Dengue Fever Virus
Authors: Author: Sophie Yacoub MRCP, Bridget Wills, M.D.
Previous Author: Bridget Wills, M.D.
Dengue is the most widely distributed mosquito-borne viral infection of humans and is emerging as a major burden on global public health. A recent study has estimated that 100 million clinically apparent dengue infections occur worldwide each year, with a further 300 million asymptomatic infections (13) (Figure 1). Typically dengue manifests as a relatively mild self-limited febrile illness characterized by fever, headache, muscle and joint pains, and skin rash. However the clinical spectrum also includes severe and occasionally life threatening disease. Potential complications include an unusual plasma leakage syndrome that may result in hypovolaemic shock, a coagulopathy sometimes accompanied by severe bleeding, and organ impairment (142).
Virology
Infection can be caused by any one of the four antigenically distinct but closely related dengue viral serotypes (DENV-1, 2, 3, and 4) that together constitute one subgroup of the genus Flavivirus, family Flaviviridae. The Flavivirus genus includes approximately 80 other viruses, many of which are also arthropod-borne human pathogens, among them Japanese encephalitis virus, West Nile virus and yellow fever virus.
The dengue virus is a single stranded, positive-sense RNA virus with a genome of approximately 11 kilobases. The mature virion consists of a nucleocapsid core comprising the capsid protein and the RNA genome enclosed within a lipid bilayer made up of the envelope and membrane structural proteins. The outer diameter of the spherical virus particles is about 50 nm (83). Recently immature viral particles have been described and intra-cellular viral processing has been characterized using cryo-electron microscopy (188). Conformational changes occur during maturation of the virus and are pH (189) and temperature dependent (190). Seven non-structural proteins are expressed in infected cells, but do not form part of the mature virion; the contribution of these proteins, in particular non-structural protein 1 (NS1), to pathogenesis is a major focus of current research efforts (111). NS1 is a glycoprotein with a molecular weight of 46-55 kDa that exists as a stable homodimer on the surface membrane of infected cells, and as a soluble hexamer in plasma, forming a barrel shape around a lipid core (53). Recent work has defined the assembly and antibody binding of NS1using cryo-electron microscopy (39). Soluble NS1 has been identified in plasma, sometimes in very high concentrations, from the first day of fever and for up to 14 days afterwards (38), and sNS1 levels in the acute phase have been found to correlate with dengue severity (93). NS1 and/or anti-NS1 antibodies have also been implicated in the pathogenesis of the vascular leakage and coagulopathy, although the evidence arises primarily from in-vitro studies or animal models (6, 7).
Humans are the primary vertebrate hosts in the life cycle of the virus, and other vertebrate species are generally not susceptible to infection. Aedes mosquitoes (principally Ae. Aegypti and Ae. Albopictus) are responsible for person-to-person transmission of all serotypes. Ae. Aegypti, the most important vector, is a highly domesticated, day-biting mosquito that is adapted to living in peri-urban and urban environments where humans are the main feeding target. It is found around the globe, mainly in tropical and sub-tropical latitudes, and is usually limited to altitudes below about 1000 metres. The mosquitoes acquire DENV when they bite infected individuals with significant plasma viraemia (116). The virus then replicates in the mosquito's mid-gut before disseminating widely, including to the saliva, after 7-12 days, following which onward transmission can occur when the mosquito takes its next blood feed (87). In recent years considerable global spread in dengue virus activity has been noted, largely reflecting expansion in the geographic distribution of the mosquito vectors. The recent spread of the vector Aedes Albopictus to new areas of the world including southern Europe, America and Africa may explain the rise in dengue transmission in these areas (164, 169).
All four viral serotypes can cause severe and fatal disease. Infection with one serotype is thought to elicit specific life-long immunity to that serotype but not to provide long-term cross-protective immunity to the remaining serotypes. Therefore persons living in a dengue-endemic area can experience several dengue infections during their lifetime. There is strong epidemiological evidence to indicate that second (or subsequent) infections with a different serotype can be associated with a more severe clinical disease phenotype (58, 163). In addition there is evidence to suggest that certain serotypes, particularly DENV-2 and DENV-3, are more virulent than others (43, 65, 117).
Within each serotype there is also considerable genetic variation and certain genotypes are associated with increased disease severity. Thus, introduction of the Southeast Asian DEN-2 genotype into Cuba in the early 1980's coincided with documentation of the first cases of dengue haemorrhagic fever (DHF) seen in the Americas (125), and spread of this strain through the region was associated with further outbreaks of severe disease. In contrast, no cases of dengue haemorrhagic fever occurred during an epidemic caused by the American genotype of DEN-2 in Peru in 1995, even though the majority of infections were secondary (170).
Epidemiology
Epidemics of dengue like disease have been reported at varying intervals over the last 200 years from different locations across Asia, Africa and North America. Contemporary reports of these early epidemics describe self limited, febrile illnesses associated with rash, vomiting, and prominent muscle and joint pains (130). Although more severe and sometimes fatal presentations were occasionally documented, the general impression of dengue fever (DF) as a disease of minor clinical significance persisted until the 1950's when a serious clinical syndrome with a high mortality began to emerge among children in Southeast Asia. This syndrome, characterized by vascular leakage and bleeding, was initially thought to be a new disease. However in the 1960's the syndrome was identified as a severe manifestation of dengue infection and was given the name dengue haemorrhagic fever (56, 60), although more recently the terminology for classification of dengue has been revised.
Over the next 20 years the incidence of dengue haemorrhagic fever increased rapidly in Southeast Asia, eventually becoming hyperendemic in most Asian cities, with major epidemics superimposed on the regular background transmission pattern at 3 to 5 year intervals.
