Fusidic Acid
Authors: Keryn J. Christiansen, MBBS
CLASS
Fusidic acid is a member of the fusidane class. The sodium salt was introduced into clinical practice in 1962.
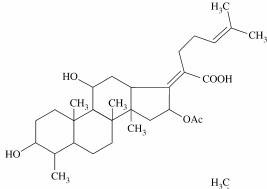
Chemical Structure (Figure 1)
Fusidic acid is a tetracyclic triterpenoid that is structurally related to cephalosporin P1 (named because of its origin from Cephalosporium acremonium – it is not related to the beta lactam cephalosporins). Fusidic acid is derived from the fungus Fusidium coccineum and differs from cephalosporin P1 by the addition of a few acetyl groups, which increase antibacterial activity. The fusidic acid nucleus has properties common to other tetracyclic structures such as the adrenocorticoids and bile salts, especially cholate and taurocholate (26). Fusidic acid is related to other antibiotic groups including the helvolic acids and the viridominic acids. Antibiotics similar or identical to fusidic acid are produced by dermatophytes such as Microsporum canis, Microsporum gypseum, and Epidermophyton floccosum (63).
Structure-Activity Relationship
The essential parts of the fusidic acid molecule related to activity are the alpha, beta – unsaturated carboxylic acid at position C20 and the acetoxyl group at C16. Other functional groups although contributory, are less vital. Many chemical modifications have been made with only 24, 25 dihydrofusidic acid having activity equivalent to fusidic acid (95).
ANTIMICROBIAL ACTIVITY
Spectrum
Fusidic acid has good in vitro activity against staphylococci, including both methicillin sensitive and resistant strains. It also has useful activity against Neisseria spp, Bordetella pertussis, Corynebacterium spp and Gram positive anaerobes such as Clostridium difficile and C. perfringens, Peptostreptococcus spp and Propionibacterium acnes (19). The MIC’s for streptococci, enterococci and Gram negative anaerobes are generally higher but at <8 mg/L may provide some activity clinically. Table 1 shows in vitro susceptibility data for the above organisms. It is not active against the Enterobacteriacae (23, 80, 82, 83). Resistance in vitro has been demonstrated for Borrelia burgdorferi (34) (MIC90 >4), and Yersinia enterocolitica (81).
Pharmacodynamic Effects
Bactericidal Effects
Fusidic acid is slowly bactericidal in vitro against S. aureus (71) and concentration-dependent inhibition has been demonstrated against Escherichia coli (25).
Effects of Subinhibitory Concentrations
There are no data on the effects of subinhibitory concentrations.
Postantibiotic Effects
At achievable serum concentrations there is a post antibiotic effect of 0.8 – 1.75 hours for S. aureus and 1hour for Streptococcus pyogenes (51).
Effects on Host Immunity
Fusidic acid immunomodulatory effects as a result of suppression of cytokine production have been shown in animal models of septic shock and insulin dependent diabetes. The clinical utility of these properties has not been verified (16).
Pharmacodynamic Correlates with Outcome
The pharmacodynamic predictors of efficacy (AUC/MIC ratio or time above MIC) have not been determined for this drug.
MECHANISMS OF ACTION
Fusidic acid interferes with the function of elongation factor G (EF-G), leading to the inhibition of protein synthesis. Elongation factor G hydrolyses GTP to GDP to provide energy for the translocation of the peptidyl – tRNA from the A site to the P site on the 50S subunit of the ribosome. In the presence of fusidic acid, EF-G remains bound to the ribosome after GTP hydrolysis, sterically blocking the next stage of protein synthesis.
MECHANISMS OF RESISTANCE
Organisms Commonly Resistant
Fusidic acid is inactive against the Enterobacteriacae and has only marginal activity against streptococci and enterococci. The prevalence of resistance in methicillin susceptibleStaphylococcus aureus varies in different countries with generally low rates reported in studies conducted up to the mid 1990’s (Denmark 0 – 1%, Australia 1-3%, Canada 0.6%) (89). Since then however, increasing resistance has been reported, particularly in the United Kingdom with reports of increases from 6 - 8% in 1995/1998 to 11.5 – 17.3% in 2001 (11, 10). An increase in the use of topical fusidic acid for skin infections has been recorded over this period suggesting resistance selection (47). In Scandinavia clonal spread of fusidic acid resistant staphylococci has been documented with an increase in resistance prevalence in Norway of 3% in 1992 to 36% in 2001. Resistance rates for methicillin resistant Staphylococcus aureus (2 -12%) (89) have remained more stable with the exception that specific clones (EMRSA –17) are reliably resistant to fusidic acid (3).
Mechanisms of Resistance
A number of mechanisms conferring resistance to fusidic acid have been reported.
Chromosomally mediated resistance is due to point mutations within the fusA gene encoding EF-G (7). Although mutations occur across a number of loci on EF-G, changes in the conserved region centered on residues 451-464 and in particular H457 (56, 58, 54) seem to be most important, suggesting a possible fusidic acid binding site. Some mutations are associated with low fitness however selected resistant clinical isolates have been shown to have a variety of compensatory mutations that restore fitness. In vitro studies suggest that compensation is more likely than reversion to the sensitive wild type thereby stabilising the resistant bacterial population. These compensatory mutations probably act by restoring the balance between the GDP and GTP conformations of EF-G of the ribosome (54, 36, 42). This type of resistance has been demonstrated in S. aureus with mutation frequencies of between one in 106 and one in 108 (89). Although not clinically relevant, altered elongation factor has also been demonstrated in Gram-negative bacteria which have MICs much higher than wild type strains (72) and inSalmonella typhimurium this has been shown to result from mutations in the fusA gene (37). The frequency of mutations in S. aureus demonstrated in vitro has lead to the recommendation for combination therapy particularly for MRSA infections. In vitro studies support this with undetectable mutations when fusidic acid is combined with rifampicin (59).
