SARS (Severe Acute Respiratory Syndrome)
Authors: David SC Hui, MBBS, M.D., FRACP, FRCP, FCCP, FHKCP, FHKAM, Joseph JY Sung, MBBS, M.D., Ph.D., FRCP, FRCPE, FRACP, FACG, FHKCP, FHKAM
GENERAL DESCRIPTION
Severe acute respiratory syndrome (SARS) is a newly emerging infectious disease in the 21st century which has posed an enormous threat to international health. During the global outbreak in 2003, the most severely affected countries were mainland China (85), Hong Kong (47), Taiwan (82), Vietnam, Canada (4), and Singapore (37). By the end of the epidemic in July 2003, 8098 probable cases were reported in 29 countries and regions with a death toll of 774 (9.6%) (85). SARS has caused considerably adverse economic impact in most of the severely affected areas as a public health threat in addition to disruption of travel and business around the world, and generation of public anxiety.
Virology
SARS is caused by a novel coronavirus (SARS-CoV) (22,41,42,61), and the genomic sequence is not closely related to any of the previously characterized human or animal coronaviruses (53,66,67). It is likely that SARS-CoV originated from wild animal reservoir in mainland China because masked palm civets (Paguma larvata) and the raccoon dog (Nyctereutes procyonoides) had a CoV almost identical to that in SARS patients. There was also a much higher sero-prevalence of SARS-CoV among wild animal handlers than controls in Guangdong (31,77). In addition, some early SARS cases were associated with animal markets (102). Interestingly, a retrospective study has detected antibodies to a SARS-CoV and/or animal SARS-CoV-like virus in 17 (1.8%) of 938 healthy persons from their serum samples collected in May 2001 in Hong Kong (105). Thus more research is needed before any definite conclusions can be drawn regarding the role of zoonotic transmission of the virus.
Epidemiology
The early cases of SARS originated from Guangdong province in southern China. In November 2002,there was an unusual epidemic of severe pneumonia of unknown etiology in Foshan, with a high rate of transmission to healthcare workers (104). A retrospective analysis of 55 patients admitted to a chest hospital with atypical pneumonia in Guangzhou between January 24 and February 18, 2003 showed positive SARS-CoV in the nasopharyngeal aspirates (NPA) whereas 48 (87%) patients had positive antibodies to SARS-CoV in their convalescent sera. Genetic analysis showed that the SARS-CoV isolates from Guangzhou shared the same origin with those in other countries, with a phylogenetic pathway that matched the spread of SARS to other parts of the world (106).
A 64-year old nephrologist from southern China, who visited Hong Kong on February 21 and died on March 4, 2003 of severe pneumonia, is believed to have been the source of infection causing subsequent outbreaks of SARS in Hong Kong (47,78), Vietnam, Singapore (37), Canada (4), and elsewhere. At least 16 hotel guests and visitors had been infected while they were either visiting friends or staying on the same floor of Hotel M, where the nephrologist was staying in Hong Kong. The nephrologist was subsequently proven to have positive RT PCR on retrospective virological analysis of both the NPA taken before death and the post mortem lung, in addition to a 4-fold rise in antibody titre against SARS-CoV (57). As a result of the relatively long incubation period of up to 10-14 days in some cases, SARS spread rapidly and globally by international travelers to their home cities without any symptoms prior to their arrival.
SARS appears to spread by close person-to-person contact via droplet transmission or fomite (5). The high infectivity of this viral illness is highlighted by the fact that 138 patients (mostly healthcare workers) were hospitalized with SARS within 2 weeks as a result of exposure to one single patient on a general medical ward at the Prince of Wales Hospital in Hong Kong (47). The use of a jet nebulizer for administering bronchodilator for its muco-ciliary clearance effects to this patient, who had presented clinically with community acquired pneumonia, could increase the viral droplet load around the patient and, together with overcrowding condition on the hospital ward and poor ventilation, had contributed to this major hospital outbreak (47,97).
The main community outbreak of SARS in Hong Kong occurred in late March at the Amoy Gardens, a private residential estate, where 329 residents were infected with 42 deaths. In retrospect, the index case of this major outbreak had suffered from both influenza A and SARS with an unusual early phase of SARS, with almost complete resolution of right lower lobe pneumonia before progression to acute respiratory distress syndrome (ARDS) (43). There are several hypotheses for this super-spreading event including passive carriage of virus by pests, drying up of bathroom U shaped floor drain, and fecal-oral viral loading through contaminated surfaces as a result of the chimney effects created by the use of exhaust fans in the presence of blockage of the contaminated sewage system (48,56).
SARS was brought to Hanoi by a Chinese-American businessman who had stayed on the same floor of Hotel M. SARS was first identified in Vietnam on February 28, 2003 by Dr Carlo Urbani, a WHO epidemiologist, who died of the disease later in Thailand (86). There were 63 cases of SARS in Hanoi and it was removed from the list of areas with local transmission on April 28, 2003.
Three guests from Singapore were infected with SARS while staying at Hotel M on February 20-21, 2003. The outbreak in Singapore was characterized by nosocomial transmission involving healthcare workers, and then spread to the community from a SARS patient to two taxi drivers and the patient’s co-workers in a wholesale market (6). There were 97 (41%) healthcare workers infected out of 238 probable SARS cases. Following prompt and decisive actions by the Singaporean health authorities in implementing contact tracing, isolation, and quarantine measures, the spread of the disease was limited, with the last case being reported on May 5, 2003.
The index case of SARS in Canada was an elderly woman who returned to Toronto on February 23, 2003 after a visit to Hong Kong and exposed to SARS during her stay on the same floor of Hotel M. She became ill after returning to Toronto and infected her family members. One of her family members was admitted to a community hospital in Toronto and resulted in a large nosocomial outbreak (83). Transmission to other persons resulted subsequently in an outbreak among 257 persons in several hospitals. On May 14, 2003, WHO removed Toronto from the list of areas with recent local SARS transmission. Unfortunately, following premature relaxation of strict infection control measures such as monitoring of fever and respiratory symptoms in hospitalized patients and visitors, there was a second wave of SARS cases among patients, visitors, and healthcare workers that occurred at a Toronto hospital approximately 4 weeks after SARS transmission was thought to have been interrupted (7). Toronto was finally declared free from local transmission on July 2, 2003.
The first case of SARS in Taiwanoccurred in a businessman who had traveled to Guangdong on February 5, 2003, and returned to Taipei via Hong Kong on February 21, 2003. He developed febrile illness on February 25, 2003 but was not hospitalized until March 8, 2003. For the first 6 weeks of the SARS outbreak, recognized spread was limited to one healthcare worker and 3 household contacts (82). There was however a late but rapid outbreak of SARS in Taiwan from mid April 2003, and appeared to be related to the visit of a resident of Amoy Gardens to Taiwan on March 26, 2003. Subsequent molecular data analysis has demonstrated that the same strain of the SARS-CoV was involved in the Amoy Gardens outbreak and the late outbreak in Taiwan (15). On July 5, 2003, WHO announced that the last known chain of human-to- human transmission of the SARS-CoV had been broken in Taiwan, and this brought an end to the initial outbreak of SARS that had begun in mid November, 2002 in southern China and spread internationally in late February, 2003 (87). The statistics of SARS in different parts of the world are shown in Table 1.
Clinical Manifestations
The incubation period of SARS is generally between 2-10 days though it has been estimated as 6.4 days (95% CI 5.2-7.7) with a mathematical model. The mean time from onset of clinical symptoms to hospital admission varied between 3 to 5 days (21). The major clinical features on presentation include persistent fever, chills/rigor, myalgia, malaise, dry cough, headache and dyspnea. Less common symptoms include sputum production, sore throat, rhinorrhea, nausea and vomiting, and diarrhea (4,37,47,82). Nevertheless, these clinical symptoms are rather non-specific and may mimic influenza or atypical pneumonia of other causes such as mycoplasma, chlamydia, and legionella.
Watery diarrhea, together with recurrence of fever, was reported in 73% of patients one week down the clinical course in the Amoy Gardens outbreak linked to a faulty sewage system, presumably due to involvement of the gastrointestinal tract via the fecal oral route (62). The diarrhea was described as watery in large volume but contained no blood or mucus. The maximum frequency of diarrhea was 6 ± 4 times daily and the duration lasted for 3.9 ± 2.3 days. The frequency of clinical features on presentation from several case series is summarized in Table 2.
SARS-CoV has been detected in the cerebrospinal fluid and serum samples of two patients who developed status epilepticus (38,46). The data suggest that a severe acute neurologic syndrome might occasionally accompany SARS.