The geographic distribution of the dengue viruses and their mosquito vectors has continued to expand and severe disease associated with dengue has emerged as a significant problem in the Pacific region and the Americas as well as in Asia (51, 99). Reports of dengue outbreaks in Africa are also increasing (33), and seroprevalence studies indicate that all 4 dengue serotypes are circulating in many locations across the African continent (42, 45, 47). However, reports of large epidemics and cases of severe dengue are rare. This may be due to under-reporting or failure to recognize the severe cases as part of the dengue spectrum, or may reflect the existence of protective genetic host factors in African populations, or low vector competence and reduced transmission efficiency (4). The eastern Mediterranean area has also seen an increase in dengue in the last three decades, and recent outbreaks in Yemen, Saudi Arabia and Pakistan. In 2012 the first European outbreak of dengue since the 1920's occurred in Madeira, Portugal, with over 2000 cases documented and 120 hospitalizations (155). At the present time, some 2.5 billion people living in tropical and sub-tropical areas of the world are at risk for infection, and an estimated 100 million clinically apparent dengue infections occur annually. Dengue is also a leading cause of hospitalization and death for children in many of the more than 100 tropical countries where the disease is now common (13). Important risk factors that are considered to influence disease severity include the serotype and strain of the infecting virus as mentioned above, as well as the age, immune status, and genetic predisposition of the patient. The age distribution for severe dengue among dengue-infected patients is bimodal. Infants commonly present at a peak age of approximately 7 months with primary antibody responses; they are always born to mothers who are dengue immune and the age at presentation appears to be related to the level of antibody acquired from the mother (25, 81, 143). Young children aged between 1 and 5 years rarely develop severe disease but a second peak occurs among older children and young adults and is almost invariably associated with secondary antibody responses. In hyperendemic situations, with repeated exposure to multiple serotypes from early childhood, the peak age for severe dengue is around 10 - 12 years. However in environments with lower endemicity adults may be the predominant group affected during an outbreak, depending on previous exposure and immunity (54). Plasma leakage is more likely to occur, and to be severe, in children than in adults (55, 61), probably because the microvasculature is intrinsically more permeable in the young and matures with age (45). Women also have a lower threshold for vascular leak than men and, although infection rates between the sexes are similar in endemic areas, a preponderance of females has been noted among those with dengue shock syndrome and among fatal cases (57). A large retrospective study of 130,000 hospitalized dengue patients in Vietnam identified female sex and age less than 5 years, as risk factors for poorer outcomes (5).
Ethnicity has been highlighted as another possible risk factor for severe dengue; black individuals were less likely to experience dengue hemorrhagic fever than white individuals during well-documented epidemics in Cuba (18, 47). Similarly nutritional status has been identified as a possible risk factor, with well-nourished children having a higher risk for severe disease than children with malnutrition (114, 134). Elderly patients and those with co-morbidities including diabetes and hypertension have also been found to be at an increased risk of severe dengue (119).
Various genetic markers for dengue hemorrhagic fever have been examined. Several groups have identified specific HLA alleles associated with both susceptibility and resistance to severe dengue disease (94, 147). In addition various polymorphic non-HLA host genetic factors that might influence susceptibility have been investigated; variations in the vitamin D receptor and Fc gamma receptor II genes conferred some protection from dengue hemorrhagic fever, but no associations were found with polymorphisms in the mannose binding lectin, interleukin-1, and interleukin-1 receptor antagonist genes and disease severity (95). Other genetic association studies have shown the potential role of ICAM-3 grabbing nonintegrin (DC-SIGN1, encoded by CD209), which is a major dengue dendritic cell receptor. A strong association between the promoter variant of CD209 (G allele variant of DCSIGN1-336) was found to be protective against severe disease (133). A genome-wide association study in Vietnam has identified two distinct loci, MICB and PLCE1 that were associated with severe disease (80), and a further study confirmed these loci were also associated with less severe forms of dengue, as well as with dengue in infants (174).
Clinical Manifestations
Dengue virus infection can cause a wide variety of clinical manifestations ranging from asymptomatic infection to life-threatening hypovolaemic shock. Previously, symptomatic disease was separated into two main clinical syndromes: dengue fever, a non-specific febrile illness; and dengue hemorrhagic fever, an illness characterized by increased vascular permeability and altered haemostasis resulting respectively in capillary leak syndrome and hemorrhage. However, recognizing that DENV infection causes a spectrum of disease manifestations rather than two separate clinical syndromes, the World Health Organization (WHO) published revised guidelines for case classification in 2009. The new WHO system classifies patients as having dengue, dengue with warning signs, or severe dengue (175) (Table 1). This set of 'warning signs' were intended to help clinicians identify patients who may develop complications during the critical phase of the illness (Figure 2).They were derived mainly from a study of 2000 patients with dengue in Asia and Latin America, but as only a small number of patients progressed to severe disease during the study, further work is ongoing to validate these findings (3).
Following the bite of an infected mosquito there is a 3-15 day incubation period (commonly 5-7days), after which symptoms begin abruptly and typically follow 3 phases – a febrile phase, a critical phase and a spontaneous recovery phase. Increased vascular permeability and altered haemostasis are the most frequent complications encountered during the critical phase, but a number of other severe manifestations involving specific organs are increasingly being recognized and will be described separately.
Febrile phase
This phase commences with sudden onset of high fever and non-specific constitutional symptoms. Anorexia, nausea and vomiting are prominent features. Adults are often more symptomatic than children, complaining of headache, retro-orbital pain, and severe myalgia and arthralgia. Other non-specific symptoms may include upper respiratory symptoms, diarrhea or constipation, and dysuria. On examination, lymphadenopathy is common, and conjunctival suffusion and an evanescent macular rash may be noted. Hepatomegaly is often present, but palpable splenomegaly is unusual. The high fever persists for about 5-7 days and usually terminates abruptly. A maculopapular rash may appear at around this time particularly in older children and adults. Sometimes this convalescent rash may be extremely florid, with an intensely erythematous appearance interspersed with islands of pale skin. The rash fades after a few days, although desquamation and pruritus may follow. Adults often complain of extreme tiredness for weeks after recovery and convalescence may be complicated by depression.
