Darunavir
Authors: Kevin C. Brown, PharmD, Angela D.M. Kashuba, BScPhm, PharmD, DABCP, Anne E. Mattson, PharmD
CLASS
Chemical Structure
The chemical formula for darunavir is C27H37N3O7S with a molecular weight of 547.664g/mol (PPI, [1]). The chemical structure of darunavir is shown in Figure 1.
Structure-Activity Relationship
Darunavir was developed to maintain activity against multi-drug resistant strains of HIV-1. Novel features include high-affinity binding to the N-H groups of Asp29 and Asp30 in the P2 pocket of the HIV-1 protease, and a large portion of the molecule resting in the substrate envelope. These features confer a unique resistance mutation pattern for darunavir.
ANTIVIRAL ACTIVITY
Spectrum
Darunavir inhibits maturation and replication of HIV-1 typically resistant to older HIV protease inhibitors.
Pharmacodynamic Effects
Darunavir is approximately 95% bound to plasma alpha-1-acid glycoprotein. The EC50 ranges from 1 to 8.5 nM, and the EC90 ranges from 2.7 to 13 nM (7). A study of 118 HIV-positive, treatment-experienced individuals calculated a median (range) inhibitory quotient (IQ) of 36.3 (0.5-1150), assuming 95% protein binding (27). Resistance to darunavir can occur both with subinhibitory darunavir exposures, and using antiretrovirals in combination regimens to which the major HIV variants have limited susceptibility.
MECHANISMS OF ACTION
Darunavir selectively blocks the cleavage of the HIV Gag-Pol polyprotein by inhibiting the HIV-1 protease enzyme. This mechanism prevents the maturation of viral particles into infectious virions.
MECHANISMS OF RESISTANCE
Organisms Commonly Resistant
The following HIV-1 protease mutations have been associated with resistance to darunavir, as identified in the Phase II POWER trials: V11I, V32I, L33F, I47V, I50V, I54L/M, G73S, L76V, I84V, and L89V. The following mutations have been associated with darunavir resistance by in vitro selection studies: L10F, V11I, I13V, I15V, G16E, L23I, V32I, L33F, S37N, M46I, I47V, I50V, F53L, L63P, A71V, G73S, L76V, V82I, I84V, T91A/S, and Q92R. The most common mutations were L10F, V32I, L33F, S37N, M46I, I47V, I50V, L63P, A71V, and I84V. Darunavir resistant strains have at least eight mutations, a 50- to 64-fold decrease in susceptibility, and an EC50 ranging from 125 nM to 3461 nM (11).
Mechanisms of Resistance
In phase II studies, individuals experiencing virologic failure while using darunavir/ritonavir 600/100 mg twice daily developed multiple amino acid substitutions associated with darunavir resistance as listed above (Section A). However, the V32I substitution appeared in more than 30% of failures (11).
Methods to Overcome or Prevent Resistance
Using appropriate doses and maintaining excellent adherence to all antiretrovirals (ARVs) in the regimen is the most effective method of preventing the development of darunavir resistance. When patients selectively or intermittently take ARVs, exposure to these drugs decreases below the IC50. Viral replication can then occur in the presence of sub-inhibitory concentrations, resulting in the development of DRV resistance mutations. Resistance to darunavir requires a regimen change to another protease inhibitor with a different pattern of resistance mutations, or switching drug classes completely (11).
PHARMACOKINETICS
Absorption
The absolute bioavailability of 600 mg darunavir is 37%. This increases to 82% when co-administered with 100 mg of ritonavir. The maximum concentration (Cmax) is achieved between 2.5 and 4 hours (Tmax). Compared to the fasted state, there is an approximate 30% increase in AUC and Cmax when darunavir/ritonavir is coadministered with food. Therefore, it is recommended that darunavir/ritonavir be administered with food (21).
Distribution
Darunavir is 95% protein bound to alpha-1-acid glycoprotein (AAG). Mean volume of distribution is approximately 131L (or 2L/kg), demonstrating moderate distribution to peripheral sites in the body (11). Darunavir is also a lipophilic compound, as it has higher solubility in organic compared to aqueous solvents, with a predicted logP of 1.89 (9).
Darunavir concentrations measured in the CSF of patients who had been taking 600/100mg DRV/r dose twice daily for a median time of 12.5 weeks (0.5-16.6) ranged from 15.9-212.9ng/mL (median concentration 34.2ng/mL) and plasma concentrations obtained simultaneously ranged from 1800-12900ng/mL (median concentration 3930ng/mL). These CSF concentrations are 0.3-18-fold above the protein adjusted IC50 for DRV (12-55ng/mL). The protein free IC50 = 1.7812ng/mL (0.003µM) (13, 30).
