. Li J, Milne RW, Nation RL, Turnidge JD, Coulthard K. Stability of colistin and colistin methanesulfonate in aqueous media and plasma as determined by high-performance liquid chromatography. Antimicrob Agents Chemother, 2003;47:1364-70. [PubMed]
109. Li J, Milne RW, Nation RL, Turnidge JD, Smeaton TC, Coulthard K. Pharmacokinetics of colistin methanesulphonate and colistin in rats following an intravenous dose of colistin methanesulphonate. J Antimicrob Chemother. 2004;53:837-40. [PubMed]
110. Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, Paterson DL.Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis, 2006;6:589-601. [PubMed]
111. Linden PK, Paterson DL. Parenteral and inhaled colistin for treatment of ventilator-associated pneumonia. Clin Infect Dis, 2006;43:s89-94. [PubMed]
112. Littlewood JM, Koch C, Lambert PA, Hoiby N, Elborn JS, Conway SP, Dinwiddie R, Duncan-Skingle F. A ten year review of colomycin. Respir Med. 2000;94:632-40. [PubMed]
113. Llobet E, March C, Giménez P, Bengoechea JA. Klebsiella pneumoniae OmpA confers resistance to antimicrobial peptides. Antimicrob Agents Chemother, 2009;53:298-302. [PubMed]
114. Lo-Ten-Foe JR, de Smet AM, Diederen BM, Kluytmans JA, van Keulen PH. Comparative evaluation of the VITEK 2, disk diffusion, etest, broth microdilution, and agar dilution susceptibility testing methods for colistin in clinical isolates, including heteroresistant Enterobacter cloacae and Acinetobacter baumannii strains. Antimicrob Agents Chemother, 2007;51:3726-30. [PubMed]
115. Loutet SA, Mussen LE, Flannagan RS, Valvano MA. A two-tier model of polymyxin B resistance in Burkholderia cenocepacia. Environ Microbiol Rep, 2011;3:278-85. [PubMed]
116. Loutet SA, Valvano MA. Extreme antimicrobial peptide and polymyxin B resistance in the genus Burkholderia. Front Cell Infect Microbiol, 2011;1:6. [PubMed]
117. Malott RJ, Steen-Kinnaird BR, Lee TD, Speert DP. Identification of hopanoid biosynthesis genes involved in polymyxin resistance in Burkholderia multivorans. Antimicrob Agents Chemother, 2012;56:464-471. [PubMed]
118. Marchand S, Frat JP, Petitpas F, Lemaître F, Gobin P, Robert R, Mimoz O, Couet W. Removal of colistin during intermittent haemodialysis in two critically ill patients. J Antimicrob Chemother, 2010;65:1836-7. [PubMed]
119. Marchand S,Lamarche I, Gobin P, Couet W. Dose-ranging pharmacokinetics of colistin methanesulphonate (CMS) and colistin in rats following single intravenous CMS doses. J Antimicrob Chemother, 2010;65:1753-8. [PubMed]
120. Markatonis SL, Markou N, Fousteri M, Sakellaridis N, Karatzas S, Alamanos I, Dimopoulou E, Baltopoulos G. Penetration of colistin into cerebrospinal fluid. Antimicrob Agents Chemother, 2009;53:4907-10. [PubMed]
121. Markou N, Apostolakos H, Koumoudiou C, Athanasiou M, Koutsoukou A, Alamanos I, Gregorakos L. Intravenous colistin in the treatment of sepsis from multiresistant Gram-negative bacilli in critically ill patients. Crit Care, 2003;7:r78-83. [PubMed]
122. Martin NI, Hu H, Moake MM, Churey JJ, Whittal R, Worobo RW, Vederas JC. Isolation, structural characterization, and properties of mattacin (polymyxin M), a cyclic peptide antibiotic produced by Paenibacillus kobensis M. J Biol Chem, 2003;278:13124-32. [PubMed]
123. Matthaiou DK, Michalopoulos A, Rafailidis PI, Karageorgopoulos DE, Papaioannou V, Ntani G, Samonis G, Falagas ME. Risk factors associated with the isolation of colistin-resistant gram-negative bacteria: a matched case-control study. Crit Care Med, 2008;36:807-111. [PubMed]
124. McPhee JB, Lewenza S, Hancock RE. Cationic antimicrobial peptides activate a two-component regulatory system, PmrA-PmrB, that regulates resistance to polymyxin B and cationic antimicrobial peptides in Pseudomonas aeruginosa. Mol Microbiol, 2003;50:205-17. [PubMed]