The differential diagnosis of dengue includes other arboviral infections, as well as measles, rubella, enterovirus infections, adenovirus infections, and influenza. Other differential diagnoses that should be considered depending on local disease prevalence include typhoid, malaria, leptospirosis, hepatitis A, rickettsial diseases, and bacterial sepsis. Certain laboratory tests may be helpful. Patients with dengue are usually leucopenic during the first few days, but may go on to develop a lymphocytosis, often including many atypical lymphocytes. Some degree of thrombocytopenia is common and hepatic transaminases are often moderately raised.
Critical Phase
The critical phase occurs around the time of defervescence, usually between days 3–7 of illness, when the temperature drops to 37.5–38oC or less. While most patients start to improve at this time, a small proportion develop complications, in particular a vasculopathy characterized by endothelial dysfunction and plasma leakage, manifested by a rising haematocrit and development of serosal effusions. Ultrasound studies have demonstrated that pleural effusions, ascites, and gall bladder wall oedema are often present in the critical phase and are associated with disease severity (12, 31, 139), but subclinical fluid accumulation can be detected as early as day 2-3 of fever (146). It is likely that a slow plasma leak occurs during the febrile phase, balanced by increased lymphatic return acting together with compensatory cardiovascular, renal and adrenal mechanisms to maintain normal plasma volume. However, as leakage progresses during the transition to the critical phase these processes result in peripheral vasoconstriction and a rise in the diastolic pressure towards the systolic pressure so that the pulse pressure narrows. When the pulse pressure narrows to ≤ 20 mm Hg the patient is classified as having dengue shock syndrome (DSS) (176). A rapid weak pulse, impaired peripheral perfusion and lethargy or restlessness are usually apparent by this stage. Interestingly however, some patients do not develop a significant tachycardia and the severity of plasma leakage may be underestimated if there is failure to monitor the blood pressure and peripheral perfusion carefully during the critical time around defervescence. If fluid resuscitation is not instituted promptly when the pulse pressure narrows the ongoing intravascular depletion of plasma becomes critical, the systolic pressure falls rapidly, and irreversible shock and death may follow.
Haemorrhagic manifestations can occur at any stage of the disease, in both uncomplicated and severe dengue, but are usually limited to presence of skin petechiae or development of bruising at sites of minor trauma. Mucosal bleeding, e.g. epistaxis, gum or gastrointestinal bleeding, is not common but may occasionally be severe. Menorrhagia is noted sometimes in post-pubertal girls, and life threatening uterine haemorrhage has been reported in pregnant women (9, 149). Intracranial haemorrhage is a very rare complication. In children severe mucosal bleeding tends to occur in those with profound or prolonged shock, and is often accompanied by metabolic acidosis and laboratory evidence of disseminated intravascular coagulation. Rarely, massive gastrointestinal bleeding causing haemorrhagic shock (as distinct from shock due to plasma leakage) has been reported in adults, and may occasionally be fatal (27, 160).
Until or unless shock develops, clinical identification of increased vascular permeability is difficult but the degree of haemoconcentration as judged by serial haematocrit measurements can be a useful marker of the severity of vascular leakage (105). Platelet counts fall over the illness course reaching a nadir around the time of maximal vascular leakage (158). Profound thrombocytopenia with counts below 20,000 platelets/mm3 is common in severe disease (17), although rarely associated with spontaneous bleeding despite the fact that the remaining platelets are also dysfunctional (107, 145). Lower platelet counts have also been found to correlate more closely with the severity of vascular leakage rather than bleeding (182). An increase in the activated partial thromboplastin time and a reduction in fibrinogen levels are the two most consistent coagulation abnormalities detected, but the true nature of the coagulopathy remains to be fully characterized (82, 100, 179). Plasma protein concentrations, particularly albumin concentrations, may be low in dengue, but severe hypoproteinaemia is usually a feature of dengue shock syndrome (180). Evidence of renal compromise, markedly raised liver enzymes, and metabolic acidosis may be seen in those with profound shock and are poor prognostic signs.
Recovery Phase
The increased vascular permeability and abnormal haemostasis are transient and usually resolve within 48 – 72 hours of becoming clinically apparent. Spontaneous reabsorption of the excess fluid from the interstitial compartment begins, usually around day 6-8 of illness, and progresses rapidly. With appropriate fluid management the outcome is generally good and convalescence is usually short and uneventful. An erythematous rash with patches of normal skin or 'islands of white' may become visible in older children and adolescents during recovery. In experienced hands and with meticulous attention to detail, mortality rates of <1 - 5% are achievable even for patients with established dengue shock syndrome managed in endemic areas. However, fluid management in infants, the elderly, and those with underlying diseases is not straightforward, and death rates are correspondingly higher in these groups. In many cases volume overload is a significant contributor to the adverse outcome. Rapid improvement in the platelet count is usually apparent during the second week of illness, along with other laboratory parameters and the general clinical improvement.
Other organ involvement
In addition to the major clinical presentations discussed above, patients may develop severe organ involvement including cardiac, hepatic or neurological syndromes, and these are included in the definition of severe dengue in the new WHO classification. Such presentations are rare however, and represent only a tiny proportion of the total disease burden (52).
A number of cardiac manifestations ranging from myocardial dysfunction to arrhythmias, and occasionally fulminant myocarditis, are now recognized to occur (186). Myocardial impairment of varying degrees has been described using several different techniques, and in some cases likely contributes to the haemodynamic instability observed during the critical phase (106, 185).
Mild to moderate hepatomegaly with a rise in a transaminase levels is frequently seen in dengue patients, with more marked derangements usually associated with more severe disease (159, 178).
Fulminant liver failure, sometimes associated with severe haemorrhage and/or encephalopathy, occurs infrequently and mortality tends to be high (112). A possible association with aspirin and/or traditional medicine consumption has led some clinicians to suspect a Reye like syndrome, and occasionally increased ammonia levels have been documented. In view of this, and the possible effects on platelets and haemostatic competence, aspirin is contraindicated in patients with dengue.
Patients with major neurological involvement usually present with coma and convulsions after several days of a non-specific febrile illness, most often indicating an encephalopathy rather than a direct neurotropic effect of the virus. Biochemical derangements, hypoxia, cerebral oedema and intracranial haemorrhage may all contribute to this picture (23). However, there is now increasing evidence that a true encephalitis with direct viral invasion of the central nervous system does occur, albeit infrequently (20, 97, 144). Given the similarities between dengue and Japanese encephalitis viruses this neurotropism is not unexpected. Spinal cord involvement and peripheral neuropathy have also been reported occasionally (74, 136).
