Angiostrongylus cantonensis and Human Angiostrongyliasis
Authors: De-Hua Lai, Ph.D., Xia-Bing Dai, M.Sc., Geoff Hide, Ph.D., Zhao-Rong Lun, Ph.D.
Authors: (First Edition, 1999 and Second Edition, 2002): Michael Martin, M.D., MPH, DTM&H, Helmut Albrecht, M.D.
PARASITOLOGY
The definitive hosts for A. cantonensis are usually rodents from the genus Rattus, such as commonly found rats, Rattus norvegicus and R. rattus. The main intermediate hosts are molluscs including snails Achatina fulica, Pocacea canaliculata (63, 64) and slugs such as Veronicella cubensis (24).
An adult female nematode in the pulmonary arteries of infected rats can lay around 15,000 eggs daily (Figure 1) (68). Eggs are carried by the bloodstream to the capillaries, where they break into the air spaces of alveolus and hatch. The first-stage larvae (the juveniles) migrate up the bronchial tree, are swallowed and are excreted out with the feces (Figure 1B). The larvae in feces are digested by an intermediate mollusc host and develop after two molts into third-stage larvae in 12 days (Figure 1C) (6). The third-stage larvae are able to infect the rat when they ingest infected molluscs. In the alimentary tract of a rat, the larvae are released from the chyme after pepsin/trypsinase digestion and enter the bloodstream. Later, the larvae reach the central nervous system (CNS) of the mammalian host, mostly in the brain, where they molt twice and become adult worms within two weeks (2). Finally, the adult worms migrate to the pulmonary arteries, become sexually mature and then lay eggs (Figure 1A). It takes approximately 6-8 weeks for an infected rat to start to excrete first-stage larvae after ingesting an infected mollusc (68).
However, infective third-stage larvae of A. cantonensis are not restricted to molluscs. A set of paratenic hosts can serve as accidental carriers of this parasite and these aremainly those animals who eat molluscs, such as fresh-water crustaceans (prawns and land crabs), terrestrial planarians, frogs, toads and monitor lizards (63). Although A. cantonensis cannot develop further in paratenic hosts, these hosts could assist in the distribution of this parasitic nematode by making them available to definitive hosts.
Human A. cantonensis infections occur after eating molluscs, paratenic hosts or vegetables, which are contained or contaminated by the third stage infective larvae of the worm (10, 53, 59, 63). The larvae are digested from the vectors and invade host intestinal tissue causing enteritis. Larval migration in mammalian host organs may cause many clinical signs, e.g. in the liver it usually causes hepatomegaly (70), while in lung it results in coughing, rhinorrhea, sore throat, malaise and fever. When the worms settle down in the central nervous system (CNS), patients may develop eosinophilic meningitis and eosinophilic pleocytosis. Occasionally, the worms can reach the eyes and cause ocular angiostrongyliasis which may occur in parallel with visual disturbances such as diplopia or strabismus in many patients (51, 53).
EPIDEMIOLOGY
The first human case of angiostrongyliasis was reported in Taiwan in 1945. Since then, several outbreaks of angiostrongyliasis in humans have been reported in the Pacific Islands. To date, more than 2,900 cases have been documented worldwide. In the last two decades, several major outbreaks of human angiostrongyliasis have been reported in endemic regions, especially in China (12 outbreaks in Mainland China and 3 outbreaks in Taiwan). In addition, western travelers have been diagnosed with eosinophilic meningitis caused by A. cantonensis after returning from endemic regions(4, 35, 37, 38, 46, 48, 55). For example, 12 American travelers developed eosinophilic meningitis after they returned from Jamaica in 2002 (55). These cases have resulted in great concern for both the general public and the physicians.
CLINICAL MANIFESTATIONS
Incubation period of human angiostrongyliasis is usually within two weeks, but may vary from one day to several months, depending on the number of the parasites involved and the age of the patients (63). After the incubation period, the onset of the disease is frequently sudden and presents as eosinophilic meningitis or meningoencephalitis. Eosinophilic meningitis is a rare condition, in relation to most other diseases, and is associated with central nervous system (CNS) injury commonly caused by parasitic agents, such as in the case of A. cantonensis (39).
Clinical symptoms appear on the sixth to the fifteenth day after infection in more than half of patients studied (51, 70). In a series of studies, clinical symptoms were analysed in 900 adult and 133 paediatric patients and they were found to have eosinophilic meningitis caused by A. cantonensis (47, 52, 63). The symptoms of headache, stiff neck, nausea and vomiting are commonly found in infected patients. Over 95% of patients suffered from headache in the early stages of the disease and it was suggested that this was caused by increased intracranial pressure or direct damage by the larvae. Furthermore, this clinical sign could be released by lumbar puncture (51, 70). Neck stiffness was observed in 43-46% of the patients. Approximately 43% of adult patients felt paraesthesia, usually expressed as pain, numbness, itching, while the frequency of this was less in children (29%). Nausea and vomiting occurred in 39% of adult and 79% paediatric patients. Fever was shown to be higher (79%) in paediatric patients than in adults (30%). Children infected with A. cantonensis were seen to also develop the following clinical symptoms: somnolence (82%), constipation (79%), abdominal pain (35%), weakness of extremities (17%), muscle twitching and convulsion (16%). The severity of symptoms is possibly related to the quantity of A. cantonensis L3 larvae ingested (44, 70) In Thailand, people are generally consumers of low larval burden Pila or Pomacea snails and tend to have milder disease than consumers of the giant African snail (A. fulica) who may develop severe or fatal disease due to the higher burdens of larvae (33, 51, 59, 70).