125. Mendes CA, Burdman EA. Polymyxins - review with emphasis on nephrotoxicity. Rev Assoc Med Bras, 2009;55:752-759. [PubMed]
126. Michalopoulos A, Kasiakou SK, Rosmarakis ES, Falagas ME. Cure of multidrug-resistant Acinetobacter baumannii bacteremia with continuous intravenous infusion of colistin. Scand J Infect Dis. 2005;37(2):142-5. [PubMed]
127. Michalopoulos AS, Tsiodras S, Rellos K, Mentzelopoulos S, Falagas ME. Colistin treatment in patients with ICU-acquired infections caused by multiresistant Gram-negative bacteria: the renaissance of an old antibiotic. Clin Microbiol Infect. 2005;11:115-21. [PubMed]
128. Michalopoulos A, Kasiakou SK, Mastora Z, Rellos K, Kapaskelis AM, Falagas ME. Aerosolized colistin for the treatment of nosocomial pneumonia due to multidrug-resistant Gram-negative bacteria in patients without cystic fibrosis. Crit Care. 2005;9:R53-9. [PubMed]
129. Michalopoulos A, Falagas ME. Colistin and polymyxin B in critical care. Crit Care Clin, 2008;24:377-91. [PubMed]
130. Moffatt JH, Harper M, Harrison P, Hale JD, Vinogradov E, Seemann T, Henry R, Crane B, St Michael F, Cox AD, Adler B, Nation RL, Li J, Boyce JD. Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob Agents Chemother, 2010;54:4971-7. [PubMed]
131. Mohamed AF, Karaiskos I, Plachouras D, Karvanen M, Pontikis K, Jansson B, Papadomichelakis E, Antoniadou A, Giamarellou H, Armaganidis A, Cars O, Friberg LE. Application of a loading dose of colistin methanesulfonate in critically ill patients: population pharmacokinetics, protein binding, and prediction of bacterial kill. Antimicrob Agents Chemother, 2012;56:4241-9.[PubMed]
132. Monarch Pharmaceuticals, Inc. Coly-mycin M Parenteral (package insert). Bristol (TN): Monarch Pharmaceuticals, Inc. 2002.
133. Moore RA, Chan L, Hancock RE. Evidence for two distinct mechanisms of resistance to polymyxin B in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1984;26:539-45.[PubMed]
134. Moskowitz SM, Ernst RK, Miller SI. PmrAB, a two-component regulatory system of Pseudomonas aeruginosa that modulates resistance to cationic antimicrobial peptides and addition of aminoarabinose to lipid A. J Bacteriol, 2004;186:575-9. [PubMed]
135. Nation RL, Velkov RL, Li J. Colistin and Polymyxin B: Peas in a Pod, or Chalk and Cheese? Clin Infect Dis, 2014. [PubMed]
136. Newton BA. The properties and mode of action of the polymyxins. Bacteriol Rev. 1956;20:14-27. [PubMed]
137. Nicas TI, Hancock RE. Outer membrane protein H1 of Pseudomonas aeruginosa: involvement in adaptive and mutational resistance to ethylenediaminetetraacetate, polymyxin B, and gentamicin. J Bacteriol. 1980;143:872-78. [PubMed]
138. Nicas TI, Hancock RE. Alteration of susceptibility to EDTA, polymyxin B and gentamicin in Pseudomonas aeruginosa by divalent cation regulation of outer membrane protein H1. J Gen Microbiol, 1983;129:509-17. [PubMed]
139. Niks M, Hanzen J, Ohlasova D, Rovna D, Purgelova A, Szovenyiova Z, Vaculikova A. Multiresistant nosocomial bacterial strains and their "in vitro" susceptibility to chloramphenicol and colistin. Klin Mikrobiol Infekc Lek. 2004;10:124-29. [PubMed]
140. Nolan K, Knight H, Clark P. NICE guidance on colistimethate sodium and tobramycin for pseudomonas lung infection in cystic fibrosis. Lancet Respir Med, 2013;1:102-103. [PubMed]
141. Nord NM, Hoeprich PD. Polymyxin B and colistin. a critical comparison. N Engl J Med. 1964;270:1030-1035. [PubMed]
142. O'Hara CM, Brenner FW, Miller JM. Classification, identification, and clinical significance of Proteus, Providencia, and Morganella. Clin Microbiol Rev, 2000;13:534-46. [PubMed]