Laboratory Diagnosis
During the febrile stage of the illness virus isolation, using mosquito inoculation or cell culture techniques, was traditionally considered the optimal method for laboratory diagnosis of dengue. However since these techniques are time consuming and require specialist laboratory facilities, detection of viral RNA by reverse transcriptase-polymerase chain reaction (RT-PCR), has now become the preferred method. Viral RNA can be detected up to about day 5-6 of illness, rarely day 8, but as the viraemia falls this method becomes less sensitive (167). Several different PCR based techniques are available which now permit identification of the serotype, and quantification of the level of plasma viraemia, with good sensitivity, specificity and limit of detection, especially for real-time RT-PCR (64, 76, 88). Multiplex real-time RT-PCR that allows detection of DENV serotypes in a single reaction has also been developed (69).
Serological diagnosis is complicated by the existence of cross-reactive antigenic determinants shared by dengue and other members of the Flavivirus family. Capture ELISA techniques are now the most commonly employed method for serological diagnosis, but in view of this cross-reactivity, ideally tests for other locally prevalent flaviviruses should be performed in parallel with dengue virus serology. In regions where Japanese encephalitis virus (JEV) co-circulates with dengue, quantitative techniques that measure the relative levels of anti-dengue and anti-Japanese encephalitis virus IgM and IgG can differentiate between the two infections (70). Although IgM can be detected as early as 3-5 days after illness onset, paired samples demonstrating seroconversion are required to make a definitive diagnosis. The pattern of antibody responses can be helpful in differentiating between primary and secondary infections; an early IgM response detectable during the febrile period or within 2-3 days of defervescence with a slower IgG response rising over several weeks suggests a primary infection, while a rapid and marked rise in IgG levels during the first two weeks of illness in association with a less marked rise in IgM suggests a secondary infection. A number of methods using different cutoffs for the IgM/IgG ratio are suggested, but since these methods do not take into account the evolving nature of the immune response over time, interpretation is difficult. Other serological tests such as haemagglutination inhibition, complement fixation and plaque reduction neutralization tests can also be used to help differentiate primary from secondary antibody responses.
Several serological tests have been developed into commercial kits intended to assist with rapid diagnosis (162, 184). In addition commercial NS1 ELISA kits have been developed, which are highly specific in early disease, although sensitivity is variable depending on the viral serotype and/or the presence of a concurrent humoral immune response (62, 157). Rapid tests combining NS1 detection with an antibody test are also available, which can extend the window of detection (44).
Pathogenesis
The pathogenic mechanisms underlying the characteristic clinical features of severe dengue remain poorly understood. Although a number of animal models for severe dengue exist, the relationships to human clinical disease are uncertain, and thus the findings from studies using these models must be interpreted with caution. Dengue viruses have been shown to infect and replicate in a wide range of cells of endothelial and epithelial derivation in vitro, but few data are available to establish the major sites of dengue virus replication in humans. Peripheral blood mononuclear cells do support infection, and dengue virus antigen has also been detected in cells of the macrophage-monocyte lineage in the lymphoid organs, lung and liver from patients dying from dengue (11, 75, 129). Infection of dendritic cells was observed in a skin biopsy from a dengue vaccine recipient who experienced a dengue-like illness (183). However, there is no evidence that the dengue virus infects endothelial cells in vivo and only minor non-specific changes have been demonstrated in histopathological studies of the microvasculature (11).
A complex interaction between host and virus seems to determine the intensity of viral replication, and it is thought that this influences disease severity. Most researchers have found higher levels of viraemia, viral RNA, and/or free circulating NS1 in patients with dengue hemorrhagic fever as compared to dengue fever (91, 163, 167). Based on well established epidemiological evidence indicating that dengue hemorrhagic fever/severe dengue occurs predominantly in patients experiencing a second infection with a dengue virus serotype different from that encountered during their first infection or in infants with transmitted maternal antibody (58), and on in vitro studies showing that dengue infection of mononuclear cells is enhanced in the presence of cross-reactive but non-neutralizing antibodies (59), the theory of "antibody-dependent enhancement (ADE) of infection" was originally proposed to explain the pathogenesis of dengue hemorrhagic fever. The antibody-dependent enhancement hypothesis suggests that heterotypic non-neutralizing antibodies bind to the virus introduced in the second infection, and form antibody-virus complexes that bind to the Fc receptors of monocytes and macrophages, favoring uptake and productive infection in these cells, and leading to an increase in viral load in the early phase of the infection. Viral antigens are then presented by the infected cells, resulting in priming and stimulation of T lymphocytes. Cytokine release and activation of other immune cells follows, and an immunopathogenic cascade is set in motion (19, 35, 141).
Rapid mobilization of serotype cross-reactive memory T cells has been suggested as an alternative mechanism to trigger the inflammatory cascade and release vasoactive molecules. In support of this theory relatively higher frequencies of cross-reactive CD8+ T cells have been found in association with more severe disease during secondary infections (109). Cells of low affinity for the infecting virus but higher affinity for other, probably previously encountered, serotypes may be less effective at clearing the infection resulting in a higher viraemia. Further work has demonstrated the T cell response was most marked to NS3 protein, with high cytokine and low CD107a (a marker of cell degranulation) predominating (36).