Darunavir concentrations measured in seminal plasma and compared to published (PC) EC50 values for wild type HIV (55 ng/mL) and (PC) EC50 for resistant HIV (550 ng/mL). Median (and interquartile range) darunavir concentrations waned slowly but steadily over the three dosing intervals - 1 to 3 hours: 5579 ng/mL (4639 to 7505) in blood, 588 ng/mL (509 to 778) in semen 4 to 6 hours: 3734 ng/mL (2935 to 4586) in blood, 490 ng/mL (479 to 640) in semen 22 to 24 hours: 2445 ng/mL (1365 to 3167) in blood, 217 ng/mL (172 to 261) in semen (25). Median semen-to-blood ratios stayed steady in the hours after dosing: 0.11 (0.09 to 0.15) at 1 to 3 hours (n = 8), 0.13 (0.07 to 0.18) at 4 to 6 hours (n = 13), and 0.11 (0.09 to 0.15) at 22 to 24 hours (n = 14) (25). Median seminal plasma to blood plasma AUC 0-24h ratios were 0.17 (0.07 to 0.19). Seminal plasma DRV exceeded the PC EC50 for wild type HIV by 11-fold (6 to 45) at 1 to 3 hours, 9-fold (3 to 21) at 4 to 6 hours, and 4-fold (2 to 16) at 22 to 24 hours (25). Darunavir has good penetration into the semen of HIV-1 infected men with concentrations approximating 10-20% of the concentrations achieved in the blood plasma at the same time points post drug ingestion (25). This data may have important implications for PI monotherapy studies, the evolution and transmission of resistant viruses, and the sexual transmission of HIV. There are no specific data on darunavir concentrations in the female genital tract.
Routes of Elimination
Darunavir is metabolized almost exclusively by the hepatic cytochrome P450 (CYP) CYP3A4 enzyme. Three major metabolites of darunavir have been identified, which have 10% of the antiviral activity against wild-type HIV compared to darunavir. Four additional minor metabolites have also been identified (23). Administration of 600 mg radio-labeled darunavir with 100 mg ritonavir demonstrated that approximately 79.5% of the dose is eliminated in the feces and 13.9% is eliminated in the urine. In the feces, 41.2% of the dose is unchanged, and in the urine, 7.7% of the dose is unchanged. The half-life of darunavir when coadministered with ritonavir is 15 hours (11).
Pharmacokinetic Parameters (Clearance, Vd Steady State, Half Life, Plasma Binding, Bioavailability, Therapeutic Range)
In a study of a single 150mg intravenous dose administered after 2 days of twice daily or ritonavir 100mg was given, the mean volume of distribution was 131 L (7). Steady-state pharmacokinetic parameters for darunavir obtained from 119 HIV-positive participants (median (range)) were as follows: AUC12 hr = 61,668 (33,857-106,490) ng*hr/mL; C0 h = 3,539 (1255-7368) ng/mL (7). Women have up to 20% higher exposures of darunavir compared to men (27).
CNS/CSF Disposition
Darunavir concentrations measured in the CSF are 0.3-18-fold above the protein adjusted IC50 for DRV (12-55ng/mL). The protein free IC50 = 1.7812ng/mL (0.003µM) (13, 30).
Effect of Disease States (hepatic disease, renal disease, shock, CHF, etc.)
HIV-1 infected patients with mild and moderate hepatic impairment taking darunavir/ritonavir 600mg/100mg twice daily had similar exposures to individuals with no hepatic impairment. In patients with mild or moderate renal dysfunction, darunavir exposures were similar to patients with normal renal function. Darunavir has not been studied in end-stage liver disease or in severe renal disease (CrCL <30 mL/min).
There are no data on darunavir pharmacokinetics for other disease states. However, disease states that affect plasma alpha-1 acid glycoprotein have the potential to alter the body clearance of darunavir.
DOSAGE
Adult (19-22)
To achieve optimal therapeutic effect, darunavir must be administered with ritonavir. Failure to do so may lead to suboptimal therapeutic levels reducing the antiviral activity of darunavir.
For treatment-naive, 800 mg by mouth once daily plus with ritonavir 100 mg by mouth once daily, with food is recommended.
For treatment-experienced Adult Patient Dosing, see Table 4.
Pediatric (21)
Darunavir is approved for pediatric patients age 3 to less than 18 years of age. Once daily dosing of darunavir is not recommended in the pediatric population. Dosing of darunavir in pediatric patients should be selected based on each child’s body weight in kilograms (kg) for children weighing at least 10 kg. This dose should not exceed the maximum daily dose recommended in treatment-experienced adults (1200 mg per day of darunavir). For children weighing at least 15 kg, assessment for the ability to swallow tablets should be done before prescribing darunavir suspension.
The weight-based dose recommendation for pediatric patients weighing less than 15 kg is darunavir 20 mg/kg by mouth twice daily with ritonavir 3 mg/kg by mouth twice daily (Table 5).
For dosing recommendations for pediatric patients weighing at least 15 kg who cannot swallow tablets, see Table 6.
Dosing recommendations for pediatric patients weighing at least 15 kg who can swallow tablets can be found in Table 7.
Renal Failures (Including CCVHD)
HIV infected patients with moderate renal failure (30 – 60 mL/min) require no dosage adjustment. Darunavir has not been studied in individuals with severe renal failure (<30 mL/min) or end-stage renal disease, and there are no recommendations for this population (11).
Hepatic Failures
Steady-state pharmacokinetic parameters of darunavir were similar after multiple dose co-administration of darunavir/ritonavir 600/100 mg twice daily to patients with normal hepatic function, mild hepatic impairment (Child-Pugh class A), and moderate hepatic impairment (Child-Pugh class B). Therefore, no dosage adjustment is necessary in individuals with mild and moderate hepatic impairment. However, the effect of severe liver impairment (Child-Pugh class C) has yet to be studied. Consequently, darunavir/ritonavir is not recommended in patients with severe hepatic impairment (11).