Older subjects may present with decrease in general well-being, poor feeding, fall/ fracture (93), and in some cases, delirium, without the typical febrile response (temperature >38C) (23,93). A sero-prevalence study has shown that subclinical or non-pneumonic SARS- CoV infection is possible and this was reflected by 3 of 400 healthy blood donors who donated during the SARS outbreak and 1 of 131 non-pneumonic pediatric inpatients having positive IgG antibodies, confirmed by western-blot assays (total 0.48% of the study population) (100).
The radiographic appearances of SARS share common features with other causes of pneumonia. About 20 to 25% of patients with SARS may have normal chest radiographs on presentation (4,47,78,94), whereas high resolution CT of thorax is useful in detecting parenchymal opacities early (95). Abnormal lung opacities occupy a peripheral or mixed peripheral and axial location in 88% of patients (94). The predominant involvement of lung periphery and the lower zone, in addition to the absence of cavitation, hilar lymphadenopathy or pleural effusion, are the more distinctive radiographic features of SARS (47,94). Radiographic progression from unilateral focal air-space opacity to either multi-focal or bilateral involvement during the second phase of the disease, followed by radiographic improvement with treatment, is commonly observed (Figure 1) (47,94). In a case series, 12% of patients developed spontaneous pneumo-mediastinum and 20% of patients developed evidence of ARDS over a period of 3 weeks (62). In general, the incidence of barotrauma (26%) in ICU admissions was higher than expected despite low volume and low pressure mechanical ventilation (28).
High resolution CT of thorax is useful in detecting lung opacities in cases with unremarkable chest radiographs. Common findings include ground-glass opacification, sometimes with consolidation, and interlobular septal and intralobular interstitial thickening, with predominantly a peripheral and lower lobe involvement (Figure 2). The characteristic peripheral alveolar opacities bear close resemblance to those found in bronchiolitis obliterans organizing pneumonia (BOOP) (47,95). The CT features of late-stage ARDS are similar to those seen in late-stage ARDS of other causes but severe SARS-induced ARDS may independently result in cyst formation (40).
Lymphopenia (destruction of both CD4 and CD8 lymphocytes), low grade disseminated intravascular coagulation (thrombocytopenia, prolonged activated partial thromboplastin time, elevated D-Dimer), elevated lactate dehydrogenase (LDH), alanine transminases (ALT), and creatinine kinase (CPK) are common laboratory features of SARS (4,47,62,78,82). Absolute lymphopenia occurs in 98% of cases of SARS during the clinical course of the disease. The CD4 and CD8 T lymphocyte counts fall early in the course of SARS, whereas low counts of CD4 and CD8 at presentation are associated with adverse clinical outcome (96).
There was also a correlation between the decrease in left ventricular ejection fraction with adverse prognostic factors such as LDH and CPK levels (50). Although the pathogenic mechanism(s) for the cardiac disturbances is unknown, it is possible that that largely reversible subclinical diastolic impairment occurs in acute SARS (50).
Mildly elevated aminotransferase levels are reported in 23–50% of SARS patients, although the clinico-pathological significance is largely unknown (47,78). Longitudinal evaluation of liver function was reported on 54 adult SARS patients. Liver dysfunction was found in 29.6% and 75.9% of patients on presentation (before ribavirin treatment) and during treatment for SARS with ribavirin and corticosteroid (99). Time to maximal radiographic damage, evaluated in the form of an overall aggregate score, correlated with time to peak ALT thus suggesting a common pathogenic mechanism(s), namely immune-mediation for disruption to the lung and liver (58).
The poor prognostic factors associated with a poor outcome (ICU admission or death) in SARS include advanced age (10,21,47,62,81), chronic hepatitis B treated with lamivudine (62), high initial LDH (81), high peak LDH (47), high neutrophil count on presentation (47,81), diabetes mellitus or other co-morbid conditions (4, 10), and low counts of CD4 and CD8 at presentation (96).
In general, young children (< 12 years of age) often run a more benign and shorter clinical course whereas teenage patients tend to have a more protracted and severe course and often present with severe constitutional features, including headache, myalgia, chills and rigors and lower respiratory tract signs, resembling those of adult SARS patients (36,47). However, pediatric patients seldom progress to ARDS. There are no fatalities in young children and teenage patients (3,16,36,70). In addition, none of the preterm or term infants born to pregnant women with SARS are found to be clinically infected or shedding the virus after birth, and all of them follow a clinical course typical of infants with similar gestations (69).
The WHO has revised the case definitions in the post outbreak period with inclusion of radiographic and laboratory findings for public health purposes (Table 3) (88). The CDC case definitions of SARS are based on clinical, epidemiologic, and laboratory criteria (Table 4) (8).The case definitions and exclusion criteria have been revised to allow exclusion of cases with a convalescent phase serum sample, collected > 28 days after symptom onset, that is negative for antibody to SARS CoV.
Laboratory Diagnosis
The detection rates for SARS CoV using conventional reverse transcriptase polymerase chain reaction (RT-PCR) are generally low in the first week of illness. The positivity rates on urine, NPA, and stool specimen have been reported to be 42%, 68% and 97% respectively on day 14 of illness whereas serology for confirmation may take 28 days to reach a detection rate above 90% (62). By optimizing RNA extraction methods and applying quantitative real-time RT-PCR techniques, the sensitivity of NPA specimens for early diagnosis of SARS can be enhanced to 80% for the first 3 days (64). Quantitative measurement of blood SARS-CoV RNA with real-time RT-PCR technique has been developed with a detection rate of 80% as early as day 1 of hospital admission but the detection rates drop to 75% and 42% on day 7 and day 14 respectively (Table 5) (30,54,55).
Pathogenesis
The pathogenesis of SARS is still poorly understood. Respiratory failure is the major complication in SARS. The clinical course of SARS appears to follow a typical pattern (62): Phase 1 (viral replication) is associated with increasing viral load and clinically characterized by fever, myalgia, and other systemic symptoms that generally improve after a few days. Phase 2 (immunopathological reaction) is characterized by recurrence of fever, oxygen desaturation, and radiological progression of pneumonia with falls in viral load. At this stage, some patients may recover spontaneously from the illness. However, hypoxemia occurs in about 50% of patients. About 20-36% of patients reaching this stage require ICU admission and 13-26% may progress into ARDS necessitating invasive ventilatory support (10,47,62,81).Based on the study by Peiris et al (62), the timing of the IgG seroconversion, which seems to start on day 10, seems to correlate with falls in viral load, which occurs from between day 10 and 15, despite the use of pulse methylprednisolone. Peiris et al (62) have shown very clearly progressive decrease in rates of viral shedding from nasophargynx, stool, and urine from day 10 to day 21 after symptom onset in the 20 patients who had serial measurements with RT-PCR. Thus clinical worsening during phase 2 cannot be explained by uncontrolled viral replication and is most likely the result of immune-mediated lung injury due to an over-exuberant host response (62).
Pulmonary histopathology in SARS cases has revealed changes of diffuse alveolar damage (DAD) without necrosis, hyaline membranes, macrophage infiltration, hemophagocytosis consistent with excess cytokine effects, cytomegaly of alveolar pneumocytes and giant cell changes (41,47,57). The pulmonary histopathology may vary with the duration of illness. For the cases of less than 10 days in duration, acute-phase DAD, airspace edema, and bronchiolar fibrin are seen whereas for cases of more than 10 days duration the lung tissue exhibited organizing-phase DAD, type II pneumocyte hyperplasia, squamous metaplasis, multinucleated giant cells, and acute bronchopneumonia (26). Although the pulmonary pathological features were dominated by DAD (26,41,47,57), BOOP-like lesions in subpleural locations were also seen (80). SARS-CoV infection of cynomologus macaques has shown early infection of type I pneumocytes that is followed by their loss and type II pneumocyte hyperplasia later in infection (25,32,42).
It has been reported that patients who were positive in NPA by qualitative RT-PCR at the time of admission were significantly more likely to have dyspnea (23% vs 8%), higher LDH (mean 287 vs 208 IU/L), and greater risk for subsequent ICU care (30% vs 10%), mechanical ventilation (24% vs 8%), and death (13% vs 3%) compared to those negative for SARS-CoV RNA (79). It is possible that higher viral levels in the upper respiratory tract may predict higher lower respiratory viral loads and/or lung damage.
Diarrhea is a marked symptom one week down the clinical course in SARS (49,62). Intestinal biopsy specimens taken by colonoscopy or autopsy revealed minimal architectural disruption but there was evidence of active viral replication within both the small and large intestines. SARS- CoV was isolated by culture from these specimens whereas SARS-CoV RNA was detected for up to 10 weeks after symptom onset (49). A higher mean SARS-CoV load in NPA obtained on day 10 after symptom onset was significantly associated with the occurrence of diarrhea (3.1 log 10 vs 1.8 log10 copies/ml; p=0.01) and mortality (6.2 vs 1.7 log10copies/ml; p<0.01) although diarrhea was not associated with mortality (13). Further investigation of the role of SARS-CoV in the pathogenesis of diarrhea is needed.