143. Okimura K, Ohki K, Sato Y, Ohnishi K, Sakura N. Chemical conversion of natural polymyxin B and colistin to their N-terminal derivatives. Bull Chem Soc Jpn, 2007;80:543-552. [PubMed]
144. Orwa JA, Govaerts C, Busson R, Roets E, Van Schepdael A, Hoogmartens J. Isolation and structural characterization of colistin components. J Antibiot (Tokyo). 2001;54:595-99.[PubMed]
145. Owen RJ, Li J, Nation RL, Spelman D.In vitro pharmacodynamics of colistin against Acinetobacter baumannii clinical isolates. J Antimicrob Chemother, 2007;59:473-7. [PubMed]
146. Ozbek B, Senturk A. Postantibiotic effects of tigecycline, colistin sulfate, and levofloxacin alone or tigecycline-colistin sulfate and tigecycline-levofloxacin combinations against Acinetobacter baumannii. Chemotherapy, 2010;56:466-71. [PubMed]
147. Padilla E, Llobet E, Doménech-Sánchez A, Martínez-Martínez L, Bengoechea JA, Albertí S. Klebsiella pneumoniae AcrAB efflux pump contributes to antimicrobial resistance and virulence. Antimicrob Agents Chemother, 2010;54:177-83. [PubMed]
148. Pamp SJ, Gjermansen M, Johansen HK, Tolker-Nielsen T. Tolerance to the antimicrobial peptide colistin in Pseudomonas aeruginosa biofilms is linked to metabolically active cells, and depends on the pmr and mexAB-oprM genes. Mol Microbiol, 2008;68:223-40. [PubMed]
149. Pankey GA, Ashcraft DS. Detection of synergy using the combination of polymyxin B with either meropenem or rifampin against carbapenemase-producing Klebsiella pneumoniae. Diagn Microbiol Infect Dis, 2011;70:561-4. [PubMed]
150. Pankuch GA, Seifert H, Appelbaum PC. Activity of doripenem with and without levofloxacin, amikacin, and colistin against Pseudomonas aeruginosa and Acinetobacter baumannii. Diagn Microbiol Infect Dis, 2010;67:191-7. [PubMed]
151. Pantopouloua A, Colistin offers prolonged survival in experimental infection by multidrug-resistant Acinetobacter baumannii: the significance of co-administration of rifampicin. Int J Antimicrob Agents, 2007:29:51-55. [PubMed]
152. Phe K, Lee Y, McDaneld PM, Prasad N, Yin T, Figueroa DA, Musick WL, Cottreau JM, Hu M, Tam VH. In Vitro Assessment and Multicenter Cohort Study of Comparative Nephrotoxicity Rates Associated with Colistimethate versus Polymyxin B Therapy. Antimicrob Agents Chemother, 2014;58:2740-6. [PubMed]
153. Piparsania S, Rajput N, Bhatambare G. Intraventricular polymyxin B for the treatment of neonatal meningo-ventriculitis caused by multi-resistant Acinetobacter baumannii--case report and review of literature. Turk J Pediatr, 2012;54:548-54.[PubMed]
154. Pitt TL, Sparrow M, Warner M, Stefanidou M. Survey of resistance of Pseudomonas aeruginosa from UK patients with cystic fibrosis to six commonly prescribed antimicrobial agents. Thorax. 2003;58:794-96. [PubMed]
155. Plachouras D, Karvanen M, Friberg LE, Papadomichelakis E, Antoniadou A, Tsangaris I, Karaiskos I, Poulakou G, Kontopidou F, Armaganidis A, Cars O, Giamarellou H. Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by gram-negative bacteria. Antimicrob Agents Chemother, 2009;53:3430-6. [PubMed]
156. Plachouras D, Giamarellos-Bourboulis EJ, Kentepozidis N, Baziaka F, Karagianni V, Giamarellou H. In vitro postantibiotic effect of colistin on multidrug-resistant Acinetobacter baumannii. Diagn Microbiol Infect Dis, 2007;57:419-22. [PubMed]
157. Poudyal A, Howden BP, Bell JM, Gao W, Owen RJ, Turnidge JD, Nation RL, Li J. In vitro pharmacodynamics of colistin against multidrug-resistant Klebsiella pneumoniae. J Antimicrob Chemother, 2008;63:1311-8. [PubMed]
158. Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem, 2002;71:635-700. [PubMed]
159. Rastogi N, Henrotte JR, David HL. Colistin (polymyxin E)--induced cell leakage in Mycobacterium aurum. Zentralbl Bakteriol Mikrobiol Hyg A, 1987;263:548-51. [PubMed]