Body Composition (Obesity, Wasting, Various Body Builds)
Redistribution of body fat as well as increased body fat has been demonstrated with darunavir based regimens (17). Patients taking darunavir/r monotherapy gained 340g (8.3%) adipose tissue in the darunavir/r monotherapy when compared to patients on a triple therapy regimen, where adipose decreased by 20g in the triple therapy arm even in patients receiving abacavir/lamivudine or tenofovir/emtricitabine (22).
Ascites/Edema
No data exists.
Chronic Diarrhea/Malabsorption
No data exists.
Malnutrition
No data exists.
Pregnancy
Pregnancy Category C: Darunavir should be used during pregnancy only if the potential benefit justifies the potential risk (11).
There have not been adequate, well-controlled studies of pregnant women receiving darunavir. However in reproduction studies with darunavir in animals, there has been no embryotoxicity or teratogenicity in mice or rats in the prescence or absence of ritonavir. This is also true in rabbits with darunavir alone. In these studies, the AUC of darunavir was higher in rats (3-fold), whereas in mice and rabbits, the AUC was lower (less than 1-fold) compared to the AUC obtained in human subjects at the recommended clinical dose of darunavir/ritonavir (11).
In a phase IIIb, nonrandomized study, darunavir/ritonavir 600/100 mg twice daily or 800/100 mg once daily was evaluated in the second and third trimesters of pregnancy in HIV-1-infected women for antiviral activity and safety. Postpartum data was also collected. Pharmacokinetic data for twice daily darunavir was presented while once daily data continues to be collected (31).
Consistent with other PIs, darunavir/ritonavir 600/100 mg exposures were lower during pregnancy compared to postpartum. The anti-viral activity, however, was similar. Darunavir/ritonavir therapy was generally well tolerated with 12% of patients discontinuing due to adverse effects (31).
Breastfeeding
It is not known whether darunavir is secreted into human breast milk, however, darunavir has been detected in the milk of lactating rats. Due to the risk of transmission of HIV through breastfeeding as well as the potential for adverse events to nursing infants, it is recommended that mothers taking darunavir/ritonavir not breastfeed (11).
ADVERSE EFFECTS
Mechanism
The most commonly report adverse effects in the phase 2b trials were diarrhea (19.8-28.2%), nausea (12.9%-18.3%), headache (15.3%-20.2%), and nasopharyngitis (10.5%-13.7%). Other adverse events include rash, vomiting, abdominal pain, and constipation (11).
Drug-induced hepatitis has been seen in 0.5% of patients during the post-marketing phase. Hepatitis occurred in patients receiving multiple concomitant medications, having pre-existing liver dysfunction (including hepatitis B or C co-infection), or developing immune reconstitution syndrome. Causality has not been established with darunavir (11).
Grade 2 or greater lab abnormalities that occurred in a higher frequency than the comparator arm in clinical trials include increased prothrombin time, decreased platelet count, hypernatremia, increased total cholesterol, increased pancreatic lipase, increased pancreatic amylase, and increased alkaline phosphatase (11).
Risk Factors
Risk factors for adverse effects include concurrent disease states, immunologic function, and treatment adherence.
Treatment and Avoidance
Patients must be instructed to contact their prescribing physician should they experience any adverse events. This is important to maintain treatment adherence and avoid erratic dosing that could lead to antiretroviral resistance. Darunavir/ritonavir-induced diarrhea and nausea can be treated with loperamide and promethazine, respectively, if necessary.
Overdoses (Manifestations and Management)
There are little data with regards to acute darunavir overdose. Healthy volunteers have been given up to 3200 mg of darunavir alone or 1600mg of darunavir in combination with ritonavir. Under these conditions, no symptoms of overdose have been observed. Supportive care should be provided along with the optional removal of unabsorbed drug by emesis, gastric lavage, or activated charcoal (11).
MONITORING REQUIREMENTS
Therapeutic Drug Monitoring
Therapeutic drug monitoring may be employed to assess patient adherence, drug absorption, or drug interactions at the clinician’s discretion. The mean protein binding adjusted darunavir EC50 for wildtype HIV-1 is 55 ng/mL. A specific target plasma trough concentration has not been established. In Phase 2 clinical trials, the median plasma trough concentration was 3539 ng/mL, with a range of 1255-7368 ng/mL. In addition, the response in highly treatment-experienced patients has been shown to correlate better with virtual inhibitory quotient (vIQ) and genotypic inhibitory quotient (gIQ) than trough concentrations or genotyping alone, although a specific IQ cut-off cannot be recommended at this time (7, 10) [Refer to section on inhibitory quotient of protease inhibitors].
Other Laboratory Monitoring
Liver enzymes, fasting cholesterol panels, and fasting blood glucose should be monitored after the initial month of therapy, and at least every 3 months thereafter. Clinicians should instruct patients on potential adverse effects and to report serious cases immediately.
DRUG INTERACTIONS
In human liver microsomes, darunavir inhibits the activity of CYP1A2, CYP2C9, CYP2C19, and CYP2D6. Darunavir demonstrates competitive inhibition of CYP3A4 with an inhibitory constant (Ki) of 0.40µmol/L (0.22 µg/mL). In human hepatocyte cultures, and in clinical trials, darunavir induces CYP3A4 activity. However, when darunavir is administered with ritonavir, the net effect is inhibition of CYP3A4 activity. Darunavir’s Ki values for There is less of a potential for clinically relevant drug interactions through CYP2B6, CYP2C9, CYP2C19, and CYP2D6, as darunavir’s Ki’s are >60 fold higher than that for CYP3A4 (9).