Fusidic acid resistance in S. aureus has also been demonstrated to be plasmid mediated. This is the predominant form of resistance for S. aureus being found in about 70% of resistant strains. This resistance, carried on a plasmid (pUB101) which also encodes a betalactamase and cadmium resistance, does not involve drug modification or protein inhibition in a cell free model. Earlier studies have described alteration in membrane permeability however alterations in membrane composition have not been observed. More recently genetic characterisation of the plasmid borne fusidic acid resistance has identified an inducible resistance gene, far1, which has similarities with fibronectin binding protein sequences in other organisms (57).
An efflux resistance mechanism has been described for both Enterobacteriacae and S. aureus. A multidrug tripartite complex, consisting of an efflux pump (AcrB), a membrane fusion protein (AcrA) and an outer membrane channel (TolC) has been shown in E. coli to confer resistance to a large number of drugs including fusidic acid, tetracycline, chloramphenicol, fluoroquinolones, beta-lactams, erythromycin, together with a number of dyes and detergents. The AcrD transporter has a more limited substrate range but includes aminoglycosides in addition to fusidic acid. The pump, AcrB or AcrD, captures its substrates from within the cytoplasmic membrane with extrusion directly into the extracellular medium via the combined action of AcrA and TolC (23). A novel efflux system, MdeA, has been described for S. aureus (31). When over expressed, resistance to fusidic acid, virginiamycin and novobiocin occurs but unlike NorA the pump does not confer resistance to fluoroquinolones. Over expression has been demonstrated to occur by spontaneous mutations in the mdeA promoter region.
Drug sequestration has been described in Enterobacteriacae that are also resistant to chloramphenicol. Type 1 chloramphenicol acetyltransferase (CAT-I) competitively binds to fusidic acid resulting in inactivation by sequestration (6). The structural basis for the binding of fusidic acid to CAT-1 has been reported (53) and is due to the presence of specific residues in the chloramphenicol binding pocket of the enzyme. Differing residues in other CAT variants result in a low binding affinity for fusidic acid and therefore a lack of resistance.
Drug inactivation of fusidic acid has been described in Streptomyces spp. The enzyme responsible is an esterase which desacetylates fusidic acid at the C16 position resulting in the inactive lactone derivative (96).
Methods to Overcome or Prevent Resistance
Selection for resistance has been demonstrated during fusidic acid monotherapy (15). In principle combination therapy may prevent resistance selection (102). Fusidic acid most often is combined clinically with beta-lactams or rifampicin. Combination therapy has been investigated in vitro with conflicting results for synergy, indifference or antagonism (19). ForStaphylococcus aureus, resistant mutant selection, in vitro, is prevented for both fusidic acid and rifampicin when these drugs are used in combination (59). Linezolid also prevents in vitroselection of fusidic acid resistant mutants for S. aureus (28). Specific clinical data are lacking.
PHARMACOKINETICS
Absorption
Sodium fusidate absorption is not very rapid as can be seen from the Tmax values in Table 2. The consistency of the Cmax values and comparable AUC values indicate that it is predictably absorbed. Food reduces the Cmax and delays the Tmax but does not alter the AUC (46). The oral formulation has a high bioavailability (91%) (84). Penetration of fusidic acid applied topically to the skin is minimal with studies showing absorption of 2% or less (94). When applied topically to the eye significant penetration occurs into the cornea, aqueous humour but not vitreous humour (86, 29). A single subconjunctival injection of 100 mg of fusidic acid produces levels above the MICs of most organisms in the cornea, aqueous, and vitreous and persists for over 24 hours, but at this concentration results in conjunctival necrosis and corneal decompensation (86).
Distribution
Fusidic acid has modest penetration into bone (16 – 24%) and synovial fluid (28-88%) and achieves levels in pus that are marginally below those in serum. Skin exudate and burn studies show good Cmax values and high fluid/serum ratios (88, 77). After systemic administration intraocular penetration is low (99, 14).
Routes of Elimination
Metabolism
Hepatic metabolism with biliary excretion is the most likely route of elimination, although renal elimination of hepatic conjugates or metabolites has not been specifically reported. Examination of biliary metabolites of fusidic acid shows that the main metabolites are a glucuronide conjugate and a dicarboxylic derivative, accounting respectively for 15% and 10% of the drug in bile. A variety of minor metabolites are produced, including a possible hydroxy metabolite, a 3-keto metabolite and three that are yet to be identified (88).
Renal Excretion
Elimination is mostly non-renal. Only very low levels have been detected in the urine in pharmacokinetic studies with < 0.5% being calculated to be excreted by the renal route following IV administration (66). Faecal excretion is similarly low.
Pharmacokinetic Parameters
The pharmacokinetic parameters, Cmax, Tmax, AUC, half life, clearance and volume of distribution, for different studies after oral and IV administration (88), are shown in Table 2. Due to its slow clearance the drug accumulates with repeated administration. When given as a 500mg dose 8 hourly the trough levels increase with reported concentrations on 4 successive days of 21mg/l, 30mg/l, 47mg/l, and 73mg/l (26). Similarly increases occur in the AUC (76). Accumulation is not demonstrated when dosing is reduced to 250mg 12 hourly (52) although a higher than expected AUC is obtained using 500mg 12 hourly (90).
Fusidic acid is highly protein bound (91-98%) and with distinct binding sites on human albumin (73) is a potent displacer of bilirubin (9).
CNS/CSF Disposition
Low levels of penetration are found in uninflamed brain (7% of the corresponding serum concentration), and uninflamed CSF (<1% of serum concentration) in humans (49). In a rabbit model percentage penetration using AUCCSF/AUCserum is 1.9% in uninflamed and 4.5% in purulent CSF (60).
Effect of Disease States
The pharmacokinetics of fusidic acid in patients with severe renal failure requiring dialysis (12) are not substantially different to patients with normal renal function as would be expected for a drug that has minimal renal clearance. The drug is not removed by haemodialysis and concentrations in peritoneal dialysis fluid are low (< 2.3mg/l). Accumulation of metabolites has not been demonstrated in these patients.
The pharmacokinetics of fusidic acid are altered in patients with coeliac disease with the AUC and Cmax being increased by 60 – 70% compared to normal subjects (61). Patients with low albumin states have increased clearance, presumably because the reduced capacity for protein binding results in greater drug availability for metabolism. Conversely decreased clearance occurs with severe cholestasis, the suggested mechanism being competition by the excess bilirubin for glucuronidation (64).