160. Rastogi N, Potar MC, David HL. Antimycobacterial spectrum of colistin (polymixin E). Ann Inst Pasteur Microbiol. 1986;137A:45-53. [PubMed]
161. Rastogi N, Potar MC, Henrotte JG, Franck G, David HL. Further studies on colistin (polymyxin E)-induced cell leakage in mycobacteria: Mg++ efflux in Mycobacterium avium and its effects on drug-susceptibility. Zentralbl Bakteriol Mikrobiol Hyg [A]. 1988;268:251-58. [PubMed]
162. Reed MD, Stern RC, O'Riordan MA, Blumer JL.The pharmacokinetics of colistin in patients with cystic fibrosis. J Clin Pharmacol, 2001;41:645-54. [PubMed]
163. Reeves PR, Hobbs M, Valvano MA, Skurnik M, Whitfield C, Coplin D, Kido N, Klena J, Maskell D, Raetz CR, Rick PD. Bacterial polysaccharide synthesis and gene nomenclature. Trends Microbiol, 1996;4:495-503. [PubMed]
164. Renard L, Sanders P, Laurentie M. [Pharmacokinetics of colistin sulfate administered by intravenous and intramuscular routes in the calf]. Ann Rech Vet. 1991;22:387-94. [PubMed]
165. Rodger KC, Nixon M, Tonning HO. Treatment of Infections with Colistimethate Sodium (Coly-Mycin). Can Med Assoc J, 1965;93:143-6. [PubMed]
166. Rosenthal AL, Rovell JM, Girard AE. Polyacrylic bone cement containing erythromycin and colistin. I. In vitro bacteriological activity and diffusion properties of erythromycin, colistin and erythromycin/colistin combination. J Int Med Res. 1976;4:296-304. [PubMed]
167. Ryan KJ, Schainuck LI, Hickman RO, Striker GE. Colistimethate toxicity. Report of a fatal case in a previously healthy child. JAMA. 1969;207:2099-101. [PubMed]
168. Sader HS, Farrell DJ, Flamm RK, Jones RN. Antimicrobial susceptibility of Gram-negative organisms isolated from patients hospitalized in intensive care units in United States and European hospitals (2009-2011). Diagn Microbiol Infect Dis 2014;78:443-448. [PubMed]
169. Sakura N, et al. The contribution of the N-terminal structure of polymyxin B peptides to antimicrobial and lipopolysaccharide binding activity. Bull. Chem. Soc. Jpn., 2004;77:1915-1924.[PubMed]
170. Salvatore CM, Abdelraouf K, Hsing DD, Tam VH. Pharmacokinetics of polymyxin B in an infant with multidrug-resistant Klebsiella pneumoniae bacteremia. Pediatr Infect Dis J, 2011;30:537-9. [PubMed]
171. Samonis G, Maraki S, Karageorgopoulos DE, Vouloumanou EK, Falagas ME. Synergy of fosfomycin with carbapenems, colistin, netilmicin, and tigecycline against multidrug-resistant Klebsiella pneumoniae, Escherichia coli, and Pseudomonas aeruginosa clinical isolates. Eur J Clin Microbiol Infect Dis, 2012;31:695-701. [PubMed]
172. Samonis G, Korbila IP, Maraki S, Michailidou I, Vardakas KZ, Kofteridis D, Dimopoulou D, Gkogkozotou VK, Falagas ME. Trends of isolation of intrinsically resistant to colistin Enterobacteriaceae and association with colistin use in a tertiary hospital. Eur J Clin Microbiol Infect Dis, 2014. [PubMed]
173. Sandri AM, Landersdorfer CB, Jacob J, Boniatti MM, Dalarosa MG, Falci DR, Behle TF, Bordinhão RC, Wang J, Forrest A, Nation RL, Li J, Zavascki AP. Population pharmacokinetics of intravenous polymyxin B in critically ill patients: implications for selection of dosage regimens. Clin Infect Dis, 2013;57:524-31. [PubMed]
174. Sandrk AM, Landersdorfer CB, Jacob J, Boniatti MM, Dalarosa MG, Falci DR, Behle TF, Saitovitch D, Wang J, Forrest A, Nation RL, Zavascki AP, Li J. Pharmacokinetics of polymyxin B in patients on continuous venovenous haemodialysis. J Antimicrob Chemother, 2013;68:674-7. [PubMed]
175. Schindler M, Osborn MJ. Interaction of divalent cations and polymyxin B with lipopolysaccharide. Biochemistry. 1979;18:4425-30. [PubMed]
176. Sekhri K, Nandha R, Mandal A, Bhasin D, Singh H. Parenteral polymyxins: assessing efficacy and safety in critically ill patients with renal dysfunction. Indian J Pharmacol, 2013;45:608-11.[PubMed]