LABORATORY DIAGNOSIS
The detection of A. cantonensis (young adults or larvae) in cerebrospinal fluid (CSF) or the ocular chamber is the gold standards for diagnosis of human angiostrongyliasis. However, a direct identification of the worm in patients is rarely successful with only 2-11% of infection cases detected (63, 70). This is due to the low probability of parasites entering the subarachnoid space and the limited volumes of CSF that are usually checked. In one study, larval recovery from CSF was increased to 41.5% of 82 patients using a pumping method (25). Other indicators can also be used for detection, these are: the clinical symptoms, medical history, CNS imaging results, laboratory detection of eosinophilia in the blood and cerebrospinal fluid and serological tests. A history of eating intermediate or paratenic hosts is very critical factor in the diagnosis of A. cantonensis infection. Furthermore, the possibility of contact with infective parasite larva such as presence of intermediate hosts in living places may also be a useful indicator for diagnosis.
For angiostrongyliasis patients, magnetic resonance imaging (MRI) examination usually reveals the presence of different types of lesions. These include leptomeningeal and multiple micronodular enhancements in brain tissues and linear enhancement in the pia mater (21, 25, 61). In addition to MRI, computed tomography (CT) is another option which may differentiate the presence of A. cantonensis from other parasitic diseases (41). However, it was reported that these findings were both highly variable and, in some cases, nonspecific in angiostrongyliasis patients and therefore could only be used as a follow up confirmation rather than the basis for differential diagnosis (27, 31, 57, 58, 61).
The appearance of CSF in angiostrongyliasis patients may be clear or coconut juice like (50, 54). The eosinophil count in the CSF of patients is often high (between 100 to 1000 per mm3) and make up at least 10% of the total white cell count (63)and 7-36% of the white cell count in peripheral blood (59). Immunological techniques, using human antibodies against A. cantonensis, have been developed to confirm a presumptive diagnosis by detection of antigens or antibodies against A. cantonensis in serum or cerebrospinal fluid. An intradermal test based on a skin reaction to A. cantonensis was developed in 1960s (1). Later, various assays were developed, including immunodiffusion (29), immuno-electrophoresis (30), indirect hemagglutination test (8), enzyme-linked immunosorbent assay (ELISA) (18) and direct and indirect immunofluorescent antibody tests (DFAT and IFAT) (66), etc.
Various ELISA methods have been developed and proved to be satisfactory with almost 100% sensitivity and specificity in laboratory diagnosis (21). A dot-blot ELISA was established which proved to be convenient for handling field samples for epidemiological surveys (20, 71). A combination of PCR techniques and immuno-methods have developed into immuno-PCR, which can successfully detected antigens from A. cantonensis in serum (14). (More related information can be obtained in References 19 and 67).
The ribosomal SSU rRNA gene was utilized to develop a nucleic acid amplification test of A. cantonensis at the Centers for Disease Control and Prevention (USA , http://www.dpd.cdc.gov). The genome of this parasite was published in 2013, which enabled a straight forward approach to developing genetic markers for distinguishing angiostrongyliasis from other parasitic diseases (42). PCR does not distinguish between different life cycle stages of the parasite and will produce a positive result for any material containing A. cantonensis DNA, including eggs, fragments of worms, and residual cells shed from adult worms.
ANTIPARASITIC THERAPY
Mild cases of angiostrongyliasis could be self-limiting and resolved spontaneously without specific therapy. However, severe cases can develop permanent, neurological sequelae without prompt and proper treatment and can even progress to coma and death. The recommended treatment is supportive and treatment of symptoms. Anthelminthics, such as albendazole, mebendazole and ivermectin, usually are not recommended for treatment of A. cantonensis infections, because dead worms may exacerbate inflammation and increase neurological symptoms. However, these compounds have been shown to have an ability to relieve symptoms and reduce the duration of the disease in patients in both China and Thailand (10, 28).
In Southeast Asia, A. cantonensis is widely distributed. At least 1,337 human infection cases have been documented in Thailand (64), which has been linked to eating raw or undercooked snails (Pila spp.) with alcohol, a popular dietary habit especially among young adult males. Cases were also found in Vietnam, Cambodia, the Philippines, Malaysia, and Indonesia, (2, 15, 63). In China (including Hong Kong and Taiwan) over 800 cases have been reported and infection has been linked to several factors: the pursuit of eating exotic and delicate foods including raw and undercooked snails and the carelessness of parents in Taiwan looking after children playing with snails (64). This is suggestive of low public concern to this parasite.