DOSAGE
Adults and Children
Oral Film Coated Tablet
The conventional dosing for adults is 250 to 500mg 8 hourly. Given the documented accumulation at these doses and greater adverse reactions, a 12 hourly dosing interval can be considered. Clinical trials in skin and soft tissue infections show similar efficacy for 8 hourly and 12 hourly dosing regimens (13, 55).
Oral Suspension
This is an aqueous suspension containing fusidic acid hemihydrate (250mg/5ml – equivalent sodium fusidate 175mg). Regimens based on conventional 8 hourly dosing are:
< 1 year: 1mL/kg/day in three divided doses,
1-5 years: 5mL 3 times daily,
6-12 years: 10mL 3 times daily
Children > 12, adults: 15mL 3 times daily
As for the tablet formulation 12 hourly dosing can be used.
Intravenous Infusion
This contains the sodium salt equivalent to 500mg of fusidic acid per vial. It is infused over no less than 2 hours. Adult dose: 500mg 8 to 12 hourly, Children: 12mg/kg up to 500mg 12 hourly or 20mg/kg/daily in three divided doses.
Topical Preparations
There are three preparations, 2% fusidic acid cream, 2% fusidic acid ointment, 2% fusidic acid gel all for use on skin surfaces twice daily. An ophthalmic preparation, 1% viscous drops, is available for instillation twice daily into the conjunctival sac.
Renal Failure
Dose modification is not required.
Hepatic Failure
As there are no safety data in patients with severely impaired hepatic function, opposing pharmacokinetics in patients with low serum albumin and hyperbilirubinemia, and potential drug hepatotoxicity it is best avoided in these patients.
Body Composition (Obesity, Wasting, Various Body Builds)
There are no data on the effects of body composition.
Ascites/Edema
Low serum albumin states may have increased drug clearance requiring doses in the higher range.
Chronic Diarrhea/Malabsorption
Patients with coeliac disease may require dose reduction.
Malnutrition
There are no data on the effects of malnutrition.
Pregnancy
There is evidence that fusidic acid can penetrate the placental barrier. Fusidic acid may cause kernicterus in babies during the first month of life by displacing bilirubin from plasma albumin. Fusidic acid should be avoided when possible during the last month of pregnancy.
ADVERSE EFFECTS
Mechanism
The most common adverse effects are gastrointestinal and are dose related (17). Nausea, vomiting or dyspepsia has been reported in up to 12% of patients particularly those receiving 500mg three times a day (13). An 8% incidence of lower abdominal pain or diarrhoea has also been reported. A rash has been reported in 5% of patients (20). The mechanisms for these adverse effects have not been reported.
Hepatotoxicity
Jaundice has been reported during both oral and intravenous therapy with fusidic acid (65, 85, 35). A study on 213 patients (with normal hepatic function) having treatment for staphylococcal bacteraemia showed a significantly higher incidence of jaundice in those treated with fusidic acid than controls (34% versus 2%). Comparison of the route of administration revealed that 48% receiving intravenous therapy became jaundiced versus 13% of those on oral fusidic acid (33). The hyperbilirubinemia induced by fusidic acid therapy is due to competitive inhibition of the ATP-dependent transport of 17beta-glucuronosyl estradiol and cholyltaurine across the canalicular membrane via the multi-drug resistance protein 2 (Mrp2) and the bile salt export pump (Bsep). In addition prolonged therapy results in a marked decrease in the hepatic Mrp2 protein (8).
Haematological Toxicity
Granulocytopenia (69, 24) and or thrombocytopenia (44, 22) have been reported as separate events with fusidic acid use. Both could be directly attributed to fusidic acid. The fall in the neutrophil count occurred after a mean of 21 days therapy and recovered completely 5-9 days after cessation of the fusidic acid. The bone marrow showed normocellularity or hypercellularity with moderate hypoplasia of the granulocyte cells (93). The thrombocytopenia in the two reported cases was severe but reversible. In one patient the mechanism was an antibody-hapten reaction rather than a direct toxic effect of fusidic acid on stem cells or megakaryocytes (22).
Skin Reactions
Contact dermatitis occurs in some patients after topical use but is uncommon (17). Most often it has been associated with use in patients with stasis ulcers.
Kernicterus in Neonates
As fusidic acid is capable of displacing bilirubin from albumin binding sites kernicterus has been cited as a possible adverse reaction to the use of fusidic acid in neonates. The data in the literature are conflicting (17).
Risk Factors
As mentioned above the gastrointestinal side effects are dose related.
Treatment and Avoidance
There are no data.
Overdoses (Manifestations and Management)
Early symptoms may include epigastric or gastric discomfit and possibly diarrhoea. Prolonged ingestion of high doses may produce jaundice and other abnormalities of liver function. There are no published reports of the treatment of accidental massive overdose and there has been no experience with any specific treatment. Treatment should be restricted to symptomatic and supportive measures. Dialysis is of no benefit, because the drug is not significantly dialysed.
MONITORING REQUIREMENTS
Therapeutic Drug Monitoring
There no requirements for drug monitoring of fusidic acid.
Other Laboratory Monitoring
Monitoring of liver function particularly during intravenous or prolonged therapy is recommended.
DRUG INTERACTIONS
Hepatic cytochrome P450 enzyme interactions have been described for fusidic acid. Levomethadone metabolism is increased during prolonged fusidic acid therapy due to induction of cytochrome P450 isoenzymes (67). Conversely fusidic acid appears to inhibit the metabolism of both ritonavir and saquinavir, with these latter two agents causing concurrent elevation of fusidic acid levels (41).
Cholestyramine binds fusidic acid in a similar fashion to the way it binds bile salts. In conventional doses cholestyramine totally prevents absorption of fusidic acid (38).
CLINICAL INDICATIONS
The predominant use of this agent is in the treatment of staphylococcal infections. The continued emergence of resistance to other classes of antibiotics such as the beta lactams makes fusidic acid of increasing importance.