It has been postulated that “cytokine storm” may play a key role in SARS (57,63) and in other severe viral pneumonias, such as avian influenza A/H5N1 infection (65). In a study of the role of T helper (Th) cell cytokines, imflammatory cytokines and chemokines in 20 adult SARS patients, we have noted marked elevation of Th1 cytokine IFN-g, inflammatory cytokines IL-1, IL-6 and IL-12 for at least 2 weeks after disease onset (92). The chemokine profile also showed significant elevation of neutrophil chemokine IL-8, monocyte chemoattractant protein-1 (MCP-1), and Th1 chemokine IFN-g-inducible protein-10 (IP-10). Interestingly, there was no significant elevation of inflammatory cytokine tumor necrosis factor TNF-a, anti-inflammatory cytokine IL-10, Th1 cytokine IL-2 and Th2 cytokine IL-4. Together, these data provide evidence for activation of Th1 cell-mediated immunity and hyperinnate inflammatory response in SARS that may occur through the accumulation of monocytes/macrophages and neutrophils in the lungs (92).
SUSCEPTIBILITY IN VITRO AND IN VIVO
Ribavirin, a nucleoside analogue that has activity against a number of viruses in-vitro, was widely used in the treatment of SARS in 2003 (4,10,33,37,47,62,81). Nevertheless, ribavirin has no significant in-vitro activity against SARS-CoV (19,73,76).
Genomic analysis of the SARS-CoV has revealed several types of enzymatic targets including the proteases (2,53,66). Chu et al (18) have demonstrated in-vitro activity against SARS-CoV for lopinavir and ribavirin at 4 ug/ml at 50 ug/ml respectively after 48 hours of incubation. Cytopathic inhibition was achieved down to a concentration of lopinavir 1 ug/ml combined with 6.25 ug/ml of ribavirin suggesting that this combination might be synergistic against SARS-CoV in vivo(18).
Type I IFN’s such as IFN-a are produced early as part of the innate immune response to virus infections. Type I IFN’s inhibit a wide range of RNA and DNA viruses (1) including SARS CoV in vitro (20,73,76). Complete inhibition of cytopathic effects of SARS-CoV in culture was observed for IFN subtypes, b-1b, a-n1, a-n3, and human leukocyte IFN-a (76). IFN-a showed an in vitro inhibitory effect on SARS-CoV starting at concentrations of 1000 IU/mL (73). In experimentally infected cynomolgus macaques with SARS-CoV, prophylactic treatment with pegylated IFN-a significantly reduces viral replication and excretion, viral antigen expression by type 1 pneumocytes and pulmonary damage, compared with untreated macaques, whereas post-exposure treatment with pegylated IFN-a yielded intermediate results (32).
There is evidence that SARS-CoV infection is initiated through binding of S1 protein to the angiotensin-converting enzyme 2 (ACE2) receptor (51). A high-affinity human monoclonal antibody (huMab) has been identified against the SARS-CoV S1 protein termed 80R that has potent neutralizing activity in vitro and in vivo(74). HuMab 80R efficiently neutralizes SARS-CoV and inhibits syncytia formation between cells expressing the S protein and those expressing the SARS-CoV receptor ACE2. HuMab 80R may be a useful viral entry inhibitor for the emergency prophylaxis and treatment of SARS (74).
Glycyrrhizin, an active component of liquorice roots, was active in inhibiting SARS- CoV in vitro but there are no data in vivo (19).
ANTIVIRAL THERAPY
Due to limited knowledge of this newly emerged disease, empirical treatment was prescribed during the outbreak in 2003. Two groups of pharmacologic agents have been used 1. anti-viral agents, and 2. immunomodulators. Ventilatory support including non-invasive positive airway pressure ventilation (NPPV) has been applied. In retrospect, none of these therapies have proven therapeutic benefit. Apart from supportive care, the appropriate treatment for SARS is unknown at present. The difficulty in devising therapy stems from the fact that up to now, no prospective randomized, placebo-controlled study of any intervention has been reported.
After 2 weeks of ribavirin treatment (1.2g tds orally), 59% of our patients experienced a fall in hemoglobin of more than 2 g/dL from baseline whereas evidence of hemolytic anemia was documented in 36% (75). The use of ribavirin for SARS in Toronto, based on a higher dosage for treating haemorrhagic fever virus, was associated with more toxicity, including elevated transaminases and bradycardia (4).
Two retrospective matched cohort studies have compared the clinical outcome of patients who received Kaletra (lopinavir 400 mg/ritonavir 100 mg) in addition to ribavirin either as initial therapy within 5 days of onset of symptoms or as rescue therapy after pulse methylprednisolone treatment for worsening respiratory symptoms versus historical controls who received ribavirin alone as initial anti-viral therapy (11,18). The addition of lopinavir/ritonavir as initial therapy was associated with reduced overall death rate (2.3%) and intubation rate (0%), when compared with a matched cohort that received standard treatment (15.6% and 11%) respectively (11). Other beneficial effects included a reduction in methylprednisolone use, less nosocomial infections, a decreasing viral load and rising peripheral lymphocyte count (18). However, the subgroup that had received lopinavir/ritonavir as rescue therapy was no better than the matched cohort, and received a higher mean dose of methylprednisolone (11). The improved clinical outcome in patients that received lopinavir/ritonavir as part of the initial therapy may be due to the fact that both peak (9.6 ug/ml) and trough (5.5 ug/ml) serum concentrations of lopinavir could inhibit the virus (39).
In an uncontrolled study in Toronto, use of IFN alfacon-1 plus corticosteroids was associated with improved oxygen saturation, more rapid resolution of radiographic lung opacities and lower levels of CPK in patients with SARS (52). These findings support clinical testing of approved IFN’s for the treatment of SARS.
Clinical studies using various herbal formulae had been tested in China during the epidemic in 2003. Results suggested a modest immuno-modulation effect of herbal medicine but randomized placebo-controlled trial data are lacking.
ADJUNCTIVE THERAPY
Non-Invasive Positive Pressure Ventilation (NPPV)
During the SARS outbreak in 2003, treatment of severe respiratory failure has incurred a heavy demand on ICU support and resources. Anecdotal reports have indicated that NPPV was effective in SARS patients with respiratory failure (71,107). Cheung et al (14) have reported the efficacy and safety of NPPV in the treatment of acute respiratory failure in SARS. NPPV was applied via face masks to 20 patients who developed severe acute hypoxemic respiratory failure without pre-existing chronic obstructive pulmonary disease in a hospital environment with adequate airflow, full personal protective equipment, and addition of a viral-bacterial filter to the exhalation port of the NPPV device. Endotracheal intubation was avoided in 14 (70%) patients, who had a much shorter length of stay in ICU than those intubated. None of the 105 healthcare workers involved in the management of the 20 patients had developed clinical evidence of SARS whereas 102 (97%) had negative SARS serology (14). As there were still 3 healthcare workers who had refused SARS serology testing, one cannot entirely eliminate the possibility of subclinical SARS infection related to use of NPPV although it seemed highly unlikely. Nevertheless, NPPV should only be applied provided there is adequate protection for the healthcare workers (i.e., adequate air exchange, contact and droplet precaution plus full personal protective equipment) because of the potential risk of viral transmission via mask leakage and flow compensation causing dispersion of contaminated aerosol. Addition of a viral-bacterial filter to the exhalation port of NPPV (14) or oxygen mask (72) may reduce risk of nosocomial transmission of SARS.
Systemic Corticosteroids
During phase 2 of SARS when there is progression of pneumonia and hypoxemia, intravenous pulse methylprednisolone has been given to prevent immunopathological lung injury (10,47,62,71,75,81,107) on the rationale that progression of the pulmonary disease may be mediated by the host inflammatory response (62). The use of pulse methylprednisolone during clinical progression was associated with favorable clinical improvement with resolution of fever and lung opacities within 2 weeks (47,75). Corticosteroids have been used because CT thorax has revealed radiological features of BOOP (47,78,95), which is a steroid-responsive condition and suggestive of an immunological phenomenon. BOOP-like lesions in subpleural locations were indeed noted histologically (80). The use of high-dose pulse methylprednisolone therapy aims to suppress the cytokine-induced lung injury in phase 2 (33,47,62,75). Corticosteroids significantly reduced IL-8, MCP-1, and IP-10 concentrations from 5 to 8 days after treatment in 20 adult SARS patients (92). In addition, in patients with fatal SARS, macrophages are the prominent leucocytes in the alveoli with evidence of haemophagocytosis in the lungs (57). Haemophagocytosis has been attributed to cytokine dysregulation (24), and intervention with steroids might modulate this cytokine response and prevent a fatal outcome, as has been proposed for other causes of ARDS (44). However, a retrospective analysis showed that the use of pulsed corticosteroids was associated with increased risk of 30-day mortality (adjusted OR 26.0, 95% CI 4.4 to 154.8) but the study cannot establish whether a causal relationship exists between use and increased risk of death (79). Furthermore, prolonged corticosteroid therapy could increase the risk of complications such as disseminated fungal disease (84) and avascular necrosis of bones. More research is needed to determine the role of systemic steroid in the treatment of immune-mediated lung injury in SARS.