There are a wide range of other countries that are also considered to be endemic areas of A. cantonensis, these include: i. East or South Asia and the Pacific Basin, Japan (Kyushu and Okinawa), India, Sri Lanka, Australia (Queensland and New South Wales), Papua New Guinea, Vanuatu Republic (New Hebrides), Fiji, American Samoa, Western Samoa, New Caledonia, French Polynesia (Tahiti), Tonga, Hawaiian Islands; ii. Africa Ivory Coast, Nigeria, Egypt, Mayotte, Madagascar, Reunion Island; iii. Caribbean, USA (Louisiana), Cuba, Jamaica, the Bahamas, Puerto Rico, Dominican Republic, Haiti; iv. South America, Brazil and Ecuador (3, 41, 45, 47, 56, 63). However, it is unknown how many additional cases there might be which have not been reported or recognized during the past decades. Travelers from non-endemic regions have been reported sporadically as infection cases after returning (63). Therefore, travelers to endemic places should avoid eating raw and undercooked molluscs. Physicians should suspect eosinophilic meningitis due to A. cantonensis when a recent traveler presents with headaches, elevated intracranial pressure, eosinophilic pleocytosis and paresthesia or hyperesthesia.
Adjunctive Therapy
Surgery is necessary to remove worms from eyes of patients with ocular angiostrongyliasis where laser treatment could be used to inactive the worms and prevent further damage (53). Analgesic drugs are administered for pain relief. Lumbar puncture is effective at relieving symptoms of eosinophilic meningitis caused by increasing intracranial pressure, such as headache and nausea. Anti-inflammation producing corticosteroid therapy was shown to be effective in the relief of symptomatic headaches caused by A. cantonensis induced eosinophilic meningitis (12). The combination of corticosteroids and anthelminthics has been successfully used for treatment of human angiostrongyliasis (9, 11, 22). (Table 1; more details are summarized in Ref. 63)
Hosts for A. cantonensis: Most species of molluscs are susceptible to and capable of transmitting A. cantonensis (32). Terrestrial and some aquatic snails and slugs are the primary intermediate hosts (40). Achatina fulica, or the African giant land snail, may contain thousands of third-stage larvae of A. cantonensis which can be a major source of human infection worldwide. A national survey in Southern China found 13.4% of A. fulica to be infected with A. cantonensis in mainland China while only 0.3% of other terrestrial snails were infected (40). In the Phitsanulok province of Thailand, the infection rate of A. fulica has been reported to be up to 12.37% (62). The global dispersal of A. cantonensis has been postulated to be associated with the spread of this snail from its native origins in Africa to the Pacific basin, South Asia, the CaribbeanIslands and North America (34).
The golden apple snail, P. canaliculata, a very successfully invasive snail from South America, has a very wide distribution in Asia (23). Unfortunately, this snail is also very susceptible to A. cantonensis and has become an important intermediate host in these regions with high infection rates of 10-69.4% (63). Most outbreaks in China have been attributed to this snail (69). A recent national survey in southern China showed 6.8% of P. canaliculata are infected with A. cantonensis, while other freshwater snails (including Bellamya aeruginosa and Cipangopaludina chinensis) have an overall infection rate of 0.05% (40). In Thailand, snails of Pila spp. (Pila polita, Pila ampullacea), which typically have low burdens of A. cantonensis, are frequently eaten by adult males and are the major intermediate hosts there (51, 70). In Okinawa and Hawaii, semi-slugs Parmarion martensi (family Helicarionidae) have a high frequency of A. cantonensis infection (e.g. 78% in Hawaii, 24). In Hawaii, 24% Cuban slugs (Veronicella cubensis, family Eronicellidae) are also frequently infected with A. cantonensis (24).
Many other species of molluscs have been found naturally infected with A. cantonensis, including Bradybaena similaris, Subulina octona, other Pomacea species (eg. Pomacea lineata), Deroceras laeve, Helix pomatia, H. aspersa and H. lucorum, Sarasinula marginata, Microparmarion malayanus and Parmarion martensi (5, 26, 43).
There is very little known about A. cantonensis in paratenic hosts such as crustaceans (prawns and land crabs), predacious land planarians (flatworms of the genus Platydemus, eg. Platydemus manokwari in Okinawa and Hawaii, 24), fish, frogs and monitor lizards. However, they have all been reported to contribute to the reservoir of infection for humans (16, 17, 36).
Rats, commonly R. rattus and R. norvegicus, are the definitive hosts and therefore are necessary for the establishment of A. cantonensis epidemics in an area. Identification of infected rats confirm an area as an endemic region. These rats are tolerant of A. cantonensis infection, surviving with relatively large infective doses (>150 parasites in brain) without any significant CNS abnormality (49). Other species of rats, such as R. exulans, R. flavipectus, found in rural and forested areas, are also reported to be natural hosts (17, 40). In addition, under laboratory conditions other species, such as Mongolian gerbils (Meriones unguiculatus), have been shown to serve as definitive hosts for A. cantonensis and support completion of the life cycle (65). The infection rates in rats were highly variable in endemic areas (63). A recent national survey showed the overall infection rate of rats (mainly R. norvegicus) with A. cantonensis was 4.2% in China (40).