Darunavir/ritonavir has known significant drug interactions with anti-infectives, HMG Co-A reductase inhibitors, phosphodiesterase inhibitors, oral contraceptives, selective serotonin reuptake inhibitors, and other antiretrovirals. Refer to Table 1 for a listing of drugs that should not be coadministered with darunavir/ritonavir. For a listing of drugs that need dose adjustment or careful monitoring, see Table 2 and Table 3.
CLINICAL INDICATIONS (Brief)
Darunavir is available as 300 mg and 600 mg film coated tablets marketed as PrezistaTM (Tibotec, Inc.) (11). Prezista was FDA approved on June 23, 2006 for treatment of HIV-1 infected patients who are highly treatment-experienced. In order to increase darunavir exposure to therapeutic concentrations, 600 mg darunavir must be taken with 100 mg ritonavir twice daily.
In the phase 3 TITAN trial, treatment-experienced HIV-1 infected patients who were also naïve to lopinavir/ritonavir therapy, were randomized to receive either 600/100 mg darunavir/ritonavir twice daily or 400/100 mg lopinavir/ritonavir twice daily with an optimized background regimen. The primary endpoint was non-inferiority. Comparing darunavir to lopinavir at 48 week of therapy, 77% versus 67% (p<0.0001) of patients, respectively, had HIV RNA < 400 copies/mL; 71% versus 60% (p=0.005) had HIV RNA <50 copies/mL, with a drop in HIV RNA from baseline of 1.95 versus 1.72 (p=0.046) log copies/mL. Darunavir/ritonavir and lopinavir/ritonavir therapy resulted in similar CD4+ T cell count responses from baseline: the median increase was 88 and 81 cells/µL, respectively. Adverse events and laboratory abnormalities were similar between both groups (6).
In the 48 week subgroup analysis of the Phase 3 POWER 1 and POWER 2 trials, darunavir/ritonavir 600/100 mg twice daily with optimized background therapy was compared to an optimized protease inhibitor regimen. In the darunavir arm, 61% had >1 log copies/mL drop in HIV RNA compared to 15% in the control protease inhibitor arm (p<0.0001). Forty-five percent versus 17% (p<0.0001) achieved HIV RNA < 50 copies/mL, respectively. The mean change in CD4+ T cell count from baseline was 102 cells/µL in the darunavir group versus 19 cells/µL in the control group (p<0.0001). Treatment-related adverse events were similar between both arms (2).
In the Phase 3 ARTEMIS trial, 800/100 mg darunavir/ritonavir once daily is being compared to 400/100 mg lopinavir/ritonavir twice daily and 800/200 mg lopinavir/ritonavir once daily in treatment-naïve HIV-1 infected patients. The 48 week data analysis demonstrated that once-daily darunavir/ritonavir was equivalent to twice daily lopinavir/ritonavir in reducing HIV RNA to less than 50 copies/mL: 84% versus 81% respectively. However, the lopinavir/ritonavir twice daily group experienced more adverse effects such as diarrhea and increased serum cholesterol and triglycerides. The rates of virologic failure were 10% in the once daily darunavir/ritonavir arm, 12% in the twice daily lopinavir/ritonavir arm, and 19% in the once daily lopinavir/ritonavir arm (3).
A preliminary 24-week data analysis from a Phase 2 trial in children and adolescents (N=80) has demonstrated that darunavir combined with low dose ritonavir is safe and effective. This trial recruited treatment-experienced HIV-1 infected patients aged 6-17 years. Patients were dosed darunavir/ritonavir by body weight according to the following scheme: 20 to <30 kg, 375/50mg twice daily; 30 to <40 kg, 450/60 mg twice daily; and ≥40 kg, 600/100 mg twice daily. A reduction in HIV RNA of at least 1 log was achieved in 74% of patients, and 64% and 50% of patients had HIV RNAs of <400 copies/mL and <50 copies/mL, respectively. There was a mean increase from baseline of 117 CD4+ cells/µL. Grade 3 or 4 adverse events were experienced by 23% of patients, which is similar to that seen in the POWER 1 and 2 trials where 20% of patients experienced at least one serious adverse event (1).