Convalescent Plasma
Convalescent plasma, donated by patients who had recovered from SARS, contains neutralizing antibody which may be clinically useful for treating other SARS patients (60,98). Research work in the preparation of SARS-CoV specific hyperimmune globulin from convalescent plasma donated by patients recovered from SARS is currently in progress.
Herbal Medicines
Herbal medicine has been used extensively in Mainland China. However, no controlled trials using placebo versus herbal therapy have been conducted.
ENDPOINTS FOR MONITORING THERAPY
As persistent fever is the major symptom whereas respiratory failure is the major complication of SARS, treatment of SARS should aim at achieving defervescence, resolution of lung consolidation and oxygen independence (75). Serial chest radiographs should be monitored regularly in addition to clinical observation (94). As several markers such as LDH (47,81), and lymphocyte subsets (96) have been reported to have prognostic implications, these are useful parameters to monitor progress. Apart from age, serum LD1 isoenzyme appears to be the best prognostic indicator for predicting death in patients with SARS compared with serum total LDH activity and blood counts (12). In addition, serum quantification of SARS-CoV RNA levels represents a useful tool not only for early diagnosis but is also important for prognostic purpose (54).
VACCINES
SARS-CoV is an enveloped RNA virus which contains several structural proteins. The spike (S) protein of SARS-CoV plays a central role in mediating viral infection via receptor binding and membrane fusion between the virion and the host cell. Currently, different vaccines such as whole killed vaccine, adenovirus vector vaccine, and recombinant spike protein vaccine are being tested. An adenoviral-based vaccine can induce strong SARS-CoV specific immune responses in rhesus macaques, and hold promise for development of a protective vaccine against SARS-CoV (27). Other research groups have reported the S gene DNA candidate vaccine could induce the production of specific IgG antibody against SARS-CoV efficiently in mice with seroconversion ratio of 75% after 3 doses of immunization (103), whereas viral replication was reduced by more than 6 orders of magnitude in the lungs of mice vaccinated with S plasmid DNA expression vectors, and protection was mediated by a humoral immune mechanism (101). Recombinant S protein exhibited the antigenicity and receptor-binding ability (35) whereas synthetic peptides for developing antibodies against SARS-CoV S protein could provide another approach for further developing SARS vaccine (17).
PREVENTION
General Preventive Measures
As the primary mode of transmission of SARS is through direct or indirect contact with infectious respiratory droplets or fomites, it is important to maintain good personal and environmental hygiene, and implement strict standard, contact, and droplet precautions among the healthcare workers (9). Prevention of spread is most important for this highly infectious disease. Public education, contact tracing, quarantine isolation for close contacts, and surveillance at border crossings including monitoring of travelers for fever are important measures to prevent community transmission (63).
Hospital Infection Control Measures
Nosocomial infection involving healthcare workers was common in SARS as viral loads increased to peak levels on day 10 from disease onset (62). It is important to designate separate wards for triage of patients, confirmed SARS cases, and step-down of patients in whom SARS has been ruled out (34). If a nosocomial outbreak is detected late, a hospital may need to be closed in order to contain spread of the disease whereas outbreaks detected early can be managed by either removing all exposed persons to a designated location or isolating them in place (29). Early case detection and isolation preferably in negative pressure room facilities, and strict droplet precaution (hand hygiene, gown, gloves, N95 masks, eye protection) among healthcare workers managing SARS patients are important measures (9). Practice of droplets precaution and contact precaution is adequate in significantly reducing the risk of infection after exposure to patients with SARS (68). Perceived inadequacy of personal protective equipment supply, infection control training <2 hrs, and inconsistent use of personal protective equipment when in contact with SARS patients are significant independent risk factors for SARS infection (45). Aerosol-generation procedures such as the use of nebulizer on general ward (47,97) should be avoided.
CONTROVERSIES, CAVEATS, or COMMENTS
The major outbreak of SARS in 2003 has created an adverse impact on health systems, tourism, business and the global economy. Although no major outbreak or secondary spread has occurred despite the re-emergence of SARS involving laboratory personnel in Singapore (89) and Taiwan (90), and more recently in 4 residents in Guangdong (59,91), the whole world is still vulnerable to another epidemic if there is any breach of laboratory biosafety guidelines or bioterrorism. Currently the appropriate treatment for different clinical stages of SARS remains uncertain. It is hoped that knowledge of the genome sequence of the SARS-CoV will facilitate efforts to develop reliable and rapid diagnostic tests, antiviral agents and effective vaccines in the long run. Randomized placebo-controlled trials of different treatment modalities must be put in place in preparation for return of this highly infectious disease.
REFERENCES
1. Ahmed R, Biron C. Immunity to viruses. In: Paul WE, editor. Fundamental Immunology. Philadelphia: Lippincott-Raven, 1999: 1295.
2. Anand K, Ziebuhr J, Wadhwani P, Mesters JR, Hilgenfeld R. Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science 2003;300:1763-1767. [PubMed]
3. Bitnun A, Allen U, Heurter H, King SM, Opavsky MA, Ford-Jones EL, Matlow A, Kitai I, Tellier R, Richardson S, Manson D, Babyn P, Read S; Other Members of the Hospital for Sick Children SARS Investigation Team. Children hospitalized with severe acute respiratory syndrome-related illness in Toronto. Pediatrics 2003;112:e261-268. [PubMed]
4. Booth CM, Matukas LM, Tomlinson GA, Rachlis AR, Rose DB, Dwosh HA, Walmsley SL, Mazzulli T, Avendano M, Derkach P, Ephtimios IE, Kitai I, Mederski BD, Shadowitz SB, Gold WL, Hawryluck LA, Rea E, Chenkin JS, Cescon DW, Poutanen SM, Detsky AS. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA 2003:289:2801-2809. [PubMed]
5. CDC. Severe acute respiratory syndrome. Fact sheet. Basic information about SARS, May 8, 2003): http://www.cdc.gov/ncidod/sars/factsheet.htm.
6. CDC. Severe acute respiratory syndrome- Singapore, 2003. MMWR 2003;52:405-411. [PubMed]
7. CDC. Severe acute respiratory syndrome- Toronto, Canada, 2003. MMWR 2003;52:547-550. [PubMed]
8. CDC. Public health guidance for community-level preparedness and response to severe acute respiratory syndrome (SARS) version 2. Supplement B: SARS surveillance. Appendix B1: Revised CSTE SARS surveillance case definition. Available from: http://www.cdc.gov/ncidod/sars/guidance/B/pdf/b.pdf.
9. CDC. SARS Supplement I: Infection control in healthcare, home and community settings. Available at http://www.cdc.gov/ncidod/sars/guidance/I/pdf/healthcare.pdf.