PREVENTION
Due to the worldwide distribution and the large population of A. cantonensis hosts (rats and molluscs), it would be almost impossible to eradicate this parasite from the environment. However, it is reasonably easy to block the transmission pathway of this parasite to humans by educating the public not to eat raw or undercooked snails, frogs, lizards or any other potential intermediate and paratenic hosts from the endemic regions. Snails are considered a very popular kind of food and are prepared in various ways. Eating raw or undercooked snails with seasonings, such as with pepper and pericarpium or alcohol, is a popular custom that has existed for generations in some endemic regions especially in Thailand and China. Most outbreaks of human A. cantonensis infections in China have been attributed to this method of preparing snails. New fashions in eating are emerging such as eating salads, raw vegetable juice and French snail-based cuisines. These may be attractive to people but could cause problems if the food supplies originate from endemic regions without specific safety considerations. Thus, education to limit or change such eating manners would be a very useful, practical and achievable intervention for control of human A. cantonensis infection. Young children, people with mental dysfunction or pica disorder, food handlers and gardeners are also at risk. Public health workers and physicians in endemic areas as well as those working in the non-endemic regions of A. cantonensis also need to be trained in the knowledge of this parasite with specific emphasis on transmission routes and the dangers to the health of the population.
In conclusion, the recommended measures for prevention in endemic regions include: (i) educating citizens/tourists to be aware of A. cantonensis and the disease caused by this parasite; (ii) education to promote only eating adequately cooked snails, slugs, small molluscs and paratenic hosts of A. cantonensis such as frogs, shrimps and land crabs; (iii) eradicating molluscan hosts near housing and vegetable gardens; (iv) education to promote not eating unwashed vegetables which may be contaminated with the infective stage larvae of A. cantonensis; (v) education to promote washing hands frequently and particularly after gardening and (vi) educating physicians in both non-endemic and endemic regions to know this parasite will help to diagnose A. cantonensis infection in humans promptly.
REFERENCES
1. Alicata JE, Brown RW. Preliminary observations on the use of intradermal test for the diagnosis of eosinophilic meningitis in man caused by Angiostrongylus cantonensis. Can J Zool 1962; 40:119–124.
2. Alicata JE. Life cycle and biology. In: Alicata JE, Jindrak K, editors. Angiostrongyliasis in the Pacific and Southeast Asia. Springfield, Illinois: C.C. Thomas 1970:17–27
3. Asato R, Taira K, Nakamura M, Kudaka J, Itokazu K, Kawanaka M. Changing epidemiology of Angiostrongyliasis cantonensis in Okinawa prefecture, Japan. Jpn J Infect Dis 2004; 57:184–186. [PubMed]
4. Bartschi E, Bordmann G, Blum J, Rothen M. Eosinophilic meningitis due to Angiostrongylus cantonensis in Switzerland. Infection 2004; 32:116–118. [PubMed]
5. Caldeira RL, Mendonça CL, Goveia CO, Lenzi HL, Graeff-Teixeira C, Lima WS, Mota EM, Pecora IL, Medeiros AM, Carvalho Odos S. First record of molluscs naturally infected with Angiostrongylus cantonensis (Chen, 1935) (Nematoda: Metastrongylidae) in Brazil. Mem Inst Oswaldo Cruz 2007; 102:887–889. [PubMed]
6. Chao D, Lin CC, Chen YA. Studies on growth and distribution of Angiostrongylus cantonensis larvae in Ampullarium canaliculatus. Southeast Asian J Trop Med Public Health 1987; 18:248–252.[PubMed]
7. Chen HT. Un nouveau nematode pulmonaire, Pulmonema cantonensis n.g., n. sp.des rats de Canton. Ann Parasitol 1935;13:312–317.
8. Chen SN, Suzuki T. Fluorescent antibody and indirect haemagglutination tests for Angiostrongylus cantonensis. J Formos Med Assoc 1974;73:393–400. [PubMed]
9. Chen WL, Zhong JM, Chen H, Wu SY, Ding L. Eosinophilic meningitis: 31 cases report. Chin J Misdiagn 2006;6:4668–4669. (In Chinese)
10. Chen XG, Li H, Lun ZR. Angiostrongyliasis, Mainland China . Emerg Infect Dis 2005; 11:1645–1647. [PubMed]
11. Chotmongkol V, Sawanyawisuth K, Sawanyawisuth K, Louhawilai S, Limpawattana P. Treatment of eosinophilic meningit is with a combination of prednisolone and mebendazole. Am J Trop Med Hyg 2006; 74:1122–1124. [PubMed]
12. Chotmongkol V, Sawanyawisuth K, Thavornpitak Y. Corticosteroid treatment of eosinophilic meningitis. Clin Infect Dis 2000; 3:660–662. [PubMed]
13. Chotmongkol V, Wongjitrat C, Sawadpanit K, Sawanyawisuth K. Treatment of eosinophilic meningitis with a combination of albendazole and corticosteroid. Southeast Asian J Trop Med Public Health 2004; 35:172–174. [PubMed]
14. Chye SM, Lin SR, Chen YL, Chung LY, Yen CM. Immuno-PCR for detection of antigen to Angiostrongylus cantonensis circulating fifth-stage worms. Clin Chem 2004; 50:51–57. [PubMed]
15. Cowie RH. Biology, systematics, life cycle, and distribution of Angiostrongylus cantonensis, the cause of rat lungworm disease. Hawaii J Med Public Health 2013;72:6–9. [PubMed]
16. Cowie RH. Pathways for transmission of angiostrongyliasis and the risk of disease associated with them. Hawaii J Med Public Health 2013;72:70–74.[PubMed]
17. Cross JH, Chen ER. Angiostrongyliasis. In: Murrell K.D., Freid B., editors. Food-Borne Parasitic Diseases. Springer US , 2007:263–290.