Skin and Soft Tissue Infection
Clinical trials show efficacy for the oral therapy of staphylococcal skin infections. A lower cure rate is obtained for the treatment of beta haemolytic streptococcal infections, particularly when low dose (250 mg twice daily) regimens are used (78). Topical fusidic acid has been used widely and has similar efficacy to other topical agents although a higher failure rate is seen with streptococcal infection (78). It has been suggested that the topical use of fusidic acid may select for resistance (47).
Treatment of Methicillin Resistant Staphylococcus aureus (MRSA)
Used in combination with rifampicin there is limited evidence of efficacy for the treatment of MRSA infection and eradication of MRSA carriage (21, 62). Current evidence does not support the use of monotherapy (97, 15)
Bone and Joint Infections
Fusidic acid, in combination with other antistaphylococcal agents, has been used successfully in the treatment of acute osteomyelitis, chronic osteomyelitis, septic arthritis, and prosthetic infections. Data are lacking on monotherapy (2).
Staphylococcus aureus Bacteraemia
Fusidic acid in combination with other antistaphylococcal agents has been reported for the treatment of non MRSA bacteraemia with favourable outcome (98) and in one study a reduction in relapse of infection (27). Data are not available for MRSA bacteraemia.
REFERENCES
1. Andrews JM: BSAC Working Party on Susceptibility testing. BSAC standardized disc susceptibility testing method. J Antimicrob Chemother 2001;48 (suppl S1);43-57. [PubMed]
2. Atkins B, Gottlieb T. Fusidic acid in bone and joint infections. Int J Antimicrob Agents. 1999 Aug;12 Suppl 2:S79-93. [PubMed]
3. Aucken HM, Ganner M, Murchan S, Cookson BD, Johnson AP. A new UK strain of epidemic methicillin-resistant Staphylococcus aureus (EMRSA-17) resistant to multiple antibiotics. J Antimicrob Chemother. 2002;50:171-175. [PubMed]
4. Bannatyne RM, Cheung R. Antimicrobial susceptibility of Bordetella pertussis strains isolated from 1960 to 1981. Antimicrob Agents Chemother. 1982;21:666-667. [PubMed]
5. Barber M, Waterworth PM. Antibacterial activity in vitro of Fucidin. Lancet 1962;1:931-932. [PubMed]
6. Bennett AD, Shaw WV. Resistance to fusidic acid in Escherichia coli mediated by the type I variant of chloramphenicol acetyltransferase. A plasmid-encoded mechanism involving antibiotic binding. Biochem J. 1983;215:29-38. [PubMed]
7. Besier S, Ludwig A, Brade V, Wichelhaus TA. Molecular analysis of fusidic acid resistance in Staphylococcus aureus. Mol Microbiol. 2003;47:463-469. [PubMed]
8. Bode KA, Donner MG, Leier I, Keppler D. Inhibition of transport across the hepatocyte canalicular membrane by the antibiotic fusidate. Biochem Pharmacol. 2002;64:151-158. [PubMed]
9. Brodersen R. Fusidic acid binding to serum albumin and interaction with binding of bilirubin. Acta Paediatr Scand. 1985;74:874-880. [PubMed]
10. Brown EM, Thomas P. Fusidic acid resistance in Staphylococcus aureus isolates. Lancet. 2002;359:803. [PubMed]
11. Brown EM, Wise R. Fusidic acid cream for impetigo. Fusidic acid should be used with restraint. BMJ. 2002;324:1394. [PubMed]
12. Brown NM, Reeves DS, McMullin CM. The pharmacokinetics and protein-binding of fusidic acid in patients with severe renal failure requiring either haemodialysis or continuous ambulatory peritoneal dialysis. J Antimicrob Chemother. 1997;39:803-809. [PubMed]
13. Carr WD, Wall AR, Georgala-Zervogiani S, Stratigos J, Gouriotou K. Fusidic acid tablets in patients with skin and soft tissue infection: a dose-finding study. Eur J Clin Res 1994;5:87-95.
14. Chadwick AJ, Jackson B. Intraocular penetration of the antibiotic fucidin. Br J Ophthalmol. 1969;53:26-29. [PubMed]
15. Chang SC, Hsieh SM, Chen ML, Sheng WH, Chen YC. Oral fusidic acid fails to eradicate methicillin-resistant Staphylococcus aureus colonization and results in emergence of fusidic acid-resistant strains. Diagn Microbiol Infect Dis. 2000;36:131-136. [PubMed]
16. Christiansen K. Fusidic acid non-antibacterial activity. Int J Antimicrob Agents. 1999;12 Suppl 2:S73-78. [PubMed]
17. Christiansen K. Fusidic acid adverse drug reactions. Int J Antimicrob Agents. 1999;12 Suppl 2:S3-9. [PubMed]
18. Cicek-Saydam C, Cavusoglu C, Burhanoglu D, Hilmioglu S, Ozkalay N, Bilgic A. In vitro susceptibility of Mycobacterium tuberculosis to fusidic acid. Clin Microbiol Infect. 2001;7:700-702. [PubMed]
19. Collignon P, Turnidge J. Fusidic acid in vitro activity. Int J Antimicrob Agents. 1999;12 Suppl 2:S45-58. [PubMed]
20. Coombs RRH, Mehtar S, Menday AP. Fusidic acid in orthopaedics. Curr Ther Res Clin Exp 1987;42:501-508 [PubMed]
21. Cox RA, Conquest C, Mallaghan C, Marples RR. A major outbreak of methicillin-resistant Staphylococcus aureus caused by a new phage-type (EMRSA-16) J Hosp Infect. 1995;29:87-106. [PubMed]
22. El-Kassar N, Kalfon P, Fromont P, Vezinet C, Godeau B, Duedari N, Bierling P. Fusidic acid induced acute immunologic thrombocytopenia. Br J Haematol. 1996;93:427-431. [PubMed]
23. Elkins CA, Nikaido H. Substrate specificity of the RND-type multidrug efflux pumps AcrB and AcrD of Escherichia coli is determined predominantly by two large periplasmic loops. J Bacteriol. 2002;184:6490-6498. [PubMed]
24. Evans DI. Granulocytopenia due to fusidic acid. Lancet. 1988;2:851. [PubMed]
25. Garrett ER, Richard AJ. Kinetics and mechanisms of drug action on microorganisms. Integrated receptor site model rationalizing observed microbial rate dependencies on drug concentration and microbial kinetics affected by sodium fusidate. J Pharm Sci. 1974;63:884-894. [PubMed]
26. Godtfredsen W, Roholt K, Tybring L. Fucidin: a new orally active antibiotic. Lancet 1962;1:928-931 [PubMed]
27. Gosden PE, Reeves BC, Osborne JR, Turner A, Millar MR. Retrospective study of outcome in patients treated for Staphylococcus aureus bacteremia. Clin Microbiol Infect. 1997;3:32-40. [PubMed]
28. Grohs P, Kitzis MD, Gutmann L. In vitro bactericidal activities of linezolid in combination with vancomycin, gentamicin, ciprofloxacin, fusidic acid, and rifampin against Staphylococcus aureus. Antimicrob Agents Chemother. 2003;47:418-420. [PubMed]
29. Hansen S. Intraocular penetration of fusidic acid with topical Fucithalmic. Eur J Drug Metab Pharmacokinet. 1985;10:329-331. [PubMed]
30. Heczko PB, Kasprowicz A, Pulverer G. Susceptibility of human skin aerobic diphtheroids to antimicrobial agents in vitro. J Antimicrob Chemother. 1977;3:141-146. [PubMed]
31. Huang J, O’Toole PW, Shen W, Jiang X, Lobo N, Palmer LM, Voelker L, Fan F, Gwynn M, McDevitt D. Identification of a novel multidrug resistance efflux system (MdeA) fromStaphylococcus aureus. Abstract # C1-427 42nd ICAAC San Diego 2002.