10. Chan JW, Ng CK, Chan YH, Mok TY, Lee S, Chu SY, Law WL, Lee MP, Li PC. Short term outcome and risk factors for adverse clinical outcomes in adults with Severe acute respiratory syndrome (SARS). Thorax 2003;58:686-689. [PubMed]
11. Chan KS, Lai ST, Chu CM, Tsui E, Tam CY, Wong MML, Tse MW, Que TL, Peiris JS, Sung J, Wong VC, and Yuen KY. Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicenter retrospective matched cohort study. Hong Kong Med J 2003;9:399-406. [PubMed]
12. Chan MH, Wong VW, Wong CK, Chan PK, Chu CM, Hui DS, Suen MW, Sung JJ, Chung SS, Lam CW. Serum LD1 isoenzyme and blood lymphocyte subsets as prognostic indicators for severe acute respiratory syndrome. J Intern Med 2004;255:512-518. [PubMed]
13. Cheng VC, Hung IF, Tang BS, Chu CM, Wong MM, Chen KH, Wu AK, Tse DM, Chan KS, Zheng BJ, Peiris JS, Sung JJ and Yuen KY. Viral replication in the nasopharynx is associated with diarrhea in patients with severe acute respiratory syndrome. Clin Inf Dis 2004;38:467-475. [PubMed]
14. Cheung TM, Yam LY, So LK, Lau AC, Poon E, Kong BM, Yung RW. Effectiveness of non-invasive positive pressure ventilation in the treatment of acute respiratory failure in severe acute respiratory syndrome. Chest 2004;126(3):845-50. [PubMed]
15. Chiu RW, Chim SS, Lo YM. Molecular epidemiology of SARS-from Amoy Gardens to Taiwan. N Engl J Med 2003;349:1875-1876. [PubMed]
16. Chiu WK, Cheung PCH, Ng KL, Ip PL, Sugunan VK, Luk DC, Ma LC, Chan BH, Lo KL, Lai WM. Severe acute respiratory syndrome in children: Experience in a regional hospital in Hong Kong. Pediatr Crit Care Med 2003;4:279-283. [PubMed]
17. Choy WY, Lin SG, Chan PK, Tam JS, Lo DY, Chu IM, Tsai SN, Zhong MQ, Fung KP, Waye MM, Tsui SK, Ng KO, Shan ZX, Yang M, Wu YL, Lin ZY, Ngai SM. Synthetic peptide studies on the severe acute respiratory syndrome (SARS) coronavirus spike glycoprotein: perspective for SARS vaccine development. Clin Chem 2004;50(6):1036-42. [PubMed]
18. Chu CM, Cheng VC, Hung IF, Wong MM, Chan KH, Chan KS, Kao RY, Poon LL, Wong CL, Guan Y, Peiris JS, Yuen KY; HKU/UCH SARS Study Group. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax 2004;59:252-256. [PubMed]
19. Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr HW. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus.Lancet 2003;361:2045-2046. [PubMed]
20. Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr HW. Treatment of SARS with human interferons. Lancet 2003; 362:293-294. [PubMed]
21. Donnelly CA, Ghani AV, Leung GM, Hedley AJ, Fraser C, Riley S, Abu-Raddad LJ, Ho LM, Thach TQ, Chau P, Chan KP, Lam TH, Tse LY, Tsang T, Liu SH, Kong JH, Lau EM, Ferguson NM, Anderson RM. Epidemiological determinants of spread of causal agent of severe acute respiratory syndrome in Hong Kong. Lancet 2003:361;1761-1766.[PubMed]
22. Drosten C, Gunther S, Preiser W, van der Werf S, Brodt HR, Becker S, Rabenau H, Panning M, Kolesnikova L, Fouchier RA, Berger A, Burguiere AM, Cinatl J, Eickmann M, Escriou N, Grywna K, Kramme S, Manuguerra JC, Muller S, Rickerts V, Sturmer M, Vieth S, Klenk HD, Osterhaus AD, Schmitz H, Doerr HW. Identification of a novel Coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 2003;348:1967-1976. [PubMed]
23. Fisher DA, Lim TK, Lim YT, Singh KS, Tambyah PA. Atypical presentations of SARS. Lancet 2003;361:1740. [PubMed]
24. Fisman DN. Hemophagocytic syndrome and infection. Emerg Infect Dis 2000; 6:601-68. [PubMed]
25. Fouchier RA, Kuiken T, Schutten M, van Amerongen G, van Doornum GJ, van den Hoogen BG, Peiris M, Lim W, Stohr K and Osterhaus AD. Aetiology: Koch’s postulates fulfilled for SARS virus. Nature 2003;423:240. [PubMed]
26. Franks TJ, Chong PY, Chui P, Galvin JR, Lourens RM, Reid AH, Selbs E, McEvoy PL, Hayden DL, Fukuoka J, Tauberberger JK and Travis WD. Lung Pathology of Severe Acute Respiratory Syndrome (SARS): A study of 8 autopsy cases from Singapore. Hum Pathol 2003;34:743-748. [PubMed]
27. Gao W, Tamin A, Soloff A, D'Aiuto L, Nwanegbo E, Robbins PD, Bellini WJ, Barratt-Boyes S, Gambotto A. Effects of a SARS-associated coronavirus vaccine in monkeys. Lancet 2003;362:1895-1896. [PubMed]
28. Gomersall CD, Joynt GM, Lam P, Li T, Yap F, Lam D, Buckley TA, Sung JJ, Hui DS, Antonio GE, Ahuja AT, and Leung P. Short-term outcome of critically ill patients with severe acute respiratory syndrome. Intensive Care Med 2004; 30:381-387. [PubMed]
29. Gopalakrishna G, Choo P, Leo YS, Tay BK, Lim YT, Khan AS, Tan CC. SARS transmission and hospital containment. Emerg Infect Dis 2004;10:395-400.
30. Grant PR, Garson JA, Teddar RS, Chan PK, Tam JS, Sung JJ. Detection of SARS coronavirus in plasma by real-time RT-PCR. N Engl J Med 2003;349:2468-2469. [PubMed]
31. Guan Y, Zheng BJ, He YQ, Liu XL, Zhuang ZX, Cheung CL, Luo SW, Li PH, Zhang LJ, Guan YJ, Butt KM, Wong KL, Chan KW, Lim W, Shortridge KF, Yuen KY, Peiris JS, Poon LL. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science 2003;302:276-278.[PubMed]
32. Haagmans BL, Kuiken T, Martina BE, Fouchier RAM, Rimmelzwaan GF, van Amerongen G, van Riel D, de Jong T, Itamura S, Chan K-H, Tashiro M and Osterhaus ADME. Pegulated interferon-a protects type I pneumocytes against SARS coronavirus infection in macaques. Nature Med 2004;10:290-293. [PubMed]
33. Ho JC, Ooi GC, Mok TY, Chan JW, Hung I, Lam B, Wong PC, Li PC, Ho PL, Lam WK, Ng CK, Ip MS, Lai KN, Chan-Yeung M, Tsang KW. High dose pulse versus non-pulse corticosteroid regimens in severe acute respiratory syndrome. Am J Respir Crit Care Med 2003; 168:1449-1456. [PubMed]
34. Ho PL, Tang XP, Seto WH. SARS: hospital infection control and admission strategies. Respirol 2003;8:S41-S45. [PubMed]
35. Ho TY, Wu SL, Cheng SE, Wei YC, Huang SP, Hsiang CY. Antigenicity and receptor-binding ability of recombinant SARS coronavirus spike protein. Biochem Biophys Res Commun 2004;313:938-947. [PubMed]
36. Hon KL, Leung CW, Cheng WT, Chan PK, Chu WC, Kwan YW, Li AM, Fong NC, Ng PC, Chiu MC, Li CK, Tam JS, Fok TF. Clinical presentations and outcome of severe acute respiratory syndrome in children. Lancet 2003;561:1701-1703. [PubMed]
37. Hsu LY, Lee CC, Green JA, Ang B, Paton NI, Lee L, Villacian JS, Lim PL, Earnest A, Leo YS. Severe acute respiratory syndrome in Singapore: Clinical features of index patient and initial contacts. Emerg Infect Dis 2003;9:713-717. [PubMed]
38. Hung EC, Chim SS, Chan PK, Tong YK, Ng EK, Chiu RW, Leung CB, Sung JJ, Tam JS, Lo YM. Detection of SARS coronavirus RNA in the cerebrospinal fluid of a patient with severe acute respiratory syndrome. Clin Chem 2003;49:2108-2109. [PubMed]
39. Hurst M, Faulds D. Lopinavir. Drugs 2000;60:1371-1381. [PubMed]
40. Joynt GM, Antonio GE, Lam P, Wong KT, Li T, Gomersall CD, Ahuja AT. Late-stage adult respiratory distress syndrome caused by severe acute respiratory syndrome: abnormal findings at thin-section CT. Radiology 2004;230:339-346. [PubMed]
41. Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, Tong S, Urbani C, Comer JA, Lim W, Rollin PE, Dowell SF, Ling AE, Humphrey CD, Shieh WJ, Guarner J, Paddock CD, Rota P, Fields B, DeRisi J, Yang JY, Cox N, Hughes JM, LeDuc JW, Bellini WJ, Anderson LJ; SARS Working Group. A Novel Coronavirus associated with severe acute respiratory syndrome. N Engl J Med 2003;348:1953-1966. [PubMed]
42. Kuiken T, Fouchier RA, Schutten M, Rimmelzwaan GF, van Amerongen G, van Riel D, Laman JD, de Jong T, van Doornum G, Lim W, Ling AE, Chan PK, Tam JS, Zambon MC, Gopal R, Drosten C, van der Werf S, Escriou N, Manuguerra JC, Stohr K, Peiris JS, Osterhaus AD. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet 2003;362:263-270. [PubMed]
43. Kwan BC, Leung CB, Szeto CC, Wang AY, Li PK. Severe acute respiratory syndrome in a Hemodialysis patient. Am J Kidney Dis 2003;42:1069-1074. [PubMed]