REFERENCES
1. Arribas J, Horban A, Gerstoft J, Fatkenheuer G, Nelson M, Clumeck N, Pulido F, Hill A, van Delft Y, Stark T, Moecklinghoff C. The MONET Trial: Darunavir/ritonavir with or without Nucleoside Analogues, for Patients with HIV RNA Below 50 copies/ml. AIDS. 2010:24:223-230. [PubMed]
2. Blanche S, Bologna R, Cahn P, Rugina S, Flynn P, Fortuny C, Vis P, Sekar V, van Baelen B, Dierynck I, Spinosa-Guzman S. Pharmacokinetics, Safety and Efficacy of Darunavir/ritonavir in Treatment-Experienced Children and Adolescents. AIDS. 2009:23:2005-2013. [PubMed]
3. Bologna R, Rugina S, Cahn P, Rugina S, Flynn P, Fortuny C, Vis P, Sekar V, van Baelen B, Dierynck I, Spinosa-Guzman S. Pharmacokinetics, safety and efficacy of darunavir/ ritonavir in treatment-experienced children and adolescents. AIDS 2009; 23:2005–2013. [PubMed]
4. Cahn P, Fourie J, Grinsztejn B, Hodder S, Molina JM, Ruxrungtham K, Workman C, Van de Casteele T, De Docker P, Lathouwers E, Tomaka F. Week 48 Analysis of Once-daily vs Twice-daily Darunavir/ritonavir in Treatment-Experienced HIV-1-Infected Patients. AIDS. 2011:25:929-939. [PubMed]
5. Clotet B, Bellos N, Molina JM, Cooper D, Goffard JC, Lazzarin A, Wöhrmann A, Katlama C, Wilkin T, Haubrich R, Cohen C, Farthing C, Jayaweera D, Markowitz M, Ruane P, Spinosa-Guzman S, Lefebvre E, POWER 1 and 2 study groups. Efficacy and safety of darunavir-ritonavir at week 48 in treatment-experienced patients with HIV-1 infection in POWER 1 and 2: a pooled subgroup analysis of data from two randomised trials. Lancet. 2007;369:1169-78. [PubMed]
6. "ClinicalTrials.gov Archive, TMC114-C214: Trial of TMC114 Administered with Low Dose Ritonavir (RTV) in HIV-1 Infected Treatement Experienced Patients" ClinicalTrials.gov, 27 Apr. 2010. Web. 01 Aug. 2010.http://clinicaltrials.gov/archive/NCT00110877/2010_04_27.
7. De Meyer S, Azijn H, Surleraux D, Jochmans D, Tahri A, Pauwels R, Wigerinck P, de Béthune MP. TMC114, a novel human immunodeficiency virus type 1 protease inhibitor active against protease-inhibitor resistant viruses, including a broad range of clinical isolates. Antimicrobial Agents Chemotherapy, 2005;49:2314-2321. [PubMed]
8. De Meyer SM, Spinosa-Guzman S, Vangeneugden TJ, de Be´thune MP, Miralles GD. Efficacy of once-daily darunavir/ ritonavir 800/100mg in HIV-infected, treatment-experienced patients with no baseline resistance-associated mutations to darunavir. J AIDS 2008; 49:179–182. [PubMed]
9. DrugBank. Darunavir (DB01264). http://www.drugbank.ca/cgi-bin/getCard.cgi?CARD=DB01264.txt Accessed May 8, 2008.
10. Food and Drug Administration. MedWatch. Available at: www.fda.gov/medwatch/index.html. Accessed July 29, 2010.
11. Janssen Services, LLC. Prezista (Darunavir) package insert June 2012.
12. Katlama, C. Valentin, MA. Algarte-Genin, M. et al. Efficacy of darunavir/ritonavir as single-drug maintenance therapy in patients with HIV-1 viral suppression: a randomized open-label non-inferiority trial, MONOI-ANRS 136. AIDS. 2010:24:2365-2374
13. Koh Y, Nakata H, Maeda K, Ogata H, Bilcer G, Devasamudram T, Kincaid JF, Boross P, Wang YF, Tie Y, Volarath P,Gaddis L, Harrison RW, Weber IT, Ghosh AK, Mitsuya H. Novel bis-Tetrahydrofuranylurethane-Containing Nonpeptidic Protease Inhibitor (PI) UIC-94017 (TMC114) with Potent Activity against Multi-PI-Resistant Human Immunodeficiency Virus In Vitro. Antimicrob Agents Chemother 2003;47(10): 3123-3129. [PubMed]
14. Madruga JV, Berger D, McMurchie M, Suter F, Banhegyi D, Ruxrungtham K, Norris D, Lefebvre E, de Béthune MP, Tomaka F, De Pauw M, Vangeneugden T, Spinosa-Guzman S; TITAN study group. Efficacy and safety of darunavir-ritonavir compared with that of lopinavir-ritonavir at 48 weeks in treatment-experienced, HIV-infected patients in TITAN: a randomised controlled phase III trial. Lancet. 2007 Jul 7;370(9581):49-58. [PubMed]
15. Maggiolo F, Ravasio L, Ripamonti D, Gregis G, Quinzan G, Arici C, et al. Similar adherence rates favor different virologic outcomes for patients treated with nonnucleoside analogues or protease inhibitors. Clin Infect Dis 2005; 40:158–163. [PubMed]
16. Marcos Bravo MC, Ocampo Hermida A, Martínez Vilela J, Pérez Rodríguez MT, Gavilán Montenegro MJ, Arenas Villarroel LJ, Miralles Alvarez C, Rodríguez Dasilva A, Martínez Vázquez C. Hypersensitivity Reaction to Darunavir and Desensitization Protocol. J Investig Allergol Clin Immunol 2009;19(3):237-252. [PubMed]
17. Mills AM, Nelson M, Jayaweera D, Ruxrungtham K, Cassetti I, Girard PM, Workman C, Dierynck I, Sekar V, Abeele CV, Lavreys L. Once-daily darunavir/ritonavir vs. lopinavir/ritonavir in treatment-naive, HIV-1-infected patients: 96-week analysis. AIDS 2009;23:1679-1688. [PubMed]
18. Moltó J, Santos JR, Pérez Alvarez N, Cedeño S, Miranda C, Khoo S, Else L, Llibre JM, Valle M, Clotet B. Inhibitory quotient predicts long-term virological response to darunavir-based salvage therapy in HIV-infected protease inhibitor-experienced patients. 9th International Workshop on Clinical Pharmacology of HIV Therapy. April 7-9, 2008. New Orleans, Louisiana, USA. Abstract P46. [PubMed]
19. New Label Information Affecting All Approved Protease Inhibhitors for Treatment of HIV." US Food and Drug Administration Home Page, 27 Apr. 2010. Web 30 July 2010. http://www.fda.gov/ForConsumers/ByAudience/ForPatientAdvocates/HIVandAIDSActivities/ucm209920.htm
20. Orkin C, DeJesus E, Khanlou H, Stoehr A, Supparatpinyo K, Van de Casteele T, Lathouwers E, Spinosa-Guzman S. ARTEMIS: 192-week Efficacy and Safety of Once-daily Darunavir/ritonavir (DRV/r) vs Lopinavir/ritonavir (LPV/r) in Treatment-Naïve HIV-1-Infected Adults. Journal of the International AIDS Society. 2010(13)Suppl 4:P3
21. PubChem Compound Database, CID 213039.Figure 1
22. Rieger, A. Banhegyi, D. Schmidt, W. et al. The MONET trial 96 week analysis: darunavir/ritonavir monotherapy versus DRV/r + 2NRTIs, for patients with HIV RNA < 50 copies/mL at baseline. XVIII International AIDS Conference (AIDS 2010). Vienna, July 18-23, 2010. Abstract THLBB209.