177. Shoji J, Kato T, Hinoo H. The structure of polymyxin S. (Studies on antibiotics from the genus Bacillus). XXI. J Antibiot (Tokyo), 1977;30:1035-41. [PubMed]
178. Shoji J, Kato T, Hinoo H. The structure of polymyxin T1. (Studies on antibiotics from the genus Bacillus. XXII). J Antibiot (Tokyo), 1977;30:1042-8. [PubMed]
179. Silaev A, et al. The structure of the antibiotic polymyxin M. Chem Nat Compd, 1975;9:278-279. [PubMed]
180. Snavely SR, Hodges GR. The neurotoxicity of antibacterial agents. Ann Intern Med, 1984;101:92-104. [PubMed]
181. Snomily AM. Comparison of E-test and disc diffusion methods for the in vitro evaluation of the antimicrobial activity of colistin in multi-drug resistant Gram-negative Bacilli. Saudi Med J, 2010;31:507-511. [PubMed]
182. Souli M, Galani I, Boukovalas S, Gourgoulis MG, Chryssouli Z, Kanellakopoulou K, Panagea T, Giamarellou H. In vitro interactions of antimicrobial combinations with fosfomycin against KPC-2-producing Klebsiella pneumoniae and protection of resistance development. Antimicrob Agents Chemother, 2011;55:2395-7. [PubMed]
183. Souli M, Rekatsina PD, Chryssouli Z, Galani I, Giamarellou H, Kanellakopoulou K. Does the activity of the combination of imipenem and colistin in vitro exceed the problem of resistance in metallo-beta-lactamase-producing Klebsiella pneumoniae isolates? Antimicrob Agents Chemother, 2009;53:2133-2135. [PubMed]
184. Stein A, Raoult D. Colistin: an antimicrobial for the 21st century? Clin Infect Dis. 2002;35:901-2. [PubMed]
185. Storm DR, Rosenthal KS, Swanson PE. Polymyxin and related peptide antibiotics. Annu Rev Biochem. 1977;46:723-63. [PubMed]
186. Sud ID, Feingold DS. Mechanism of polymyxin B resistance in Proteus mirabilis. J Bacteriol, 1970;104:289-94. [PubMed]
187. Tam VH, Schilling AN, Vo G, Kabbara S, Kwa AL, Wiederhold NP, Lewis RE. Pharmacodynamics of polymyxin B against Pseudomonas aeruginosa. Antimicrob Agents Chemother, 2005;49:3624-30. [PubMed]
188. Tamm M, Eich C, Frei R, Gilgen S, Breitenbucher A, Mordasini C. [Inhaled colistin in cystic fibrosis]. Schweiz Med Wochenschr. 2000;130:1366-72. [PubMed]
189. Tan TH, Ng SY. Comparison of Etest, Vitek and agar dilution for susceptibility testing of colistin. Clin Microbiol Infect, 2007;13:541-4. [PubMed]
190. Tan CH, Li J, Nation RL. Activity of colistin against heteroresistant Acinetobacter baumannii and emergence of resistance in an in vitro pharmacokinetic/pharmacodynamic model.Antimicrob Agents Chemother, 2007;51:3413-5. [PubMed]
191. Tan TY, Lim TP, Lee WH, Sasikala S, Hsu LY, Kwa AL. In vitro antibiotic synergy in extensively drug-resistant Acinetobacter baumannii: the effect of testing by time-kill, checkerboard, and Etest methods. Antimicrob Agents Chemother, 2011;55:436-438. [PubMed]
192. Tascini C, Gemignani G, Ferranti S, Tagliaferri E, Leonildi A, Lucarini A, Menichetti F. Microbiological activity and clinical efficacy of a colistin and rifampin combination in multidrug-resistant Pseudomonas aeruginosa infections. J Chemother, 2004:16:282-287. [PubMed]
193. Tascini C, Ferranti S, Messina F, Menichetti F. In vitro and in vivo synergistic activity of colistin, rifampin, and amikacin against a multiresistant Pseudomonas aeruginosa isolate. Clin Microbiol Infect. 2000;6:690-691. [PubMed]
194. Teng CB, Koh PT, Lye DC, Ang BS. Continuous versus intermittent infusion of polymyxin B in the treatment of infections caused by multidrug-resistant Gram-negative bacteria. Int J Antimicrob Agents, 2008;31:80-82. [PubMed]
195. Theuretzbacher U. Product information for parenteral colistin varies substantially across Europe. J Antimicrob Chemother, 2014;69:1987-92. [PubMed]