44. Lai KN, Leung JC, Metz CN, Lai FM, Bucala R, Lan HY. Role for macrophage migration inhibitory factor in acute respiratory distress syndrome. J Pathol 2003;199:496-508. [PubMed]
45. Lau JT, Fung KS, Wong TW, Kim JH, Wong E, Chung S, Ho D, Chan LY, Lui SF, Cheng A. SARS transmission among hospital workers in Hong Kong. Emerg Infect Dis 2004;10:280-286. [PubMed]
46. Lau KK, Yu WC, Chu CM, Lau ST, Sheng B, Yuen KY. Possible central nervous system infection by SARS coronavirus. Emerg Infect Dis 2004; 10:342-344.[PubMed]
47. Lee N, Hui DS, Wu A, Chan P, Cameron P, Joynt GM, Ahuja A, Yung MY, Leung CB, To KF, Lui SF, Szeto CC, Chung SC, Sung JY. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med 2003; 348:1986-1994. [PubMed]
48. Lee SH. The SARS epidemic in Hong Kong. J Epidemiol Community Health 2003;57:652-654. [PubMed]
49. Leung WK, To KF, Chan PK, Chan HL, Wu AK, Lee N, Yuen KY, Sung JJ. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterol 2003; 125:1011-1017. [PubMed]
50. Li SS, Cheng CW, Fu CL, Chan YH, Lee MP, Chan JW, Yiu SF. Left ventricular performance in patients with severe acute respiratory syndrome: a 30-day echocardiographic follow-up study. Circulation 2003;108:1798-1803. [PubMed]
51. Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003; 426:450-454. [PubMed]
52. Loutfy MR, Blatt LM, Siminovitch KA, Ward S, Wolff B, Lho H, Pham DH, Deif H, LaMere EA, Chang M, Kain KC, Farcas GA, Ferguson P, Latchford M, Levy G, Dennis JW, Lai EK, Fish EN. Interferon Alfacon-1 plus corticosteroids in severe acute respiratory syndrome. A Preliminary Study. JAMA 2003;290:3222-3228.[PubMed]
53. Marra MA, Jones SJ, Astell CR, Holt RA, Brooks-Wilson A, Butterfield YS, Khattra J, Asano JK, Barber SA, Chan SY, Cloutier A, Coughlin SM, Freeman D, Girn N, Griffith OL, Leach SR, Mayo M, McDonald H, Montgomery SB, Pandoh PK, Petrescu AS, Robertson AG, Schein JE, Siddiqui A, Smailus DE, Stott JM, Yang GS, Plummer F, Andonov A, Artsob H, Bastien N, Bernard K, Booth TF, Bowness D, Czub M, Drebot M, Fernando L, Flick R, Garbutt M, Gray M, Grolla A, Jones S, Feldmann H, Meyers A, Kabani A, Li Y, Normand S, Stroher U, Tipples GA, Tyler S, Vogrig R, Ward D, Watson B, Brunham RC, Krajden M, Petric M, Skowronski DM, Upton C, Roper RL. The genome sequence of the SARS-associated coronavirus. Science 2003:300:1399-1404. [PubMed]
54. Ng EK, Hui DS, Chan KC, Hung EC, Chiu RW, Lee N, Wu A, Chim SS, Tong YK, Sung JJ, Tam JS, Lo YM. Quantitative analysis and prognostic implication of SARS coronavirus in the plasma and serum of patients with severe acute respiratory syndrome. Clin Chem 2003;49:1976-1980. [PubMed]
55. Ng EK, Ng PC, Hon KL, Cheng WT, Hung EC, Chan KC, Chiu RW, Li AM, Poon LL, Hui DS, Tam JS, Fok TF, Lo YM. Serial analysis of the plasma concentration of SARS coronavirus RNA in pediatric patients with severe acute respiratory syndrome. Clin Chem 2003;49:2085-2088. [PubMed]
56. Ng SK. Possible role of an animal vector in the SARS outbreak at Amoy Gardens. Lancet 2003;362:570-572. [PubMed]
57. Nicholls JM, Poon LL, Lee KC, Ng WF, Lai ST, Leung CY, Chu CM, Hui PK, Mak KL, Lim W, Yan KW, Chan KH, Tsang NC, Guan Y, Yuen KY, Peiris JS. Lung pathology of fatal severe acute respiratory syndrome. Lancet 2003:361:1773-1778. [PubMed]
58. Ooi CG, Khong PL, Lam B, Ho JC, Yiu WC, Wong WM, Wang T, Ho PL, Wong PC, Chan RH, Lam WK, Lai KN, Tsang KW. Severe acute respiratory syndrome: relationship between radiologic and clinical parameters. Radiology 2003;229:492-499. [PubMed]
59. Parry J. WHO confirms SARS in Chinese journalist. Brit Med J 2004;328:65. [PubMed]
60. Pearson H, Clarke T, Abbott A, Knight J, Cyranoski D. SARS: what have we learned? Nature 2003;424:121-126. [PubMed]
61. Peiris JS, Lai ST, Poon LL, Guan Y, Yam LY, Lim W, Nicholls J, Yee WK, Yan WW, Cheung MT, Cheng VC, Chan KH, Tsang DN, Yung RW, Ng TK, Yuen KY; SARS study group. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 2003;361:1319-25. [PubMed]
62. Peiris JS, Chu CM, Cheng VC, Chan KS, Hung IF, Poon LL, Law KI, Tang BS, Hon TY, Chan CS, Chan KH, Ng JS, Zheng BJ, Ng WL, Lai RW, Guan Y, Yuen KY; HKU/UCH SARS Study Group. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet 2003;361:1767-1772. [PubMed]
63. Peiris JS, Yuen KY, Osterhaus AD, Stohr K. The severe acute respiratory syndrome. N Engl J Med 2003;349:2431-2441. [PubMed]
64. Poon LL, Chan KH, Wong OK, Yam WC, Yuen KY, Guan Y, Lo YM, Peiris JS. Early diagnosis of SARS coronavirus infection by real time RT-PCR. J Clin Virol 2003;28:233-238. [PubMed]
65. Rimmelzwaan GF, Kuiken T, van A, Bestebroer TM, Fouchier RA, Osterhaus AD. Pathogenesis of influenza A (H5N1) virus infection in a primate model. J Virol 2001; 75:6687-6691. [PubMed]
66. Rota PA, Oberste MS, Monroe SS, Nix WA, Campagnoli R, Icenogle JP, Penaranda S, Bankamp B, Maher K, Chen MH, Tong S, Tamin A, Lowe L, Frace M, DeRisi JL, Chen Q, Wang D, Erdman DD, Peret TC, Burns C, Ksiazek TG, Rollin PE, Sanchez A, Liffick S, Holloway B, Limor J, McCaustland K, Olsen-Rasmussen M, Fouchier R, Gunther S, Osterhaus AD, Drosten C, Pallansch MA, Anderson LJ, Bellini WJ. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science 2003:300:1394-1399. [PubMed]
67. Ruan YJ, Wei CL, Ee LA, Vega VB, Thoreau H, Su ST, Chia JM, Ng P, Chiu KP, Lim L, Zhang T, Peng CK, Lin EO, Lee NM, Yee SL, Ng LF, Chee RE, Stanton LW, Long PM, Liu ET. Comparative full-length genome sequence analysis of 14 SARS coronavirus isolates and common mutations associated with putative origins of infection. Lancet 2003:361:1779-1785. [PubMed]
68. Seto WH, Tsang D, Yung RW, Ching TY, Ng TK, Ho M, Ho LM, Peiris JS, and advisors of Expert SARS group of Hospital Authority. Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS). Lancet 2003; 361:1519-1520.[PubMed]
69. Shek CC, Ng PC, Fung GP, Cheng FW, Chan PK, Peiris MJ, Lee KH, Wong SF, Cheung HM, Li AM, Hon EK, Yeung CK, Chow CB, Tam JS, Chiu MC, Fok TF. Infants born to mothers with severe acute respiratory syndrome. Pediatrics 2003;112:e254-256. [PubMed]
70. Sit SC, Yau EKC, Lam YY, Ng DK, Fong NC, Hui YW, Cheng WF, Leung CW, Chiu MC. A young infant with severe acute respiratory syndrome. Pediatrics 2003;112:e257-260. [PubMed]
71. So LK, Lau AC, Yam LY, Cheung TM, Poon E, Yung RW, Yuen KY. Development of a standard treatment protocol for severe acute respiratory syndrome. Lancet 2003;361:1615-1617. [PubMed]
72. Somogyi R, Vesely AE, Azami T, Preiss D, Fisher J, Correia J, Fowler RA. Dispersion of respiratory droplets with open vs closed oxygen delivery masks. Implications for the transmission of severe acute respiratory syndrome. Chest 2004;125:1155-1157. [PubMed]
73. Stroher U, DiCaro A, Li Y, Strong JE, Aoki F, Plumier F, Jones SM, Feldmann H. Severe acute respiratory syndrome-related coronavirus is inhibited by interferon-a. J Infect Dis 2004;189:1164-1167. [PubMed]
74. Sui J, Li W, Murakami A, Matthews LJ, Wong SK, Moore MJ, Tallarico AS, Olurinde M, Choe H, Anderson LJ, Bellini WJ, Farzan M, Marasco WA. Potent neutralization of severe acute respiratory syndrome (SARS) coronavirus by a human mAb to S1 protein that blocks receptor association. Proc Nat’l Acad Sci 2004:101:2536-2541. [PubMed]
75. Sung JJ, Wu A, Joynt GM, Yuen KY, Lee N, Chan PK, Cockram CS, Wong VW, Ahuja AT, Yu LM, Hui DS. Severe Acute Respiratory Syndrome: Report of treatment and outcome after a major outbreak. Thorax 2004;59:414-20. [PubMed]
76. Tan EL, Ooi EE, Lin CY, Tan HC, Ling AE, Lim B and Stanton LW. Inhibition of SARS coronavirus infection in vitro with clinically approved antiviral drugs. Emerg Infect Dis[serial online]2004 Apr [accessed on 6 Mar 2004]. Available from: http://www.cdc.gov/ncidod/EID/vol10no4/03-0458.htm. [PubMed]
77. The Chinese SARS Molecular Epidemiology Consortium. Molecular Evolution of the SARS coronavirus during the course of the SARS epidemic in China. Science 2004;303:1666-1669. [PubMed]