However, although a range of markers of immune activation have been found in association with severe disease during secondary infections (10, 48), these response patterns do not differ significantly from those occurring in other viral infections which do not progress to a shock syndrome. Secondly, as yet no specific pathway has been identified linking these immunopathogenic mechanisms with the profound derangements in microvascular permeability and haemostatic control that are seen. In recent years considerable advances have been made towards understanding the mechanisms underlying microvascular permeability, with the current concept that intrinsic permeability is regulated by the endothelial surface glycocalyx as much as by endothelial cells themselves (105). This highly anionic proteoglycan matrix is located on the luminal surface of the vascular endothelium, anchored in the plasma membrane of endothelial cells, and forms an electrostatic barrier repelling negatively charged plasma proteins away from the endothelial surface and thus effectively restricting access to underlying cellular transport mechanisms. Preliminary evidence from children with dengue shock syndrome suggests that structural or functional changes in this layer may be important in pathogenesis (180), through the binding of heparan sulfate by the DENV E protein and the NS1 antigen (6, 26). Circulating levels of free heparan sulfate are raised in plasma of dengue patients and can be detected early in the disease (151), but further efforts to link aspects of the inflammatory response to effector mechanisms at the vascular endothelial level are needed.
Complement dysregulation has been suggested to play a role in the pathogenesis through the generation of anaphylatoxins and the terminal complement complex SC5b-9 by soluble and membrane-associated NS1 (7, 16). High levels of NS1 and SC5b-9 in the plasma of dengue patients correlated with severity and were also found in large amounts in pleural fluid of dengue shock syndrome patients along with the anaphylatoxin C5a. Other possible pathogenic mechanisms for which there is supporting data include differences in viral virulence (89, 124, 125). molecular mimicry (30, 41, 102), virus-specific plasmablast responses (46), and immune complexes (121, 167, 168). It seems likely that a complex interaction of many of these factors, together with host genetic factors, contributes to the overall disease phenotype. However, since the severe manifestations occur relatively late in the disease course, often when the infecting virus is no longer detectable in plasma, immune mediated mechanisms are thought to play a significant role in pathogenesis.
ANTIMICROBIAL THERAPY
At present no effective antimicrobial therapy exists for the treatment of dengue infections, but several different classes of viral inhibitor are under consideration, including experimental agents that inhibit viral binding and entry, intracellular multiplication and maturation, or secretion of viral particles (90, 150, 171). However the work is hampered by lack of a suitable animal model for dengue with most studies being carried out in cell culture systems that bear little resemblance to natural infections. Primates and rodents can be infected experimentally and do develop detectable viraemia but do not usually demonstrate the clinical features seen during human infection; however the use of immune deficient and humanized mice has shown some promise in better representing human pathology (152, 191). Several drugs already licensed for human use have been investigated. Ribavirin, a drug with significant in vivo activity against some RNA viruses, had little effect on viraemia in a blinded placebo-controlled study of ribavirin pre-treatment of rhesus monkeys subsequently infected with dengue (101). Pegylated recombinant alpha interferon 2a was shown to lower viraemia levels significantly and improve viral clearance as compared to placebo, when given as a single dose one day after onset of experimental DEN-2 viraemia in rhesus monkeys, but evidence from human studies is lacking (1). Another drug with potential antiviral properties is chloroquine; apart from its well-known anti-malarial effects, chloroquine exerts inhibitory effects on several viruses, such as retroviruses, coronaviruses and flaviviruses (135). However a randomized controlled trial of chloroquine in early dengue showed no clinical benefit, change in viraemia or NS1 clearance times (156). Two more recent randomized and blinded human trials assessing the safety and efficacy of other antiviral agents were also disappointing; neither balapiravir in Vietnam (115), nor celgosivir in Singapore (115), demonstrated any clinical benefit in patients with confirmed dengue, or altered virological or host inflammatory kinetics.
The search for effective dengue therapeutics continues, and with several novel treatments under investigation plus improved animal models and human infection models in development, this is a rapidly changing field (172).
ADJUNCTIVE THERAPY
The only treatment currently available for symptomatic dengue infections is supportive, with a particular focus on careful fluid management. No specific pharmacological therapy has been shown to be beneficial but the options investigated to date have been limited. Carabazochrome, an agent thought to influence haemostasis and vascular function, did not influence the severity of plasma leakage in a randomized controlled trial (154). Corticosteroid therapy, often employed for the treatment of dengue hemorrhagic fever in the early days, showed no convincing benefit on mortality from shock in several small randomized controlled trials during the 1970's and 1980's (148, 153). More recently a randomized, blinded, placebo-controlled trial of oral prednisolone in early dengue infection, with the primary goal of assessing safety during the phase of active viral replication, failed to show any effect on clinical outcome (151). Although the trial did not show evidence of harm with early prednisolone use compared to placebo, there was also no evidence of efficacy in preventing dengue shock syndrome. Currently, there is an ongoing study of statin therapy in early dengue, and it is speculated that the anti-viral and endothelial stabilizing properties of lovastatin could modulate disease severity (103, 173).
The basic principles of fluid management are described below. Paracetamol (10-15 mg/kg/4-6 hourly) is the preferred antipyretic agent for control of fever. Aspirin and non-steroidal anti-inflammatory drugs should not be administered because of their anti-platelet effects and, in the case of aspirin, because of the possible association with Reye's syndrome and severe hepatic involvement.
Parenteral fluid therapy
During the first few days of illness oral fluids should be encouraged to offset losses from high fever and vomiting. Although patients with dengue without shock may require small volumes of parenteral fluid, particularly if vomiting is severe, the primary indication for parenteral fluid therapy is in patients with significant vascular leakage leading to cardiovascular compromise. Prompt restoration of an adequate circulating plasma volume is essential, followed by fluid therapy to support the circulation at a level just sufficient to maintain critical organ perfusion until vascular permeability reverts to normal. Attempts to restore the plasma volume to normal are likely to precipitate significant fluid overload and to contribute to mortality, especially in health-care settings without access to advanced respiratory support.
The current WHO management guidelines recommend use of crystalloid solutions in the first instance, followed by plasma or colloid solutions for dengue shock syndrome patients with profound or unresponsive shock (175). Formal research studies broadly support this approach, although there are some indications that early intervention with colloid solutions may improve outcome in those with severe shock (37, 113, 181). The majority of children with shock respond to 10-20 ml/kg/hr of a crystalloid solution given for 1-2 hours, followed by a schedule reducing to maintenance levels over 6-8 hours. However, children have a relatively high requirement for fluid, and it is important for adult patients, the obese, the elderly, or those with underlying diseases, that the volume of fluid given is carefully adjusted to suit the individual circumstances and thus limit the likelihood of precipitating fluid overload. Only isotonic crystalloid solutions, which distribute primarily within the extracellular fluid compartment and thus provide the greatest intravascular support, should be used. Intravenous fluid should be stopped as soon as the patient stabilizes, ideally before the reabsorption phase of the illness begins, in most cases within 24 - 48 hours of the onset of shock.