196. Toome L, Ringmets I, Andresson P, Ilmoja ML, Saik P, Varendi H. Changes in care and short-term outcome for very preterm infants in Estonia. Acta Paediatr, 2012;101:390-6. [PubMed]
197. Tumbarello M, De Pascale G, Trecarichi EM, De Martino S, Bello G, Maviglia R, Spanu T, Antonelli M. Effect of aerosolized colistin as adjunctive treatment on the outcomes of microbiologically documented ventilator-associated pneumonia caused by colistin-only susceptible gram-negative bacteria. Chest 2013;144:1768-75. [PubMed]
198. Tunkel AR, Hartman BJ, Kaplan SL, Kaufman BA, Roos KL, Scheld WM, Whitley RJ. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis, 2004;39:1267-84. [PubMed]
199. Tuon FF, Rigatto MH, Lopes CK, Kamei LK, Rocha JL, Zavascki AP. Risk factors for acute kidney injury in patients treated with polymyxin B or colistin methanesulfonate sodium. Int J Antimicrob Agents, 2014;43:349-52. [PubMed]
200. Vaara M, Sader HS, Rhomberg PR, Jones RN, Vaara T. Antimicrobial activity of the novel polymyxin derivative NAB739 tested against Gram-negative pathogens. J Antimicrob Chemother, 2013;68:636-639. [PubMed]
201. Vaara M, Siikanen O, Apajalahti J, Fox J, Frimodt-Møller N, He H, Poudyal A, Li J, Nation RL, Vaara T. A novel polymyxin derivative that lacks the fatty acid tail and carries only three positive charges has strong synergism with agents excluded by the intact outer membrane. Antimicrob Agents Chemother, 2010;54:3341-6. [PubMed]
202. Vaara M, Siikanen O, Apajalahti J, Frimodt-Møller N, Vaara T. Susceptibility of carbapenemase-producing strains of Klebsiella pneumoniae and Escherichia coli to the direct antibacterial activity of NAB739 and to the synergistic activity of NAB7061 with rifampicin and clarithromycin. J Antimicrob Chemother, 2010;65:942-945. [PubMed]
203. Vaara M, Vaara T. The novel polymyxin derivative NAB739 is remarkably less cytotoxic than polymyxin B and colistin to human kidney proximal tubular cells. Int J Antimicrob Agents, 2013;41:292-293. [PubMed]
204. Valour F, Dutronc H, Dinh A, Cazorla C, Pavèse P, Lesens O, Uçkay I, Chidiac C, Ferry T, Colistin BJIs Study Group. Difficult-to-treat Gram-negative bone and joint infections: efficacy and safety of prolonged intravenous colistin. Int J Antimicrob Agents, 2013;41:197-199. [PubMed]
205. Van der Heijden IM, Levin AS, De Pedri EH, Fung L, Rossi F, Duboc G, Barone AA, Costa SF. Comparison of disc diffusion, Etest and broth microdilution for testing susceptibility of carbapenem-resistant P. aeruginosa to polymyxins. Ann Clin Microbiol Antimicrob, 2007;6:8. [PubMed]
206. Vidaillac C, Benichou L, Duval RE. In vitro synergy of colistin combinations against colistin-resistant Acinetobacter baumannii, Pseudomonas aeruginosa, and Klebsiella pneumoniae isolates.Antimicrob Agents Chemother, 2012;56:4856-61. [PubMed]
207. Viljanen P. Vaara M. Susceptibility of gram-negative bacteria to polymyxin B nonapeptide. Antimicrob Agents Chemother, 1984;25:701-705. [PubMed]
208. Warner DM, Levy SB. Different effects of transcriptional regulators MarA, SoxS and Rob on susceptibility of Escherichia coli to cationic antimicrobial peptides (CAMPs): Rob-dependent CAMP induction of the marRAB operon. Microbiology, 2010;156(Pt2):570-578. [PubMed]
209. Warren HS, Kania SA, Siber GR. Binding and neutralization of bacterial lipopolysaccharide by colistin nonapeptide. Antimicrob Agents Chemother, 1985;28:107-112. [PubMed]
210. Witzke NM, Hedein H. Broad-spectrum derivatives of polymyxin B and colistin. J Antibiot (Tokyo), 1976;29:1349-50. [PubMed]
211. Wright WW, Welch H. Chemical, biological and clinical observations on colistin. Antibiot Annu, 1959;7:61-74. [PubMed]
212. Yahav D, Farbman L, Leibovici L, Paul M. Colistin: new lessons on an old antibiotic. Clin Microbiol Infect, 2012;18:18-29. [PubMed]