78. Tsang KW, Ho PL, Ooi GC, Yee WK, Wang T, Chan-Yeung M, Lam WK, Seto WH, Yam LY, Cheung TM, Wong PC, Lam B, Ip MS, Chan J, Yuen KY, Lai KN. A cluster of cases of severe acute respiratory sundrome in Hong Kong. N Engl J Med 2003: 348:1977-1985. [PubMed]
79. Tsang OT, Chau TN, Choi KW, Tso EY, Lim W, Chiu MC, Tong WL, Lee PO, Lam BH, Ng TK, Lai JY, Yu WC, Lai ST. Coronavirus-positive nasopharyngeal aspirate as predictor for severe acute respiratory syndrome mortality. Emerg Infect Dis 2003;9:1381-1387. [PubMed]
80. Tse GM, To KF, Chan PK, Lo AW, Ng KC, Wu A, Lee N, Wong HC, Mak SM, Chan KF, Hui DS, Sung JJ, Ng HK. Pulmonary pathological features in coronavirus associated severe acute respiratory syndrome (SARS). J Clin Pathol 2004;57:260-265. [PubMed]
81. Tsui PT, Kwok ML, Yuen H, Lai ST. Severe acute respiratory syndrome: Clinical outcome and prognostic correlates. Emerg infect Dis 2003;9:1064-1069.[PubMed]
82. Twu SJ, Chen TJ, Chen CJ, Olsen SJ, Lee LT, Fisk T, Hsu KH, Chang SC, Chen KT, Chiang IH, Wu YC, Wu JS, Dowell SF. Control measures for severe acute respiratory syndrome (SARS) in Taiwan. Emerg Infect Dis 2003;9:718-720. [PubMed]
83. Varia M, Wilson S, Sarwal S, McGeer A, Gournis E, Galanis E, Henry B; Hospital Outbreak Investigation Team. Investigation of a nosocomial outbreak of severe acute respiratory syndrome (SARS) in Toronto, Canada. CMAJ 2003;169:285-292. [PubMed]
84. Wang H, Ding Y, Li X, Yang L, Zhang W, Kang W. Fatal aspergillosis in a patient with SARS who was treated with corticosteroids. N Engl J Med 2003; 349:507-508. [PubMed]
85. WHO. Summary of probable SARS cases with onset of illness from 1 November to 31 July 2003. Available from:http://www.who.int/csr/sars/country/table2003_09_23/en.
86. WHO. Update 95-SARS: Chronology of a serial killer. Available from: http://www.who.int/csr/don/2003_07_04/en.
87. WHO. Summary table of areas that experienced local transmission of SARS during the outbreak period from 1 November 2002 to 31 July 2003. Available from:http://www.who.int/csr/sars/areas/areas2003_11_21/en.
88. WHO. Alert, verification and public health management of SARS in the post outbreak period: http://www.who.int/csr/sars/postoutbreak/en/print.html.
89. WHO. Severe acute respiratory syndrome (SARS) in Singapore- Update 2. Available at http://www.who.int/csr/don/2003_09_24/en/.
90. WHO. Severe acute respiratory syndrome (SARS) in Taiwan, China. Available at http://www.who.int/csr/don/2003_12_17/en/.
91. WHO. New case of laboratory confirmed SARS in Guangdong, China- Update 5. Available at http://www.who.int/csr/don/2004_01_31/en/.
92. Wong CK, Lam CWK, Wu AK, Ip WK, Lee NL, Chan IH, Lit LC, Hui DS, Chan MH, Chung SS, Sung JJ. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol 2004; 136:95-103. [PubMed]
93. Wong KC, Leung KS, Hui M. Severe acute respiratory syndrome (SARS) in a geriatric patient with a hip fracture. A case report. J Bone Joint Surg Am 2003;85A:1339-1342. [PubMed]
94. Wong KT, Antonio GE, Hui DS, Lee N, Yuen EH, Wu A, Leung CB, Rainer TH, Cameron P, Chung SS, Sung JJ, Ahuja AT. Severe Acute Respiratory Syndrome: Radiographic appearances and pattern of progression in 138 Patients. Radiology 2003:228:401-406. [PubMed]
95. Wong KT, Antonio GE, Hui DS, Lee N, Yuen EH, Wu A, Leung CB, Rainer TH, Cameron P, Chung SS, Sung JJ, Ahuja AT. Thin section CT of Severe Acute Respiratory Syndrome: Evaluation of 73 patients exposed to or with the disease. Radiology 2003;228:395-400. [PubMed]
96. Wong RS, Wu A, To KF, Lee N, Lam CW, Wong CK, Chan PK, Chan WY , Yu LY, Hui DS, Tam J, Cheng G, Sung JJ. Haematological manifestations in patients with severe acute respiratory syndrome: retrospective analysis. Brit Med J 2003;326:1358-1362. [PubMed]
97. Wong RS, Hui DS. Index patient and SARS outbreak in Hong Kong. Emerg Infect Dis 2004;10:339-341. [PubMed]
98. Wong VW, Dai D, Wu AK, Sung JJ. Treatment of severe acute respiratory syndrome with convalescent plasma. Hong Kong Med J 2003;9:199-201. [PubMed]
99. Wong WM, Ho JC, Hung IF, Mok T, Chan J, Hung IF, Ng W, Lam YM, Tam WO, Wong BC, Wong PC, Ho PL, Lai CL, Lam WK, Lam SK, Tsang KW. Temporal patterns of hepatic dysfunction and disease severity in patients with SARS. JAMA 2003;290:2663-2665. [PubMed]
100. Woo PC, Lau SK, Tsoi HW, Chan KH, Wong BH, Che XY, Tam VK, Tam SC, Cheng VC, Hung IF, Wong SS, Zheng BJ, Guan Y, Yuen KY. Relative rates of non-pneumonic SARS coronavirus infection and SARS coronavirus pneumonia. Lancet 2004;363:841-845. [PubMed]
101. Yang ZY, Kong WP, Huang Y, Roberts A, Murphy BR, Subbarao K, Nabel GJ. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature 2004;428:561-564. [PubMed]
102. Yu D, Li H, Xu R, He J, Lin J, Li W, Xu H, Huang S, Huang J. CDC. Prevalence of IgG antibody to SARS-associated coronavirus in animal traders- Guangdong province, China, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:986-987. [PubMed]
103. Zhao P, Ke JS, Qin ZL, Ren H, Zhao LJ, Yu JG, Gao J, Zhu SY, Qi ZT. DNA vaccine of SARS-CoV S gene induces antibody response in mice. Acta Biochim et Biophysica Sinica 2004;36:37-41. [PubMed]
104. Zhao Z, Zhang F, Xu M, Huang K, Zhong W, Cai W, Yin Z, Huang S, Deng Z, Wei M, Xiong J, Hawkey PM. Description and clinical treatment of an early outbreak of severe acute respiratory syndrome (SARS) in Guangzhou, PR China. J Med Microbiol 2003;52:715-720. [PubMed]
105. Zheng BJ, Guan Y, Wong KH, Zhou J, Wong KL, Young BWY, Lu LW and Lee SS. SARS-repated virus predating SARS outbreak, Hong Kong. Emerg Infect Dis 2004; 10:176-178. [PubMed]
106. Zhong NS, Zheng BJ, Li YM, Poon, Xie ZH, Chan KH, Li PH, Tan SY, Chang Q, Xie JP, Liu XQ, Xu J, Li DX, Yuen KY, Peiris, Guan Y. Epidemiology and cause of severe acute respiratory syndrome in Guangdong, People’s Republic of China, in Feb 2003. Lancet 2003;362:1353-1358. [PubMed]
107. Zhong NS, Zeng GQ. Our strategies for fighting severe acute respiratory syndrome. Am J Respir Crit Care Med 2003:168:7-9. [PubMed]
Table 1. Summary of Probable SARS Cases With Onset of Illness From November 1, 2002 to July 31, 2003 (85)
| Areas | Cumulative n | Deaths n | Case fatality ratio (%) | Age (median range) yrs | Health Care Workers n(%) |
|---|---|---|---|---|---|
| Australia | 6 | 0 | 0 | 15(1-45) | 1 (16) |
| Canada | 251 | 43 | 17 | 49 (1-98) | 109 (43) |
| China | 5327 | 349 | 7 | pending | 1002 (19) |
| Hong Kong | 1755 | 299 | 17 | 40 (0-100) | 386 (22) |
| Taiwan | 346 | 37 | 11 | 42 (0-93) | 68 (20) |
| Malaysia | 5 | 2 | 40 | 30 (26-84) | 0 |
| Philippines | 14 | 2 | 14 | 41 (29-73) | 4 (29) |
| Singapore | 238 | 33 | 14 | 35 (1-90) | 97 (41) |
| Thailand | 9 | 2 | 22 | 42 (2-79) | 1 (11) |
| UK | 4 | 0 | 0 | 59 (28-74) | 0 |
| USA | 29 | 0 | 0 | 33 (0-83) | 0 |
| Vietnam | 63 | 5 | 8 | 43 (20-76) | 36 (57) |
| Global | 8098 | 774 | 9.6 | N/A | 1007 (21) |
Table 2. Clinical Features of SARS on Presentation (4,37,47,78)
| Symptom | % of patients with symptom |
|---|---|
| Persistent fever > 38C | 99-100 |
| Non-productive cough | 57-75 |
| Myalgia | 45-61 |
| Chills/rigor | 15-73 |
| Headache | 20-56 |
| Dyspnea | 40-42 |
| Malaise | 31-45 |
| Nausea and vomiting | 20-35 |
| Diarrhea | 20-25 |
| Sore throat | 13-25 |
| Dizziness | 4.2-43 |
| Sputum production | 4.9-29 |
| Rhinorrhea | 2.1-23 |
| Arthralgia | 10.4 |
Table 3. WHO Case Definitions of SARS in the Post-Outbreak Period (88)
Clinical case definition of SARS:
A person with a history of :
Fever ≥ 38C
AND
one or more symptoms of lower respiratory tract illness (cough, difficulty breathing, shortness of breath)
AND
Radiographic evidence of lung infiltrates consistent with pneumonia or Respiratory distress syndrome (RDS) OR autopsy findings consistent with the pathology of pneumonia or RDS without an identifiable cause.