32. Huebner J, Kropec A, Engels I, Daschner F. In vitro susceptibility of methicillin-resistant Staphylococcus aureus and slime-producing and non-slime-producing coagulase-negative staphylococci to fusidic acid. Chemotherapy. 1992;38:206-210. [PubMed]
33. Humble MW, Eykyn S, Phillips I. Staphylococcal bacteraemia, fusidic acid, and jaundice. Br Med J. 1980;280:1495-1498. [PubMed]
34. Hunfeld KP, Weigand J, Wichelhaus TA, Kekoukh E, Kraiczy P, Brade V. In vitro activity of mezlocillin, meropenem, aztreonam, vancomycin, teicoplanin, ribostamycin and fusidic acid against Borrelia burgdorferi. Int J Antimicrob Agents. 2001;17:203-208. [PubMed]
35. Iwarson S, Fasth S, Olaison L, Hulten L. Adverse reactions to intravenous administration of fusidic acid. Scand J Infect Dis. 1981;13:65-67. [PubMed]
36. Johanson U, Aevarsson A, Liljas A, Hughes D. The dynamic structure of EF-G studied by fusidic acid resistance and internal revertants. J Mol Biol. 1996;258:420-432. [PubMed]
37. Johanson U, Hughes D. Fusidic acid-resistant mutants define three regions in elongation factor G of Salmonella typhimurium Gene. 1994;143:55-59. [PubMed]
38. Johns WH, Bates TR. Drug-cholestyramine interactions. II. Influence of cholestyramine on GI absorption of sodium fusidate. J Pharm Sci. 1972;61:735-739. [PubMed]
39. Jones RN, Barry AL. Antimicrobial activity of coumermycin and recommendations for disk diffusion tests with 5- and 15-micrograms disks. Diagn Microbiol Infect Dis. 1987;7:77-82. [PubMed]
40. Kanazaki H, Akiyama H, Kanamoto A, Abe Y, Yamada T, Arata J, Umemura S, Ikeda M. Outbreak of fusidic acid resistant Staphylococcus aureus. Nippon Hifuka Gakkai Zasshi. 1989;99:507-510. [PubMed]
41. Khaliq Y, Gallicano K, Leger R, Foster B, Badley A. A drug interaction between fusidic acid and a combination of ritonavir and saquinavir. Br J Clin Pharmacol. 2000;50:82-83. [PubMed]
42. Laurberg M, Kristensen O, Martemyanov K, Gudkov AT, Nagaev I, Hughes D, Liljas A. Structure of a mutant EF-G reveals domain III and possibly the fusidic acid binding site. J Mol Biol. 2000;303:593-603. [PubMed]
43. Leclercq R, Bismuth R, Casin I, Cavallo JD, Croize J, Felten A, Goldstein F, Monteil H, Quentin-Noury C, Reverdy M, Vergnaud M, Roiron R. In vitro activity of fusidic acid against streptococci isolated from skin and soft tissue infections. J Antimicrob Chemother. 2000;45:27-29. [PubMed]
44. Leibowitz G, Golan D, Yeshurun D, Brezis M. Leukopenia and thrombocytopenia due to fusidic acid. Postgrad Med J. 1991;67:591-592. [PubMed]
45. Leroi MJ, Siarakas S, Gottlieb T. E test susceptibility testing of nosocomial Clostridium difficile isolates against metronidazole, vancomycin, fusidic acid and the novel agents moxifloxacin, gatifloxacin, and linezolid. Eur J Clin Microbiol Infect Dis. 2002;21:72-74. [PubMed]
46. MacGowan AP, Greig MA, Andrews JM, Reeves DS, Wise R. Pharmacokinetics and tolerance of a new film-coated tablet of sodium fusidate administered as a single oral dose to healthy volunteers. J Antimicrob Chemother. 1989;23:409-415. [PubMed]
47. Mason BW, Howard AJ, Magee JT. Fusidic acid resistance in community isolates of methicillin-susceptible Staphylococcus aureus and fusidic acid prescribing. J Antimicrob Chemother. 2003;51:1033-1036. [PubMed]
48. Miles RS, Moyes A. Comparison of susceptibility of Neisseria meningitidis to sodium sulphadiazine and sodium fusidate in vitro. J Clin Pathol. 1978;31:355-358. [PubMed]
49. Mindermann T, Zimmerli W, Rajacic Z, Gratzl O. Penetration of fusidic acid into human brain tissue and cerebrospinal fluid. Acta Neurochir. 1993;121:12-14. [PubMed]
50. Moorhouse EC, Mulvihill TE, Jones L, Mooney D, Falkiner FR, Keane CT. The in-vitro activity of some antimicrobial agents against methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 1985;15:291-295. [PubMed]
51. Munckhof WJ, Turnidge JD. The postantibiotic effect of fusidic acid against gram-positive bacteria. J Antimicrob Chemother. 1997;40:433-436. [PubMed]
52. Munkholm P, Hey H, Rasmussen SN, Johansen PB. Antibiotic activity in serum following single and repeated oral administration of sodium fusidate in volunteers. Eur J Drug Metab Pharmacokinet. 1994;19:337-341. [PubMed]
53. Murray IA, Cann PA, Day PJ, Derrick JP, Sutcliffe MJ, Shaw WV, Leslie AG. Steroid recognition by chloramphenicol acetyltransferase: engineering and structural analysis of a high affinity fusidic acid binding site. J Mol Biol. 1995;254:993-1005. [PubMed]
54. Nagaev I, Bjorkman J, Andersson DI, Hughes D. Biological cost and compensatory evolution in fusidic acid-resistant Staphylococcus aureus. Mol Microbiol. 2001;40:433-439. [PubMed]
55. Nordin P, Mobacken H. A comparison of fusidic acid and flucloxacillin in the treatment of skin and soft tissue infection. Eur J Clin Res 1994;5:97-109.