213. Yoon J, Urban C, Terzian C, Mariano N, Rahal JJ. In vitro double and triple synergistic activities of Polymyxin B, imipenem, and rifampin against multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother, 2004;48:753-7. [PubMed]
214. Zavascki AP, Goldani LZ, Cao G, Superti SV, Lutz L, Barth AL, Ramos F, Boniatti MM, Nation RL, Li J. Pharmacokinetics of intravenous polymyxin B in critically ill patients. Clin Infect Dis, 2008;47:1298-304. [PubMed]
215. Zhai B, Lin X. Evaluation of the anticryptococcal activity of the antibiotic polymyxin B in vitro and in vivo. Int J Antimicrob Agents, 2013;41:250-254. [PubMed]
216. Zhou F, Peng Z, Murugan R, Kellum JA. Blood purification and mortality in sepsis: a meta-analysis of randomized trials. Crit Care Med, 2013;41:2209-2220. [PubMed]
217. Ziaka M, Markantonis SL, Fousteri M, Zygoulis P, Panidis D, Karvouniaris M, Makris D, Zakynthinos E. Combined intravenous and intraventricular administration of colistin methanesulfonate in critically ill patients with central nervous system infection.Antimicrob Agents Chemother, 2013;57:1938-40. [PubMed]
218. Zusman O, Systematic review and meta-analysis of in vitro synergy of polymyxins and carbapenems. Antimicrob Agents Chemother, 2013;57:5104-5111. [PubMed]
Tables
Figure 1 (a,b). Structure of colistin and polymyxin B. The fatty acid molecule is 6-methyloctanoic acid for colistin A and Polymyxin B1, and 6-methylheptanoic acid for colistin B and polymyxin B2. (α,γ)L-Dab. Diaminobutyric acid, α and γ indicate the respective –NH2 involved in the peptide linkage.
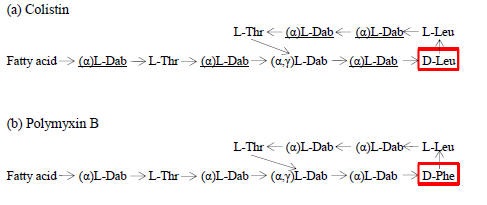
Table 1. Minimum inhibitory concentrations for polymyxin B and colistin according to CLSI and EUCAST.
| CLSI 2014 | EUCAST 2014 | ||||
|---|---|---|---|---|---|
| Micro-organism | Susceptible | Intermediate | Resistant | Susceptible | Resistant |
| Enterobacteriaceae* | ≤2 μg/ml | 4 μg/ml | ≥8 μg/ml | ≤2 μg/ml | >2 μg/ml |
| P. aeruginosa | ≤2 μg/ml | 4 μg/ml | ≥8 μg/ml | ≤4 μg/ml | >4 μg/ml |
| A. baummanni | ≤2 μg/ml | --- | ≥4 μg/ml | ≤2 μg/ml | >2 μg/ml |
* Does not refer to isolates inherently resistant to polymyxins
EUCAST does not provide breakpoints for polymyxin B.
Abbreviations: CLSI - Clinical and Laboratory Standards Institute, EUCAST - European Committee on Antimicrobial Susceptibility Testing
Table 2. Major differences between colistin and polymyxin B, which may affect clinical outcomes.
| COLISTIN | POLYMYXIN B |
|---|---|
| Administered as pro-drug (CMS), requires conversion to colistin | Administered as the active antibiotic, no conversion is required |
| Considerable inter-individual variation in plasma steady-state concentration exists | Low inter-individual variation in plasma concentrations |
| CMS excreted in urine, conversion results to high colistin urine concentration | It is not excreted in the urine, low urine concentration |
| CMS is converted slowly to colistin, its plasma concentration increases slowly* | Plasma concentration increases sufficiently |
| Loading dose required on PK grounds, improves time to bactericidal concentration | Loading dose required on PK grounds, improves rapidly time to bactericidal concentration |
| Adjustment for renal function is mandatory | No adjustment is required |
| Removed by hemodialysis, dose adjustment is required | Removed to a minor extent, dose adjustment is not required |
| Therapeutic drug monitoring requires stringent procedures in the collection, storage and transportation of the sample to prevent conversion of CMS to colistin after collection | Difficulties not present |
* Potential for inadequate treatment for several hours/days (it is unknown if this is associated with increased mortality and development of resistance, both are possible).