23. Rittweger M, Arastéh K. Clinical Pharmacokinetics of Darunavir. Clin Pharmacokinet 2007;46:739-756. [PubMed]
24. Solas C, Colson P, Drogoul M, Obry-Roguet V, Tamalet C, Lacarelle B, Poizot-Martin I. Darunavir genotypic inhibitory quotient : a predictive factor of darunavir/ritonavir virological response in highly treatment-experienced patients. 9th International Workshop on Clinical Pharmacology of HIV Therapy. April 7-9, 2008. New Orleans, Louisiana, USA. Abstract P48.
25. Taylor S, Jayasuriya A, Dufty N, et al. Darunavir concentrations exceed the protein-corrected EC50 for wild type HIV in the semen of HIV-1-infected men. 17th Conference on Retroviruses and Opportunistic Infections. February 16-19, 2010. San Francisco. Abstract 611. http://www.retroconference.org/2010/PDFs/611.pdf. [PubMed]
26. Valantin, M.A. Flandre, P. Kolta, S. et al. Darunavir Monotherapy Increased Limb Fat - Fat Tissue Distribution Changes in HIV-infected Patients with Viral Suppression Treated with Darunavir/ritonavir (DRV/r) monotherapy versus 2 NRTIs + DRV/r in the MONOI-ANRS 136 Randomized Trial : Results at 48 weeks. 17th Conference on Retroviruses and Opportunistic Infections. February 16-19, 2010. San Francisco, CA.
27. Vanitha Sekar, S.D.M., T Vangeneugden, E Lefebvre, M De Pauw, D Van Baelen, E De Paepe, M P De Bethune, R Hoetelmans, W Parys, Pharmacokinetic/ Pharmacodynamic Analyses of TMC114 in the POWER 1 and POWER 2 Trials in Treatment-experienced HIV-infected Patients, in 13th Conference on Retroviruses and Opportunistic Infections. 2006: Denver, Colorado, USA. Abstract.
28. Violari A, Bologna R, Kimutar R, et al. ARIEL: 24-week safety and efficacy of DRV/r in treatment-experienced 3- to 6-year-old patients. Poster 713, 18th CROI 2011 Feb 27–Mar 2, Boston, USA.
29. Yasuhiro, Nakata, Hirotomo, Maeda, Kenji, et al. Novel bis-Tetrahydrofuranylurethane-Containing Nonpeptidic Protease Inhibitor (PI) UIC-94017 (TMC114) with Potent Activity against Multi-PI-Resistant Human Immunodeficiency Virus In Vitro. Antimicrobial Agents & Chemotherapy 2003;47(10):3123-3129. [PubMed]
30. Yilmaz, A et al. Danrunivir Concentrations in Cerebrospinal Fluid and Blood in HIV-1 Infected Patients. AIDS Research and Human Retroviruses. 2009;(4):457-461.
31. Zorrilla C, Wright R, Olayemi O, Yasin S, Baugh B, Brown K, Coate B, Verboven P, Hillewaert V, Kakuda T. Total an Unbound Daunavir (DRV) Pharmacokinetics (PK) in HIV-1-Infected Pregnant Women. 19th Conference on Retroviruses and Opportunistic Infections. 2012: Seattle, Washington, USA. Abstract.