56. O'Brien FG, Botterill CI, Endersby TG, Lim RL, Grubb WB, Gustafson JE. Heterogeneous expression of fusidic acid resistance in Staphylococcus aureus with plasmid or chromosomally encoded fusidic acid resistance genes. Pathology. 1998;30:299-303. [PubMed]
57. O'Brien FG, Price C, Grubb WB, Gustafson JE. Genetic characterization of the fusidic acid and cadmium resistance determinants of Staphylococcus aureus plasmid pUB101. J Antimicrob Chemother. 2002;50:313-321. [PubMed]
58. O'Neill AJ, Bostock JM, Moita AM, Chopra I. Antimicrobial activity and mechanisms of resistance to cephalosporin P1, an antibiotic related to fusidic acid. J Antimicrob Chemother. 2002;50:839-848. [PubMed]
59. O'Neill AJ, Cove JH, Chopra I. Mutation frequencies for resistance to fusidic acid and rifampicin in Staphylococcus aureus. J Antimicrob Chemother. 2001;47:647-650. [PubMed]
60. Ostergaard C, Yieng-Kow RV, Knudsen JD, Frimodt-Moller N, Espersen F. Evaluation of fusidic acid in therapy of experimental Staphylococcus aureus meningitis. J Antimicrob Chemother. 2003;51:1301-1305. [PubMed]
61. Parsons RL, Hossack G, Paddock G. The absorption of antibiotics in adult patients with coeliac disease. J Antimicrob Chemother. 1975;1:39-50. [PubMed]
62. Pearman JW, Christiansen KJ, Annear DI, Goodwin CS, Metcalf C, Donovan FP, Macey KL, Bassette LD, Powell IM, Green JM, Harper WE, McKelvie MS . Control of methicillin-resistant Staphylococcus aureus (MRSA) in an Australian metropolitan teaching hospital complex. Med J Aust. 1985;142:103-108. [PubMed]
63. Perry MJ, Hendricks-Gittins A, Stacey LM, Adlard MW, Noble WC. Fusidane antibiotics produced by dermatophytes. J Antibiot. 1983;36:1659-1663. [PubMed]
64. Peter JD, Jehl F, Pottecher T, Dupeyron JP, Monteil H. Pharmacokinetics of intravenous fusidic acid in patients with cholestasis. Antimicrob Agents Chemother. 1993;37:501-506. [PubMed]
65. Portier H. A multicentre, open, clinical trial of a new intravenous formulation of fusidic acid in severe staphylococcal infections. J Antimicrob Chemother. 1990;25 Suppl B:39-44. [PubMed]
66. Reeves DS. The pharmacokinetics of fusidic acid. J Antimicrob Chemother. 1987;20:467-476. [PubMed]
67. Reimann G, Barthel B, Rockstroh JK, Spatz D, Brockmeyer NH. Effect of fusidic acid on the hepatic cytochrome P450 enzyme system. Int J Clin Pharmacol Ther. 1999;37:562-566. [PubMed]
68. 1996 Report of the Comité de l’Antibiogramme de la Société Francaise de Microbiologie. Clin Microbiol Infect. 1996;2(supp 1):S48. [PubMed]
69. Revell P, Nicholson F, Pearson TC. Granulocytopenia due to fusidic acid. Lancet. 1988;2:454-455. [PubMed]
70. Reyn A, Benzon MW. A study of the relationships between the sensitivities of Neisseria gonorrhoeae to sodium penicillin G, four semi-synthetic penicillins, spiramycin, and fusidic acid. Br J Vener Dis. 1968;44:140-150. [PubMed]
71. Reynaud AE, Espaze EP, Coste-Burel M, Richet H. In vitro kinetics of the activity of fusidic acid and fluoroquinolones combinations against staphylococci according to methicillin-resistance phenotype. Implications for the treatment of osteoarticular infections. Pathol Biol. 1992;40:466-470. [PubMed]
72. Richter Dahlfors AA, Kurland CG. Novel mutants of elongation factor G. J Mol Biol. 1990 Oct 20;215(4):549-57. [PubMed]
73. Rieutord A, Bourget P, Troche G, Zazzo JF. In vitro study of the protein binding of fusidic acid: a contribution to the comprehension of its pharmacokinetic behaviour. Int J Pharm 1995;119:57-64.
74. Robbins M, Marais R, Wilson AP, Felmingham D. Activity of sodium fusidate in vitro against anaerobic bacteria. Eur J Clin Microbiol. 1987;6:326-327. [PubMed]
75. Schepky G, Busch U, Heinzel G, Jost K, Lechner U, Mielenz H, Su ChA. Influence of enteric coating on drug delivery and absorption of fusidic acid film-coated tablets. Pharmazeutische Industrie 1991;53:135-139.