Abbreviations: CMS - colistimethate sodium, PK - pharmacokinetic
Table 3. Recommended maintenance dose for intravenous polymyxins in Europe and USA, as provided by the manufacturers and PK studies.
| Recommendation by | Antibiotic | Degree of renal impairment | ||||
|---|---|---|---|---|---|---|
| Normal (≥80 ml/min) | Mild (CrCl 50-79 ml/min) | Moderate (CrCl 30-49 ml/min) | Severe (CrCl 10-29 ml/min) | Renal replacement | ||
| FDA USA, manufacturers# | Colistin base* | 2.5-5 mg/kg (divided in 2-4 doses) | 2.5-3.8 mg/kg (divided in 2 doses) | 2.5 mg/kg (in 1 or 2 doses) | 1.5 mg/kg (in 1 dose every 36h) | --- |
| Manufacturers Europe | CMS* | 4-10 mg/kg (in 2-3 divided doses) | 2.3-10 mg/kg (in 2-3 divided doses) | 1-6 mg/kg (in 1-3 divided doses) | 1-6 mg/kg (divided in doses every 8-36 h) | --- |
| PK studies | Colistin base* | 2.5-5 mg/kg (in 2-3 divided doses) | Dose adjustment according to the formula: colistin Css,avg targetx (1.50 x CrCL + 30) | CVVHD: 192 mg for every 1μg//ml Css,avg | ||
| Manufacturer | Polymyxin B | 1.5-2.5 mg/kg (divided in 2 doses) | The dose should be reduced from 1.5 mg/kg downwards according to renal impairment | --- | ||
| PK studies | Polymyxin B | 1.5-2.5 mg/kg (divided in 2 doses) | Dose adjustment is not required | |||
* 1 mg of colistin base is equal to 2.4 mg of CMS, 1 mg of CMS is 12500 IU
# % of urea clearance is used rather than creatinine clearance in the FDA and most manufacturers; the provided values approximate those in FDA
Abbreviations: CMS -colistimethate sodium, CrCl - creatinine clearance, CVVHD - continuous venovenous hemodialysis, Css, avg - mean colistin steady state concentration
GUIDED MEDLINE SEARCH FOR:
Pharmacokinetics/Pharmacodynamics
What's New
Bard JD, et al. Rationale for Eliminating Staphylococcus Breakpoints for β-lactam Agents Other Than Penicillin, Oxacillin or Cefoxitin, and Ceftaroline. Clin Infect Dis 2014;58:1287-1296.
Sandri AM, et al. Population Pharmacokinetics of Intravenous Polymyxin B in Critically Ill Patients: Implications for Selection of Dosage Regimens. Clin Infect Dis 2013;57:524.
Akajagbor DS, et al. Higher Incidence of Acute Kidney Injury with Intravenous Colistimethate Sodium Compared with Polymyxin B in Critically Ill Patients at a Tertiary Care Medical Center. Clin Infect is 2013;57:1300.
Ziaka M, et al. Combined Intravenous and Intraventricular Administration of Colistin Methanesulfonate in Critically Ill Patients with Central Nervous System Infection. Antimicrobial Agents and Chemotherapy 2013;57:1938-1940.
Gauthier TP, et al. Incidence and Predictors of Nephrotoxicity Associated with Intravenous Colistin in Overweight and Obese Patients. Antimicrob Agents and Chemotherapy.2012;56:2392-6.
Kwa ALH, et al. Pharmacokinetics of Polymyxin B in a Patient with Renal Insufficiency: A Case Report. Clinical Infectious Diseases. 2011;52:1280-1.
Colistin vs. Colistimethate: serious dosing error [http://www.ashp.org/DocLibrary/Policy/PatientSafety/NANAlert-Colistimethatesodium.aspx]
FDA Warning: Colistin in Nebulizer Solution.