Tables
Figure 1: Chemical Structure of Darunavir
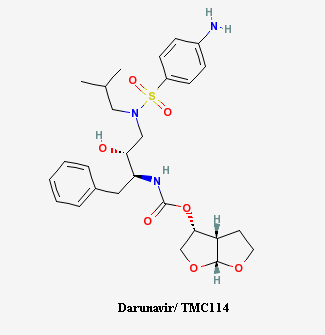
Table 1: Drugs That Are Contraindicated when administered with darunavir/ritonavir (adapted from the PrezistaTM package insert)
| Drug Class: Drug Name | Clinical Comment |
|---|---|
| Alpha 1 Adrenoreceptor Antagonist: Alfuzosin | Potential for serious and/or life-threatening reactions such as hypotension. |
| Anticonvulsants: Carbamazepine, Phenobarbital, phenytoin | Carbamazepine, phenobarbital and phenytoin induce CYP450 enzymes. Co-administration may cause decreases in darunavir plasma concentrations, resulting in loss of therapeutic effect to darunavir. |
| Antihistamines: Astemizole, Terfenadine | CONTRAINDICATED due to co-administration may cause life-threatening cardiac arrhythmias. |
| Antimycobacterial: Rifampin | Rifampin induces CYP450 enzymes. Co-administration may cause decreases in darunavir plasma concentrations, resulting in loss of therapeutic effect to darunavir. |
| Ergot Derivatives: Dihydroergotamine, Ergonovine, Ergotamine, Methylergonovine | CONTRAINDICATED due to co-administration my cause life-threatening ergot toxicity |
| Gastrointestinal Motility Agent: Cisapride | CONTRAINDICATED due to co-administration may cause life-threatening cardiac arrhythmias. |
| Herbal Products: St. John’s wort (Hypericum perforatum) | St. John’s wort induces CYP450 enzymes. Co-administration may cause decreases in darunavir plasma concentrations, resulting in loss of therapeutic effect to darunavir. |
| HMG-CoA Reductase Inhibitors: Lovastatin, Simvastatin | Darunavir/rtv may increase statin concentrations leading to adverse events such as myopathy (rhabdomyoloysis). |
| Neuroleptic: Pimozide | CONTRAINDICATED due to co-administration may cause life-threatening cardiac arrhythmias. |
| PDE-5 Inhibitors: Sildenafil & Tadalafil for treatment of pulmonary arterial hypertension | A safe and effective dose for the treatment of pulmonary arterial hypertension has not been established with darunavir/ritonavir. There is an increased potential for tadalafil/sildenafil-associated adverse events which include visual disturbances, hypotension, prolonged erection, and syncope. Tadalafil in patients on darunavir/ritonavir: After receiving darunavir/ritonavir for at least one week, initiate tadalafil 20 mg once daily & titrate to 40 mg once daily based upon individual tolerability. Darunavir/ritonavir in patients on tadalafil: Avoid use of tadalafil during the initiation of darunavir/ritonavir. Discontinue tadalafil > 24 hours prior to darunavir/ritonavir initiation. After >one week following the initiation of darunavir/ritonavir, resume tadalafil at 20 mg once daily & titrate to 40 mg once daily based upon individual tolerability. |
| Sedative/Hypnotics: Midazolam, Triazolam | CONTRAINDICATED due to co-administration may lead to increased sedation or respiratory depression |
Table 2: Antiretrovirals that interact with darunavir/ritonavir (adapted from the PrezistaTM package insert)
| Concomitant Antiretroviral | Effect | Clinical Comment |
|---|---|---|
| Efavirenz | darunavir, efavirenz | Use with caution 13% and 31% decrease in darunavir AUC and Cmin 21% and 17% increase in efavirenz AUC and Cmin |
| Maraviroc | ↑Maraviroc | When used in combination with darunavir, maraviroc should be decreased to 150 mg twice daily. |
| Nevirapine | Nevirapine | No dosage adjustment |
| Tenofovir disoproxil fumarate | Tenofovir | No dosage adjustment |
| Atazanavir (boosted) | No change | Can be co-administered |
| Indinavir (boosted) | darunavir indinavir | Use with caution |
| Lopinavir/ritonavir | darunavir | Co-administration not recommended |
| Saquinavir | darunavir | Co-administration not recommended |
Table 3: Drugs That Interact with Darunavir/Ritonavir (adapted from the PrezistaTM package insert)
| Concomitant Drug Class | Effect | Clinical Comment |
|---|---|---|
| Antiarrhythmics: Bepridil, Lidocaine (systemic), Quinidine, Amiodarone diogoxin | antiarrhythmics digoxin |
Use with caution Therapeutic drug monitoring of antiarrhythmics is recommended if available Initiation of digoxin with lowest dose and titrate to clinical concentrations while monitoring serum concentrations of digoxin |
| Anticoagulant: Warfarin | warfarin | INR should be closely monitored during dosing changes of either warfarin or darunavir |
| Antidepressant: Trazodone | trazodone | Use with caution; lower dose of trazodone may need to be used to prevent adverse effects such as nausea, dizziness, hypotension and syncope |
| Anti-infective: Clarithromycin | clarithromycin | No dosage adjustment if patient has normal renal function CRCL 30-60 mL/min: reduce clarithromycin dose by 50% CRCL < 30 mL/min: reduce clarithromycin dose by 75% |