76. Singlas E, Kitzis MD, Guibert J, Taburet AM, Acar JF. Pharmacokinetics of sodium fusidate in man after single and repeated infusions. J Pharmacie Clinique 1988 Suppl II: 3341.
77. Somekh E, Golan T, Tanay A, Poch F, Dan M. Concentration and bactericidal activity of fusidic acid and cloxacillin in serum and synovial fluid. J Antimicrob Chemother. 1999 Apr;43:593-596. [PubMed]
78. Spelman D. Fusidic acid in skin and soft tissue infections. Int J Antimicrob Agents. 1999;12 Suppl 2:S59-66. [PubMed]
79. Steinkraus GE, McCarthy LR. In vitro activity of sodium fusidate against anaerobic bacteria. Antimicrob Agents Chemother. 1979;16:120-122. [PubMed]
80. Stock I. Natural antibiotic susceptibility of Proteus spp., with special reference to P. mirabilis and P. penneri strains. J Chemother. 2003;15:12-26. [PubMed]
81. Stock I, Henrichfreise B, Wiedemann B. Natural antibiotic susceptibility and biochemical profiles of Yersinia enterocolitica-like strains: Y. bercovieri, Y. mollaretii, Y. aldovae and 'Y. ruckeri'. J Med Microbiol. 2002;51:56-69. [PubMed]
82. Stock I, Gruger T, Wiedemann B. Natural antibiotic susceptibility of strains of the Enterobacter cloacae complex. Int J Antimicrob Agents. 2001;18:537-545. [PubMed]
83. Stock I, Wiedemann B. Natural antibiotic susceptibilities of Edwardsiella tarda, E. ictaluri, and E. hoshinae. Antimicrob Agents Chemother. 2001;45:2245-2255. [PubMed]
84. Taburet AM, Guibert J, Kitzis MD, Sorensen H, Acar JF, Singlas E. Pharmacokinetics of sodium fusidate after single and repeated infusions and oral administration of a new formulation. J Antimicrob Chemother. 1990;25 Suppl B:23-31. [PubMed]
85. Talbot J, Beeley L. Fusidic acid and jaundice. Br Med J. 1980;281:308. [PubMed]
86. Taylor PB, Burd EM, Tabbara KF. Corneal and intraocular penetration of topical and subconjunctival fusidic acid. Br J Ophthalmol. 1987;71:598-601. [PubMed]
87. Toma E, Barriault D. Antimicrobial activity of fusidic acid and disk diffusion susceptibility testing criteria for gram-positive cocci. J Clin Microbiol. 1995;33:1712-1715. [PubMed]
88. Turnidge J. Fusidic acid pharmacology, pharmacokinetics and pharmacodynamics. Int J Antimicrob Agents. 1999;12 Suppl 2:S23-34. [PubMed]
89. Turnidge J, Collignon P. Resistance to fusidic acid. Int J Antimicrob Agents. 1999;12 Suppl 2:S35-44. [PubMed]
90. Vaillant L, Machet L, Taburet AM, Sorensen H, Lorette G. Levels of fusidic acid in skin blister fluid and serum after repeated administration of two dosages (250 and 500 mg). Br J Dermatol. 1992;126:591-595. [PubMed]
91. Van Caekenberghe D. Comparative in-vitro activities of ten fluoroquinolones and fusidic acid against Mycobacterium spp. J Antimicrob Chemother. 1990;26:381-386. [PubMed]
92. Verbist L. The antimicrobial activity of fusidic acid. J Antimicrob Chemother. 1990;25 Suppl B:1-5. [PubMed]
93. Vial T, Gontier D, Pinede L, Cance P, Pofilet C, Evreux JC. Neutropenia during treatment with fusidic acid: analysis of 5 cases. Therapie. 1995;50:447-450. [PubMed]
94. Vickers CF. Percutaneous absorption of sodium fusidate and fusidic acid. Br J Dermatol. 1969;81:902-908. [PubMed]
95. von Daehne W, Godtfredsen WO, Rasmussen PR. Structure-activity relationships in fusidic acid-type antibiotics. Adv Appl Microbiol. 1979;25:95-146. [PubMed]
96. von der Haar B, Walter S, Schwapenheuer S, Schrempf H. A novel fusidic acid resistance gene from Streptomyces lividans 66 encodes a highly specific esterase. Microbiology. 1997;143 ( Pt 3):867-874. [PubMed]
97. Whitby M. Fusidic acid in the treatment of methicillin-resistant Staphylococcus aureus. Int J Antimicrob Agents. 1999;12 Suppl 2:S67-71. [PubMed]
98. Whitby M. Fusidic acid in septicaemia and endocarditis. Int J Antimicrob Agents. 1999;12 Suppl 2:S17-22. [PubMed]
99. Williamson J, Russell F, Doig WM, Paterson RW. Estimation of sodium fusidate levels in human serum, aqueous humour, and vitreous body. Br J Ophthalmol. 1970;54:126-130. [PubMed]
100. Wise R, Pippard M, Mitchard M. The disposition of sodium fusidate in man. Br J Clin Pharmacol. 1977;4:615-619. [PubMed]
101. Witzig RS, Franzblau SG. Susceptibility of Mycobacterium kansasii to ofloxacin, sparfloxacin, clarithromycin, azithromycin, and fusidic acid. Antimicrob Agents Chemother. 1993;37:1997-1999. [PubMed]
102. Zhao X, Drlica K. Restricting the selection of antibiotic-resistant mutant bacteria: measurement and potential use of the mutant selection window. J Infect Dis. 2002;185:561-565. [PubMed]
Tables
Table 1. In Vitro Activity of Fusidic Acid.