18. Cross JH, Chi JC. ELISA for the detection of Angiostrongylus cantonensis antibodies in patients with eosinophilic meningitis. Southeast Asian J Trop Med Public Health1982;13:73–76. [PubMed]
19. Eamsobhana P, Yong HS. Immunological diagnosis of human angiostrongyliasis due to Angiostrongylus cantonensis (Nematoda: Angiostrongylidae). Int J Infect Dis 2009;13:425–431. [PubMed]
20. Eamsobhana P, Yoolek A, Kreethapon N. Blinded multi-laboratory evaluation of an in-house dot-blot ELISA kit for diagnosis of human parastrongyliasis.Southeast Asian J Trop Med Public Health2003; 34:1–6. [PubMed]
21. Graeff-Teixeira C, da Silva AC, Yoshimura K. Update on eosinophils meningoencephalitis and its clinical relevance. Clin Microbiol Rev 2009; 22:322. [PubMed]
22. Han JH, Zhu YH, Jie WZ, Li Y, Yan Y, Ying M, Xiong J, Liu YX. Eosinophilic meningitis: 28 cases report. J Patho Biol 2006; 1(Suppl):2–3. (In Chinese)
23. Hollingsworth RG, Cowie RH. Apple snails as disease vectors. In: Joshi RC, Sebastian LC, editors. Global advances in ecology and management of golden apple snails. Nueva Ecija, Philippines: Philippine Rice Institute, 2006:121–132.
24. Hollingsworth RG, Howe K, Jarvi SI. Control measures for slug and snail hosts of Angiostrongylus cantonensis, with special reference to the semi-slug Parmarion martensi. Hawaii J Med Public Health 2013; 72(6 Suppl 2):75–80.[PubMed]
25. Hwang KP, Chen ER. Clinical studies on angiostrongyliasis cantonensis among children in Taiwan. Southeast Asian J Trop Med Public Health 1991; 22(Suppl):194–199. [PubMed]
26. Jarvi SI, Farias EM, Howe K, Jacquier S, Hollingsworth R, Pitt W. Quantitative PCR estimates Angiostrongylus cantonensis (rat lungworm) infection levels in semi-slugs (Parmarion martensi). Mol Biochem Parasitol 2012; 185:174–176. [PubMed]
27. Jin E, Ma D, Liang Y, Ji A, Gan S. MRI findings of eosinophilic myelomeningoencephalitis due to Angiostrongylus cantonensis. Clin Radiol 2005; 60:242–250. [PubMed]
28. Jitpimolmard S, Sawanyawisuth K, Morakote N, Vejjajiva A, Puntumetakul M, Sanchaisuriya K, Tassaneeyakul W, Tassaneeyakul W, Korwanich N. Albendazole therpy for eosinophilic meningitis caused by Angiostrongylus cantonensis. Parasitol Res 2007; 100:1293–1296. [PubMed]
29. Kamiya M, Kanda T. Immunodiffusion and haemagglutination test results with antigens obtained from whole worms, lung lesions and incubates of adult Angiostrongylus cantonensis. Jpn J Exp Med 1974; 23:12–17. [PubMed]
30. Kamiya M, Tharavanij S, Harinasuta C. Antigenicity for haemagglutination and immuno-electrophoresis tests in fractionated antigens from Angiostrongylus cantonensis. Southeast Asian J Trop Med Public Health 1973; 4:187–194. [PubMed]
31. Kanpittaya J, Jitpimolmard S, Tiamkao S, Mairiang E. MR findings of eosinophilic meningoencephalitis attributed to Angiostrongylus cantonensis. AJNR Am J Neuroradiol 2000; 21:1090–1094. [PubMed]
32.Kim JR, Hayes KA, Yeung NW, Cowie RH. Definitive, intermediate, paratenic, and accidental hosts of Angiostrongylus cantonensis and its molluscan intermediate hosts in Hawaii. Hawaii J Med Public Health 2013;72:10. [PubMed]
33. Kliks MM, Kroenke K, Hardman JM. Eosinophilic radiculomyeloencephalitis: an angiostrongyliasis outbreak in American Samoa related to ingestion of Achatina fulica snails. Am J Trop Med Hyg 1982; 31:1114–1122. [PubMed]
34. Kliks MM,.Palumbo NE. Eosinophilic meningitis beyond the Pacific Basin: the global dispersal of a peridomestic zoonosis caused by Angiostrongylus cantonensis, the nematode lungworm of rats. Soc Sci Med 1992; 34:199–212. [PubMed]
35. Kumar V, Kyprianou I, Keenan JM. Ocular Angiostrongyliasis: removal of a live nematode from the anterior chamber. Eye 2005;19:229–230. [PubMed]