Further research is needed to determine whether children with profound dengue shock syndrome benefit from first-line treatment with colloid solutions. However, current practice is to give patients already being treated for dengue shock syndrome who fail to respond to crystalloid infusions (no improvement in pulse pressure or pulse rate, persisting peripheral shutdown, a rising haematocrit) 10 ml/kg of a colloid solution over one hour. Some patients with severe vascular leakage experience repeated episodes of cardiovascular decompensation over the 24-36 hours after onset of dengue shock syndrome, and may require repeated supplementary treatment with small infusions of 5-10 ml/kg of colloid. The volume given on each occasion should be kept to a minimum. The selection of which colloid to use depends on local availability and personal familiarity, but an iso-oncotic medium molecular weight preparation that combines a good initial osmotic effect with reasonable intravascular persistence is probably the most appropriate choice (49). Hyper-oncotic solutions are best avoided in patients with hypovolaemic shock in view of the recognised association with acute renal failure (14, 42). Other concerns relating to the use of colloid solutions include the possibility of adverse effects on blood coagulation, particularly given the intrinsic coagulopathy associated with dengue, and the propensity of all colloids to cause allergic reactions (165). In many dengue-endemic countries hydroxyethyl starch (HES) solutions have become the standard colloid used for managing severe dengue, since dextran solutions are no longer available and gelatin based solutions have a high potential to cause allergic reactions and are perceived to be ineffective. However in the light of recent large-scale fluid intervention studies that indicate an increased risk for acute kidney injury in adults with severe sepsis treated with hydroxyethyl starch solutions, the use of any semisynthetic colloids for fluid resuscitation in critically ill patients has become controversial (138). Dengue shock syndrome is unique however, in that the population affected typically consists of young, previously healthy persons presenting with critical dysfunction of a single organ system; given the huge global burden of disease, and the limited access to ventilatory support in many dengue-endemic areas, resuscitation with crystalloids alone might well result in increased global morbidity and mortality secondary to fluid overload and respiratory failure. Formal evaluation of the indications for and usage of colloid therapy for dengue shock syndrome is warranted.
Monitoring
All patients hospitalized with dengue warning signs must be observed closely, including regular pulse and blood pressure monitoring, until they have been afebrile for at least 24 hours without antipyretics. The critical period for the development of shock is around the time of defervescence. Assessment of the degree of haemoconcentration using serial capillary haematocrit measurements is the simplest and most accessible method for monitoring the development and severity of the vascular leak syndrome. In the first few days of the illness the haematocrit should be checked daily or twice daily, but if it starts to rise rapidly or the patient's clinical condition deteriorates, it should be checked every few hours. Similarly, a platelet count should be performed on admission and then every one to two days, but if the patient develops significant mucosal bleeding or severe thrombocytopenia is documented (platelet count < 20-30,000 mm3), more frequent measurements are advisable.
Among patients with dengue shock syndrome frequent observation of pulse rate and pulse pressure (at least hourly), peripheral perfusion, mental state and urine output, together with serial haematocrit measurements are used to assess the response to treatment and guide decision making with respect to the need for additional fluid therapy. However it is important to recognize that it is the clinical response that matters, rather than normalization of the haematocrit. If the patient is warm, well perfused and has good urine output, then a relatively high but stable haematocrit is acceptable.
Management of haemorrhagic complications
In children clinically significant bleeding occurs only rarely, usually in association with profound and prolonged shock. However major mucosal bleeding (gastrointestinal, vaginal) may occur in adults without obvious vascular leakage. For most patients close inpatient observation with regular monitoring of the platelet count is all that is necessary, although nasal packing may be required for persistent epistaxis. Individuals with profound thrombocytopenia (<20,000 platelets/mm3) but without major bleeding should be managed expectantly with bed rest and protection from trauma. Platelet concentrates are not indicated and may be harmful; thrombocytopenia improves spontaneously within a few days, the half-life of transfused platelets is markedly reduced such that they are only effective for a few hours, and the patient may develop fluid overload (79, 83, 84, 98).
Major bleeding is almost always associated with very severe or prolonged shock, and is usually from the gastrointestinal tract. Contributing factors include profound thrombocytopenia, tissue hypoxia, metabolic acidosis and disseminated intravascular coagulation. Internal bleeding may not become apparent for many hours until the first melaena stool is passed. It should be considered in all those with dengue shock syndrome who fail to improve clinically after appropriate fluid resuscitation, particularly if the haematocrit is stable or falling and the abdomen is distended and tender. Transfusion should be undertaken with extreme care because of the risk of fluid overload in these circumstances; in the event that major internal bleeding is suspected in a patient with severe dengue shock syndrome, a small volume of fresh whole blood (5-10ml/kg) should be given over 1-2 hours and the response observed. Further small transfusions may be given subsequently if there is a good clinical response and significant bleeding is confirmed. Use of platelet concentrates, fresh frozen plasma and other blood products should be guided by the platelet count and coagulation profile. Two small studies indicate that recombinant activated factor VII may be helpful in controlling life threatening bleeding but further research is needed (28, 29)
Significant mucosal bleeding is more common in adults with dengue infection, often related to the presence of gastritis or peptic ulcer disease, and may contribute to shock (27, 160). More aggressive transfusion is often necessary although care is still required to avoid the pitfalls of fluid overload.