| Organism | N | Country (year of report) | MIC50 | MIC90 | MIC range | Reference |
|---|---|---|---|---|---|---|
| Gram positive aerobes | ||||||
| Staphylococcus aureus: Methicillin susceptible | 162 | USA (1987) | 0.03 | 0.06 | 0.008-0.25 | (39) |
| 72 | Japan (1987) | 0.2 | 1.56 | 0.1 – 3.13 | (40) | |
| 20 | Belgium (1990) | 0.06 | 0.06 | 0.06 – 0.12 | (92) | |
| Staphylococcus aureus Methicillin resistant | 185 | Ireland (1985) | >32 | >32 | 0.03 – 64 | (50) |
| 111 | USA (1987) | 0.03 | 0.12 | 0.008 – 4 | (39) | |
| 108 | Belgium (1990) | 0.06 | 0.06 | 0.03 – 8 | (92) | |
| 100 | Germany (1992) | 0.125 | 4 | 0.03 - 8 | (32) | |
| Staphylococcus spp: Coagulase negative | 100 | Germany (1992) | 0.25 | 0.25 | 0.03 – 8 | (32) |
| 197 | Canada (1995) | 0.25 | 0.5 | 0.12 - 32 | (87) | |
| Streptococcus pyogenes | 102 | France (2000) | 4 | 8 | 1 - 32 | (43) |
| Group G streptococci | 69 | France (2000) | 8 | 8 | 0.25 – 128 | (43) |
| Streptococcus agalactiae | 50 | France (2000) | 16 | 32 | 1 - 64 | (43) |
| Group C streptococci | 10 | France (2000) | 4 | 16 | 4 - 32 | (43) |
| Enterococcus faecalis | 152 | Canada (1995 | 4 | 8 | 1.0 - 32 | (87) |
| Corynebacterium spp | 118 | Germany (1977) | 0.04 | 2 | 0.04 – 12.5 | (30) |
| Gram negative aerobes | ||||||
| Bordetella pertussis | 100 | Canada (1960-81) | 0.1 | 0.2 | 0.03 – 0.5 | (4) |
| Moraxella catarrhalis | 9 | UK (1962) | 0.12 | 0.12 | 0.06 – 0.12 | (5) |
| Neisseria gonorrhoeae | 96 | Denmark (1968) | 0.6 | 2 | 0.25 - 2 | (70) |
| Neisseria meningitidis | 100 | UK (1978) | 0.03 | 0.12 | 0.015 – 0.5 | (48) |
| Mycobacterium spp | ||||||
| M. tuberculosis | 170 | Turkey (2001) | 16 | 16 | 16 - 256 | (18) |
| M. tuberculosis | 64 | Belgium (1990) | 8 | 16 | 4 - 32 | (91) |
| M. avium | 22 | Belgium (1990) | 32 | 64 | 1 - > 128 | (91) |
| M. chelonei | 17 | Belgium (1990) | 64 | 128 | 32 - >128 | (91) |
| M. fortuitum | 19 | Belgium (1990) | 64 | >128 | 16 - >128 | (91) |
| M. kansasii | 19 | USA (1992) | 32 | 32 | 2.0 - 64 | (101) |
| Anaerobic bacteria | ||||||
| Clostridium difficile | 80 | Australia (2002) | 0.75 | 2 | 0.125 - 4 | (45) |
| Clostridium perfringens | 39 | USA (1979) | 0.12 | 0.5 | ≤0.06 - 1 | (79) |
| Peptostreptococcus anaerobius | 34 | USA (1979) | 0.25 | 0.5 | ≤0.06 - 2 | (79) |
| Propionibacterium acnes | 25 | USA(1979) | 0.25 | 1 | ≤0.06 - 2 | (79) |
| Bacteroides fragilis | 100 | UK (1987) | 2 | 2 | 0.5 - 4 | (74) |
| Prevotella intermedia | 31 | USA (1979) | 0.25 | 1 | ≤0.06 - 1 | (79) |
| Porphyromonas asaccharolytica | 31 | USA (1979) | 0.25 | 1 | ≤0.06 - 1 | (79) |
| Fusobacterium spp | 15 | UK (1987) | 1 | 64 | ≤0.25 - >128 | (74) |
Notes:
- Susceptibility breakpoints have not been determined by NCCL
- The Comite de l’Antibiogramme de la Societe Francaise de Microbiologie (68)give MIC breakpoints ≤ 2mg/L = susceptible and >16 mg/L = resistant for non fastidious organisms
- The BSAC (1) MIC breakpoints for staphylococci are ≤ 1mg/L = susceptible, ³ 2mg/L = resistant
Table 2. Pharmacokinetics of Fusidic Acid/Sodium Fusidate After Oral or IV Administration in Adults.
| Route | Number subjects | Cmax mg/L | Tmax h | C 8 h mg/L | C12 h mg/L | AUC range | AUC mg.h/l | t½β h | Cl ml/min | Vd l/kg |
|---|---|---|---|---|---|---|---|---|---|---|
| oral (100) | 6 | 31.4 | 3 | ~12 | 0-8h | 162 | ||||
| oral (46), fasting | 12 | 30.6 | 2.2 | 0 - ∞ | 329 | 8.9 | ||||
| oral (46), after food | 12 | 22.7 | 3.2 | 0 - ∞ | 276 | 9.5 | ||||
| oral (84) | 8 | 33.3 | 2.1 | ~12.5 | ~10 | 0 - ∞ | 368 | 16.0 | ||
| oral (52) | 10 | 30 | ~2 | 8.5 | 0 - ∞ | 315 | 11.0 | 33 | 0.52 (b) | |
| IV (2h infusion) (76) | 8 | 52.4 | 2 | ~15 | ~10 | 411 | 9.8 | 21 | 0.3 | |
| IV (2h infusion) (75) | 12 | 23.6 | 2 | ~11 | ~8 | 204 | 14.5 | 42 | 0.46 |
Cmax = peak concentration, Tmax = time to peak concentration, C8h = concentration 8 hours after dosing, C12h = concentration 12 hours after dosing, AUC = area-under-the-curve, t½ß = elimination half-life, Cl = clearance, Vd = volume of distribution, ß = elimination half-life volume of distribution
Figure 1. Chemical Structure of Fusidic Acid.
GUIDED MEDLINE SEARCH FOR:
Therapeutic Effects
Dosage
Adverse Effects
Drug Interactions
Pharmacokinetics/Pharmacodynamics
Pharmacoeconomics
GUIDED MEDLINE SEARCH FOR RECENT REVIEWS
Therapeutic Effects
Dosage
Adverse Effects
Drug Interactions
Pharmacokinetics/Pharmacodynamics
Pharmacoeconomics