36. Lai CH, Yen CM, Chin C, Chung HC, Kuo HC, Lin HH. Eosinophilic meningitis caused by Angiostrongylus cantonensis after ingestion of raw frogs. Am J Trop Med Hyg 2007; 76:399–402. [PubMed]
37. Leone S, De MM, Ghirga P, Nicastri E, Esposito M, Narciso P. Eosinophilic meningitis in a returned traveler from Santo Domingo: case report and review. J Travel Med 2007; 14:407–410. [PubMed]
38. Lo Re V, III, Gluckman SJ. Eosinophilic meningitis due to Angiostrongylus cantonensis in a returned traveler: case report and review of the literature. Clin Infect Dis 2001; 33:e112–e115. [PubMed]
39. Lo Re V, III, Gluckman SJ. Eosinophilic meningitis. Am J Med2003; 114:217–223. [PubMed]
40. Lv S, Zhang Y, Liu HX, Hu L, Yang K, Steinmann P, Chen Z, Wang LY, Utzinger J, Zhou XN. Invasive snails and an emerging infectious disease: results from the first national survey on Angiostrongylus cantonensis in China. PLoS Negl Trop Dis 2009;3:e368.[PubMed]
41. Martins YC, Tanowitz HB, Kazacos KR. Central nervous system manifestations of Angiostrongylus cantonensis infection. Acta Trop 2015;141(PtA):46-5. [PubMed]
42. Morassutti AL, Perelygin A, DE Carvalho MO, Lemos LN, Pinto PM, Frace M, Wilkins PP, Graeff-Teixeira C, DA Silva AJ. High throughput sequencing of the Angiostrongylus cantonensis genome: a parasite spreading worldwide. Parasitol 2013;140:1304–1309. [PubMed]
43. Morassutti AL, Thiengo SC, Fernandez M, Sawanyawisuth K, Graeff-Teixeira C. Eosinophilic meningitis caused by Angiostrongylus cantonensis: an emergent disease in Brazil. Mem Inst Oswaldo Cruz 2014; 109:399–407. [PubMed]
44. Murphy GS, Johnson S. Clinical Aspects of Eosinophilic Meningitis and Meningoencephalitis caused by Angiostrongylus cantonensis, the Rat Lungworm. Hawaii J Med Public Health 2013;72:35–40. [PubMed]
45. Nishimura K, Mogi M, Okazawa T, Sato Y, Toma H, Wakibe H. Angiostrongylus cantonensis infection in Ampullarius canaliculatus in Kyushu, Japan. Southeast Asian J Trop Med Public Health 1986; 17:595–600. [PubMed]
46. Noskin GA, McMenamin MB, Grohmann SM. Eosinophilic meningitis due to Angiostrongylus cantonensis. Neurology 1992; 42:1423–1424. [PubMed]
47. Oehler E, Ghawche F, Delattre A, Berberian A, Levye M, Valour F. Angiostrongylus cantonensis eosinophilic meningitis: A clinical study of 42 consecutive cases in French Polynesia. Parasitol Int 2014; 63:544–549. [PubMed]
48. Podwall D, Gupta R, Furuya EY, Sevigny J, Resor SR. Angiostrongylus cantonensis meningitis presenting with facial nerve palsy. J Neurol., 2004;25:1280–1281. [PubMed]
49. Prociv P, Spratt DM, Carlisle MS. Neuro-angiostrongyliasis: unresolved issues. Int J Parasitol 2000;30:1295–1303. [PubMed]
50. Punyagupta S, Bunnag T, Juttijudata P, Rosen L. Eosinophilic meningitis in Thailand. Epidemiologic studies of 484 typical cases and the etiologic role of Angiostrongylus cantonensis. Am J Trop Med Hyg 1970; 19:950–958. [PubMed]
51. Punyagupta S, Juttijudata P, Bunnag T. Eosinophilic meningitis in Thailand. Clinical studies of 484 typical cases probably caused by Angiostrongylus cantonensis. Am J Trop Med Hyg 1975; 24:921–931. [PubMed]
52.Sawanyawisuth K, Chindaprasirt J, Senthong V, Limpawattana P, Auvichayapat N3, Tassniyom S, Chotmongkol V, Maleewong W, Intapan PM. Clinical manifestations of Eosinophilic meningitis due to infection with Angiostrongylus cantonensis in children. Korean J Parasitol 2013; 51:735–738. [PubMed]
53. Sawanyawisuth K, Kitthaweesin K, Limpawattana P, Intapan PM, Tiamkao S, Jitpimolmard S, Chotmongkol V. Intraocular angiostrongyliasis: clinical findings, treatments and outcomes. Trans R Soc Trop Med Hyg 2007; 107:497–501 [PubMed]