Management of other complications
Invasive procedures should generally be avoided in patients with severe dengue because of the thrombocytopenia and coagulopathy that is usually present. However, patients with dengue shock syndrome who develop severe fluid overload sometimes require haemodynamic monitoring, artificial ventilation, drainage of pleural effusions and ascites etc. Very occasionally inotropic support is required; in a large series of dengue shock syndrome patients managed over a 10 year period from a single hospital in Vietnam, only 4% required additional haemodynamic support, with the majority recovering with standard crystalloid resuscitation or following a single colloid infusion (86). Few hospitals in the tropics have sophisticated intensive care facilities where respiratory failure can be safely managed, so every effort must be made to ensure that severe overload does not occur. Fortunately, in the majority of cases fluid overload is not severe and can be successfully treated with oxygen, bed rest, and diuretic therapy once the patient is cardiovascularly stable. Diuresis proceeds rapidly as soon as the reabsorption phase of the illness commences.
Management of unusual manifestations such as dengue encephalopathy, myocarditis or fulminant hepatitis is similar to standard management for these disorders.
VACCINES
Development of a safe and effective vaccine is essential for long-term control of dengue. A combination vaccine that simultaneously induces sustained protective immunity against all four serotypes is needed to avoid creating a situation where vaccinees might be sensitized to develop severe disease in the future rather than being protected.
The first ever phase III dengue vaccine studies have recently been reported assessing a tetravalent live attenuated vaccine (CYD-TDV Sanofi-Pasteur) in approximately 30,000 participants across Asia (22) and Latin America (166). The vaccine was generally well tolerated and showed an overall efficacy of 56.5% in children aged 2-14 years in Asia and 64.7 % in children aged 9-16 years in Latin America. However, although the neutralizing antibodies to all 4 serotypes were demonstrated among vaccines participating in a substudy this did not translate into balanced protection against all 4-serotypes, with efficacy for DENV-2 of only 35% in the Asian study and 42.3% in Latin America. Long term follow-up to assess continuing safety and further work to improve our knowledge on the immunological correlates of disease will be key next steps. If, as is likely, this vaccine proceeds to full licensure in the near future, careful consideration will be needed to decide how to deploy it safely in different epidemiological situations.
Several other live attenuated vaccines have shown promise in early human trials (40, 118). There are some concerns about possible reversion to wild-type and/or recombination between vaccine virus and circulating wild-type flaviviruses allowing the emergence of recombinants with increased virulence (137). Long-term studies looking at the genetic stability of the vaccine viruses will be essential. Various strategies are also being explored to develop genetically engineered vaccines, among them recombinant subunit vaccines, chimeric vaccines, and DNA vaccines (24, 68). With the recent discovery of a new class of broadly neutralizing antibodies that is capable of potently neutralizing all four dengue serotypes, this has opened up a new avenue for the development of a future sub-unit vaccine targeting a single conserved epitope (32).
PREVENTION
Until a safe, balanced immunogenic vaccine is widely available, prevention of epidemics will continue to rely on eradication of Ae. Aegypti and other vectors by a) environmental management to eliminate potential breeding sites, and b) biological and chemical vector control. Well-organized and rapidly responsive entomological, clinical and virological surveillance systems are essential if vector control is to be effective. Biological control methods include treatment of water containers with larvivorous fish, bacteria that kill mosquito larvae but are not toxic to humans, or copepods that consume the larva while leaving the water potable. Programs using copepods of the genus Mesocyclops have proved to be successful in the short term in several countries (34, 78). However a very high level of community participation is required for such programs to be effective (77). Also, some groups remain concerned about "contamination" of their water supplies with live organisms. Another technology showing great promise for dengue control is release of biologic and/or genetically modified mosquitoes designed to invade and displace wild mosquito populations. The intracellular bacterium Wolbachia, when introduced into Aedes mosquitoes can influence the ability of the insects to transmit dengue virus, indirectly by reducing the mosquito life span and directly by reducing viral replication in the mosquito (104, 110). Studies investigating the feasibility of Wolbachia infected mosquitoes in wild mosquito populations in Australia and Vietnam are currently underway (67, 72). Genetically modified mosquitoes are also entering field trials, with promising results from engineered male Aedes mosquitoes in the Cayman Islands (63).
Chemical control of adult vectors involves use of residual insecticide sprays and both indoor and outdoor fogging techniques. Unfortunately many mosquito species have developed resistance to the commonly used insecticides following decades of use.
In the end it is likely that a combination of host protective strategies and vector control initiatives will be required if the spread of dengue is to be contained or reversed (127).
REFERENCES
1. Ajariyakhajorn C, Mammen MP Jr, Endy TP, Gettayacamin M, Nisalak A, Nimmannitya S, Libraty DH. Randomized, placebo-controlled trial of nonpegylated and pegylated forms of recombinant human alpha interferon 2a for suppression of dengue virus viremia in rhesus monkeys.Antimicrob Agents Chemother 2005;49(11):4508-14. [PubMed]