| Antifungals: Ketoconazole Itraconazole (not studied) Voriconazole (not studied) | ketoconazole darunavir | Ketoconazole and itraconazole daily doses should not exceed 200 mg. Co-administration of ritonavir (100mg BID) and voriconazole decreased the AUC of voriconazole by 39%. Use only if benefit outweighs risk. |
| Anti-Gout: colchicine | ↑ colchicine | Treatment of gout-flarescolchicine & darunavir /ritonavir: 0.6 mg (1 tablet) x 1 dose, followed by 0.3 mg (half tablet) 1 hour later. Treatment course to be repeated no earlier than 3 days. Prophylaxis of gout-flarescolchicine & darunavir /ritonavir: If the original regimen was 0.6 mg twice a day, the regimen should be adjusted to 0.3 mg once a day. If the original regimen was 0.6 mg once a day, the regimen should be adjusted to 0.3 mg once every other day. |
| Antimycobacterial: Rifabutin | rifabutin, darunavir | Rifabutin induces CYP450 enzymes. Dose 150 mg rifabutin once every other day when co-administered with darunavir/rtv. |
| Calcium Channel Blockers: Felodipine, Nifedipine, Nicardipine | calcium channel blockers | Use with caution; close clinical monitoring is recommended. |
| Corticosterois: Dexamethasone, Fluticasone propionate | darunavir fluticasone propionate | Use with caution; dexamethasone (systemic) induces CYP3A. Darunavir/rtv may increase systemic concentrations of inhaled fluticasone; consider using an alternative |
| Endothelin receptor antagonists: Bosentan | ↑ bosentan | Patients requiring bosentan therapy: discontinue / do not initiate therapy until they have been continuously taking darunavir for 10 days. |
| HMG-CoA Reductase Inhibitors: Pravastatin, Atorvastatin Rosuvastatin | pravastatin atorvastatin rosuvastatin | Use the lowest dose. Titrate dose to achieve beneficial effect with close monitoring of side-effects and liver function |
| H2-Receptor Antagonists and Proton Pump Inhibitors: Ranitidine, Omeprazole | No change | No dosage adjustment |
| Immunosuppressants: Cyclosporine, Tacrolimus, Sirolimus | immunosuppressants | Therapeutic drug monitoring of immunosupressants is recommended. |
| Narcotic Analgesic: Methadone | methadone | No dosage adjustment; monitor therapy and make adjustments |
| Oral Contraceptives/estrogen: Ethinyl estradiol Norethindrone | ethinyl estradiol, norethindrone | Ritonavir induces clearance of oral contraceptives; barrier contraceptives are recommended |
| PDE-5 inhibitors: Sildenafil, Vardenafil, Tadalafil | Use with caution. Sildenafil: do not exceed 25 mg in 48 hour Vardenafil: do not exceed 2.5mg in 72 hours Tadalafil: do not exceed 10 mg in 72 hours | |
| Selective Serotonin Reuptake Inhibitors (SSRIs): Sertraline, Paroxetine | sertraline, paroxetine |
Table 4. Treatment-Experienced Adult Patient Dosing.
Treatment-Experienced Adult Patient Dosing |
|
|---|---|
| With no darunavir resistance associated substitutions* | Darunavir 800 mg by mouth once daily with ritonavir 100 mg by mouth once daily and with food |
| With at least one darunavir resistance associated substitution* | Darunavir 600 mg by mouth twice daily with ritonavir 100 mg by mouth twice daily and with food. |
V11I, V321, L33F, I47V, I50V, I54L, I54M, T74P, L76V, I84V and L89V
Table 5. Pediatric Dosing with Darunavir Oral Suspension (100 mg/mL) and Ritonavir Oral, Solution (80 mg/mL) for Patients Weighing 10 kg to Less Than 15 kg/p>
| Body Weight (kg) | Dose (by mouth twice daily and with food) |
|---|---|
| Greater than or equal to 10 kg to less than 11 kg | Darunavir 200 mg (2 mL) with ritonavir 32 mg (0.4 mL) |
| Greater than or equal to 11 kg to less than 12 kg | Darunavir 220 mg (2.2 mL) with ritonavir 32 mg (0.4 mL) |
| Greater than or equal to 12 kg to less than 13 kg | Darunavir 240 mg (2.4 mL) with ritonavir 40 mg (0.5 mL) |
| Greater than or equal to 13 kg to less than 14 kg | Darunavir 260 mg (2.6 mL) with ritonavir 40 mg (0.5 mL) |
| Greater than or equal to 14 kg to less than 15 kg | Darunavir 280 mg (2.8 mL) with ritonavir 48 mg (0.6 mL) |
Table 6. Pediatric Dosing with Darunavir Oral Suspension (100 mg/mL) and Ritonavir Oral Solution (80 mg/mL) for Patients Weighing at Least 15 kg
| Body Weight (kg) | Dose (by mouth twice daily and with food) |
|---|---|
| Greater than or equal to 15 kg to less than 30 kg | Darunavir 375 mg (3.8 mL) with ritonavir 50 mg (0.6 mL) |
| Greater than or equal to 30 kg to less than 40 kg | Darunavir 450 mg (4.6 mL) with ritonavir 60 mg (0.75 mL) |
| Greater than or equal to 40 kg | Darunavir 375 mg (6 mL) with ritonavir 100 mg (1.25 mL) |
Table 7. Pediatric Dosing with Darunavir tablets and Ritonavir Oral Solution (80 mg/mL) or Tablets/Capsules (100 mg) for Patients Weighing at Least 15 kg
| Body Weight (kg) | Dose (by mouth twice daily and with food) |
|---|---|
| Greater than or equal to 15 kg to less than 30 kg | Darunavir 375 mg with ritonavir 50 mg (0.6 mL) |
| Greater than or equal to 30 kg to less than 40 kg | Darunavir 450 mg with ritonavir 60 mg (0.75 mL) |
| Greater than or equal to 40 kg | Darunavir 375 mg with ritonavir 100 mg |