54. Senthong V, Chindaprasirt J, Sawanyawisuth K. Differential diagnosis of CNS angiostrongyliasis: a short review. Hawaii J Med Public Health 2013; 72:52–54. [PubMed]
55. Slom TJ, Cortese MM, Gerber SI, Jones RC, Holtz TH, Lopez AS, Zambrano CH, Sufit RL, Sakolvaree Y, Chaicumpa W, Herwaldt BL, Johnson S. An outbreak of eosinophilic meningitis caused by Angiostrongylus cantonensis in travelers returning from the Caribbean. N Engl J Med 2002; 346:668–675. [PubMed]
56. Thiengo SC, Simões RO, Fernandez MA, Maldonado Júnior A. Angiostrongylus cantonensis and rat lungworm disease in Brazil. Hawaii J Med Public Health 2013;72:18–22. [PubMed]
57. Tsai HC, Chen YS, Yen CM. Human parasitic meningitis caused by Angiostrongylus cantonensis infection in Taiwan. Hawaii J Med Public Health 2013; 72:26–27. [PubMed]
58. Tsai HC, Liu YC, Kunin CM, Lai PH, Lee SS, Chen YS, Wann SR, Lin WR, Huang CK, Ger LP, Lin HH, Yen MY. Eosinophilic meningitis caused by Angiostrongylus cantonensis associated with eating raw snails: correlation of brain magnetic resonance imaging scans with clinical findings. Am J Trop Med Hyg 2003; 68:281–285. [PubMed]
59. Tsai HC, Liu YC, Kunin CM, Lee SS, Chen YS, Lin HH, Tsai TH, Lin WR, Huang CK, Yen MY, Yen CM. Eosinophilic meningitis caused by Angiostrongylus cantonensis: report of 17 cases. Am J Med 2001; 111:109–114. [PubMed]
60. Tsai HC, Lee SS, Huang CK, Yen CM, Chen ER, Liu YC. Outbreak of eosinophilic meningitis associated with drinking raw vegetable juice in southern Taiwan. Am J Trop Med Hyg 2004; 71:222–226. [PubMed]
61.Tsai HC, Tseng YT, Yen CM, Chen ER, Sy CL, Lee SS, Wann SR, Chen YS. Brain magnetic resonance imaging abnormalities in eosinophilic meningitis caused by Angiostrongylus cantonensis infection. Vector Borne Zoonotic Dis 2012;12:161–166. [PubMed]
62.Vitta A, Polseela R, Nateeworanart S, Tattiyapong M. Survey of Angiostrongylus cantonensis in rats and giant African land snails in Phitsanulok province, Thailand. Asian Pac J Trop Med 2011;4:597–599. [PubMed].
63. Wang QP, Lai DH, Zhu XQ, Chen XG, Lun ZR. Human angiostrongyliasis. Lancet Infect Dis 2008; 8:621–630. [PubMed]
64. Wang QP, Wu ZD, Wei J, Owen RL, Lun ZR. Human Angiostrongylus cantonensis: an update. Eur J Clin Microbiol Infect Dis 2012; 31:389–395. [PubMed]
65. Wei Y, Hong Q, Chen D, Liang C, Liu H, Luo X, Zhu X. Permissibility of Mongolian gerbil for Angiostrongylus cantonensis infection and utility of this animal model for anthelmintic studies. Parasitol Res 2014; 113:1687–1693. [PubMed]
66. Welch JS, Dobson C. Immunodiagnosis of parasitic zoonoses: comparative efficacy of three immunofluorescence tests using antigens purified by affinity chromatography. Trans R Soc Trop Med Hyg 1978; 72:282–288. [PubMed]
67. Wilkins PP, Qvarnstrom Y, Whelen AC, Saucier C, da Silva AJ, Eamsobhana P. The current status of laboratory diagnosis of Angiostrongylus cantonensis infections in humans using serologic and molecular methods. Hawaii J Med Public Health 2013;72:55–57. [PubMed]
68. Wu GH. Angiostrongylus cantonensis. In: Tang JQ, editor. Nature-Borne Diseases. Beijing: Science Press 2006:1182–1189. (In Chinese) [PubMed]
69. Yang TB, Wu ZD, Lun ZR. The apple snail Pomacea canaliculata, a novel vector of the rat lungworm, Angiostrongylus cantonensis: its introduction, spread, and control in China. Hawaii J Med Public Health 2013;72:23–25 [PubMed]
70. Yii CY. Clinical observations on eosinophilic meningitis and meningoencephalitis caused by Angiostrongylus cantonensis on Taiwan. Am J Trop Med Hyg 1976; 25:233–249. [PubMed]
71. Zhu P, Xiong Z, Chunyun Wu C, Wu J. Detection of Angiostrongylus cantonensis antibody by dot-ELISA. Chin J Zoonoses 2002; 18:51–53.