AND
No alternative diagnosis can fully explain the illness.
Laboratory case definition of SARS:
A person with symptoms and signs that are clinically suggestive of SARS AND with positive laboratory findings for SARS CoV based on one or more of the following diagnostic criteria:
a) PCR positive for SARS CoV
PCR positive using a validated method from:
-
At least 2 different clinical specimens (eg nasopharyngeal aspirate or stool) OR
-
The same clinical specimen collected on 2 or more occasions during the course of the illness (eg sequential nasopharyngeal aspirates) OR
-
Two different assays or repeat PCR using a new RNA extract from the original clinical sample on each occasion of testing.
b) Seroconversion by ELIZA or IFA
-
Negative antibody test on acute serum followed by positive antibody test on convalescent phase serum tested in parallel OR
-
Fourfold or greater rise in antibody titre between acute and convalescent phase sera tested in parallel.
c) Virus isolation
Isolation in cell culture of SARS CoV from any specimen AND PCR confirmation using a validated method.
Table 4. CDC Updated Interim Case Definition for SARS (8).
Clinical criteria:
Early illness
· Presence of 2 or more of the following features: fever (might be subjective), chills, rigors, myalgia, headache, diarrhoea, sore throat, rhinorrhoea.
Mild to moderate respiratory illness
· Temp >100.4F or 38C) and at least one lower respiratory illness (eg cough, dyspnoea, difficulty breathing)
Severe respiratory illness
· Meets clinical criteria of mild to moderate respiratory illness, and
· One or more of the following findings:
radiographic evidence of pneumonia, or acute respiratory distress syndrome, or autopsy findings consistent with pneumonia, or acute respiratory distress syndrome without an identifiable cause.
Epidemiologic criteria:
Possible exposure to SARS CoV
At least one of the following exposures in the 10 days before onset of symptoms:
· Travel to a foreign or domestic location with documented or suspected recent transmission of SARS, or
· Close contact with a person with mild-to-moderate or severe respiratory illness and with history of travel in the 10 days before onset of symptoms to a foreign or domestic location with documented or suspected recent transmission of SARS CoV.
Likely exposure to SARS CoV
One of the following exposures in the 10 days before onset of symptoms:
· Close contact with a confirmed case of SARS CoV disease or
· Close contact with a person with mild-to-moderate or severe respiratory illness for whom a chain of transmission can be linked to a confirmed case of SARS CoV disease in the 10 days before onset of symptoms.
Laboratory criteria:
· Detection of serum antibody to SARS-CoV by a test validated by CDC (eg enzyme immunoassay), or
· Isolation in cell culture of of SARS-CoV from a clinical specimen, or
· Detection SARS-CoV RNA by reverse- transcriptase polymerase chain reaction (RT-PCR) test validated by CDC and with subsequent confirmation in a reference laboratory (eg CDC).
Exclusion criteria
A person may be excluded as a SARS report under investigation if any of the following applies:
· An alternative diagnosis can fully explain the illness.
· Absence of antibody to SARS CoV in a serum specimen obtained > 28 days after symptom onset.
· The case was reported on the basis of contact with a person who was excluded subsequently as a case of SARS CoV disease; then the reported case is also excluded provided other epidemiologic or laboratory criteria are absent.
SARS disease Classification:
· Probable case of SARS CoV disease: in a person who meets the clinical criteria for severe respiratory illness and the epidemiologic criteria for likely exposure to SARS-CoV.
· Confirmed case of SARS CoV disease: in a person who has a clinically compatible illness (ie early, mild-to-moderate, or severe) that is laboratory confirmed.
Table 5. Diagnostic Tests for SARS-CoV
| RT-PCR | Detection rate |
|---|---|
| Nasophargyneal aspirate | Conventional RTPCR (62): 32% Day 3, 68% Day 14 Second-generation with real-time quantitative RTPCR assay (64): 80% during first 3 days |
| Stool (62) | 97% Day 14 |
| Urine (62) | 42% Day 15 |
| Real-time quantitative Serum SARS CoV RNA (30,54,55) | 80% Day 1, 75% Day 7, 45% Day 14 |
| Serology (62) IgG seroconversion to SARS-CoV | 15% Day15 60% Day 21 >90% Day 28 |
Figure 1. Serial chest radiographs of a 30 year-old male patient with SARS. Initial chest radiograph on day 3 of illness showed right lower zone infiltrate. He developed ARDS on day 9 requiring invasive ventilatory support. His condition improved following 3 pulses of 0.5g per day of methylprednisolone.
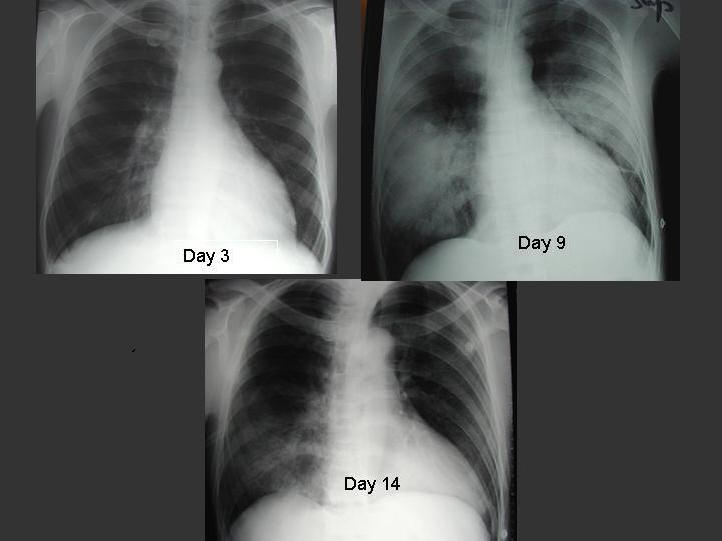
Figure 2. High resolution CT of thorax of a 32 year-old male patient showing bilateral ground-glass opacification with interlobular septal and intralobular interstitial thickening.

Hui DS, et al. Severe acute respiratory syndrome vs. the Middle East respiratory syndrome. Curr Opin Pulm Med 2014;20(3):233-241
Guided Medline Seach For:
Guided Medline Search For Recent Reviews
Berger S. Emergence of Infectious Diseases into the 21st Century, 2008.