Tables
Table 1: Treatment for human angiostrongyliasis and itseffectiveness (modified from 63)
| Ref. | Number of patients treated | Medicine Treatment (dosage per day) and duration | Outcome |
|---|---|---|---|
22 (age 15-43) |
Albendazole (400-1200 mg) and dexamethasone (10-20 mg) for 10-20 days. | All patients recovered, serious side-effects were not detected. | |
9 (age 15-43) |
Praziquantel (400-1200 mg) and dexamethasone (10-20 mg) for 10-20 days. | All patients recovered, serious side-effects were not detected. | |
28 (age 25-63) |
Albendazole (15-20 mg) and dexamethasone (10 mg) for 9-27 days. | All patients recovered, serious side-effects were not detected, two recurred in a month. | |
5 (age 30-57) |
Dexamethasone (15 mg) for 7 days and prednisolone (60 mg) for another 7 days. | All patients recovered in 3 weeks, but two cases had side-effects. | |
8 (age 23-39) |
Mebendazole (200 mg), dexamethasone and prednisolone for 4-11 days. | All patients recovered. | |
41 (age ≥15) |
Mebendazole (10 mg/kg) and prednisolone (60 mg) for 14 days. | Median duration of headache was 3 days; 8% had headache; no serious side-effects. | |
34 (age ≥15) |
Albendazole (15 mg/kg). | Mean duration of headache was 8.9 days; 21% of headaches persisted; no serious side-effects. | |
32 (age ≥15) |
Placebo. | Mean duration of headache was 16.2 days. | |
26 (age ≥15) |
Albendazole (15 mg/kg) and prednisolone (60 mg) for 14 days. | Median duration of headache was 4 days; 12% had headache; no serious side-effects. | |
32 (age ≥15) |
Placebo. | Mean duration of headache was 8.9 days; 41% had persistent headache; no serious side-effects. | |
55 (age ≥15) |
Albendazole (15 mg/kg) for 14 days. | Mean duration of headache was 5 days, 9% of cases had persistent headache; no serious adverse effects. | |
55 (age 15) |
No drug treatment. | Mean duration of headache was 13 days; 45% cases had persistent headache. | |
284* |
Analgesic for 14 days. | 35% patients had headache relief. | |
96* |
Analgesic and prednisone (30-60 mg) for 14 days. | 26% patients had headache relief. | |
56* |
Penicillin (3.0-4.5 g) or tetracycline (2 g) for 14 days. | 34% of patients had headache relief. | |
9 (age 21-28) |
Analgesic with or without non-steroidal anti-inflammatory agents. | All patients recovered, 67% had headache for at least 4 weeks. | |
3 (age 21-28) |
Corticosteroid. | Symptoms were markedly improved. | |
| *The ages of these patients were not defined in the study. However, 82% of the patients were older than 20 years. | |||
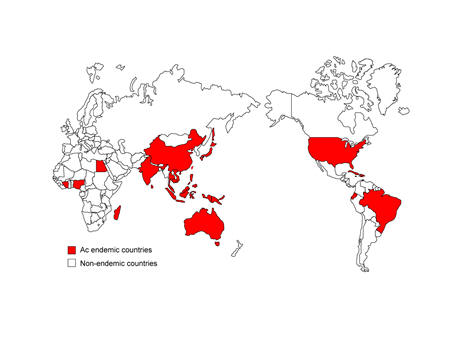
Figure 1. The life cycle of Angiostrongylus cantonensis.
Rats, as definitive hosts, acquire A. cantonensis when the third-stage infective larvae are ingested. The larvae enter the bloodstream and reach the central nervous system (CNS), where they undergo two molts to become adult worms in two weeks. The adult worms migrate to the pulmonary arteries and develop to sexual maturity and lay eggs (A). An adult female nematode produces ~15,000 eggs daily. Eggs are carried to the capillaries and break into the air spaces where they hatch. The first-stage larvae (the juveniles) migrate up the trachea and are swallowed, and are excreted out with the feces (B). Approximately six to eight weeks after infection, the rat excretes the first-stage larvae. The larvae in feces are swallowed by intermediate host molluscs (snails or slugs) and develop into third-stage (infective) larvae in 12 days (C). The third-stage larvae could be transmitted to the paratenic hosts such as shrimps, land crabs and predacious land planarians (D) or contaminate vegetables (E). Humans occasionally acquire A. cantonensis when they eat snails, slugs and sometimes, land crabs, frogs, freshwater shrimps, containing infective larvae, or contaminated vegetables. The larvae are digested from tissues and enter bloodstream in intestine (F). The larvae finally reach central nervous system (CNS) and cause eosinophilic meningitis (G) or move to eye chamber and cause ocular angiostrongyliasis.

Figure 2. Global endemic regions of A. cantonensis
What's New
Park SY. Angiostrongylus cantonensis: Epidemiology in the Continental United States and Hawai'i. Hawaii J Med Public Health 2013;72(6 Suppl 2):34.
Ramirez-Avila L et al. Eosinophilic Meningitis due to Angiostrongylus and Gnathostoma Species.Clin Infect Dis. 2009; 48:322–327.
Lv S, Zhang Y, et al. Emerging Angiostrongyliasis in Mainland China. Emerg Infect Dis 2008;14:161-164.
Guided Medline Search For:
Reviews
Diao Z, et al. International Symposium on Angiostrongylus and Angiostrongyliasis, 2010. Emerg Infect Dis 2011:17 (7):e1.