2. Alcon S, Talarmin A, Debruyne M, Falconar A, Deubel V, Flamand M. Enzyme-linked immunosorbent assay specific to Dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in the blood during the acute phase of disease in patients experiencing primary or secondary infections. J ClinMicrobiol 2002;40(2):376-81. [PubMed]
3. Alexander N, Balmaseda A, Coelho IC, Dimaano E, Hien TT, Hung NT, Janisch T, Kroeger A, Lum LC, Martinez E, Siqueira JB, Thuy TT, Villalobos I, Villegas E, Wills B; European Union, World Health Organization (WHO‐TDR) supported DENCO Study Group. Multicentre prospective study on dengue classification in four South-east Asian and three Latin American countries. Trop Med Int Health;2011;16:936-948. [PubMed]
4. Amarasinghe A, Kuritsk JN, Letson GW, Margolis HS. Dengue virus infection in Africa. Emerg Infect Dis 2011;17:1349-1354. [PubMed]
5. Anders KL, Nguyet NM, Chau NV, Hung NT, Thuy TT, Lien le B, Farrar J, Wills B, Hien TT, Simmons CP. Epidemiological factors associated with dengue shock syndrome and mortality in hospitalized dengue patients in Ho Chi Minh City, Vietnam. Amer J Trop Med Hyg 2011;84:127-134. [PubMed]
6. Avirutnan P, Zhang L, Punyadee N, Manuyakorn A, Puttikhunt C, Kasinrerk W, Malasit P, Atkinson JP, Diamond MS. Secreted NS1 of dengue virus attaches to the surface of cells via interactions with heparan sulfate and chondroitin sulfate E. PLoS Pathogens 2007;3:e183.[PubMed]
7. Avirutnan P, Punyadee N, Noisakran S, Komoltri C, Thiemmeca S, Auethavornanan K, Jairungsri A, Kanlaya R, Tangthawornchaikul N, Puttikhunt C et al: Vascular leakage in severe dengue virus infections: a potential role for the nonstructural viral protein NS1 and complement. J Infect Dis 2006;193:1078-1088.[PubMed]
8. Balmaseda A, Hammond SN, Perez MA, Cuadra R, Solano S, Rocha J, Idiaquez W, Harris E. Assessment Of The World Health Organization Scheme For Classification Of Dengue Severity In Nicaragua. Am J Trop Med Hyg 2005;73:1059-1062. [PubMed]
9. Basurko C, Carles G, Youssef M, Guindi WE. Maternal and fetal consequences of dengue fever during pregnancy. Eur J Obstet Gynecol Reprod Biol. 2009;147:29-32. [PubMed]
10. Bethell DB, Flobbe K, Cao XT, Day NP, Pham TP, Buurman WA, Cardosa MJ, White NJ, Kwiatkowski D. Pathophysiologic and prognostic role of cytokines in dengue hemorrhagic fever. J Infect Dis 1998;177:778-82. [PubMed]
11. Bhamarapravati N. Pathology of Infection. In: Gubler DJ, Kuno G, editors. Dengue and Dengue Hemorrhagic Fever. Wallingford: CAB International; 1997;pg 115-132. [PubMed]
12. Bharath Kumar Reddy KR, Laksmana RR, Veerappa BG, Shivananda. Ultrasonography as a tool in predicting the severity of dengue fever in children--a useful aid in a developing country. Pediatr Radiol 2013;43:971-977. [PubMed]
13. Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI: The global distribution and burden of dengue. Nature. 2013;496:504-7.[PubMed]
14. Biesenbach G, Kaiser W, Zazgornik J. Incidence of acute oligoanuric renal failure in dextran 40 treated patients with acute ischemic stroke stage III or IV. Ren Fail 1997;19:69-75. [PubMed]
15. Blaylock JM, Maranich A, Bauer K, Nyakoe N, Waitumbi J, Martinez LJ, Lynch J. The seroprevalence and seroincidence of dengue virus infection in western Kenya. Travel Med Infect Dis 2011;9:246-248. [PubMed]16. Bokisch VA, Top FH, Russell PK, Dixon FJ, Muller-Eberhard HJ. The potential pathogenic role of complement in dengue hemorrhagic shock syndrome. N Engl J Med 1973;289:996-1000. [PubMed]
17. Brasier AR, Ju H, Garcia J, Spratt HM, Victor SS, Forshey BM, Halsey ES, Comach G, Sierra G, Blair PJ, A three-component biomarker panel for prediction of dengue hemorrhagic fever. The American journal of tropical medicine and hygiene 2012;86:341-348. [PubMed]
18. Bravo JR, Guzman MG, Kouri GP. Why dengue haemorrhagic fever in Cuba? 1. Individual risk factors for dengue haemorrhagic fever/dengue shock syndrome (DHF/DSS). Trans R Soc Trop Med Hyg 1987;81:816-20. [PubMed]
19. Butthep P, Chunhakan S, Yoksan S, Tangnararatchakit K, Chuansumrit A. Alteration of cytokines and chemokines during febrile episodes associated with endothelial cell damage and plasma leakage in dengue hemorrhagic fever. Pediatric Infect Dis J 2012;31:e232-238.[PubMed]
20. Cam BV, Fonsmark L, Hue NB, Phuong NT, Poulsen A, Heegaard ED. Prospective case-control study of encephalopathy in children with dengue hemorrhagic fever. Am J Trop Med Hyg 2001;65:848-51. [PubMed]
21. Cao XT, Ngo TN, Wills B, Kneen R, Nguyen TT, Ta TT, Tran TT, Doan TK, Solomon T, Simpson JA, White NJ, Farrar JJ; Dong NaiPaediatric Hospital Study Group. Evaluation of the World Health Organization standard tourniquet test and a modified tourniquet test in the diagnosis of dengue infection in Viet Nam. Trop Med Int Health 2002;7:125-32. [PubMed]
22. Capeding MR, Tran NH, Hadinegoro SR, Ismail HI, Chotpitayasunondh T, Chua MN, Luong CQ, Rusmil K, Wirawan DN, Nallusamy R. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet 2014;384:1358-1365. [PubMed]
23. Carod-Artal FJ, Wichmann O, Farrar J, Gascon J. Neurological complications of dengue virus infection. Lancet Neurology 2013;12:906-919. [PubMed]
24. Chang GJ, Kuno G, Purdy DE, Davis BS. Recent advancement in flavivirus vaccine development. Expert Rev Vaccines 2004;3:199-220. [PubMed]
25. Chau TN, Quyen NT, Thuy TT, Tuan NM, Hoang DM, Dung NT, Lien le B, Quy NT, Hieu NT, Hieu LT, Hien TT, Hung NT, Farrar J, Simmons CP: Dengue in Vietnamese infants--results of infection-enhancement assays correlate with age-related disease epidemiology, and cellular immune responses correlate with disease severity. J Infect Dis 2008;198:516-524. [PubMed]
26. Chen Y, Maguire T, Hileman RE, Fromm JR, Esko JD, Linhardt RJ, Marks RM. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nature Med 1997;3:866-871. [PubMed]
27. Chiu YC, Wu KL, Kuo CH, Hu TH, Chou YP, Chuah SK, Kuo CM, Kee KM, Changchien CS, Liu JW, Chiu KW. Endoscopic findings and management of dengue patients with upper gastrointestinal bleeding. Am J Trop Med Hyg 2005;73:441-4. [PubMed]
28. Chuansumrit A, Tangnararatchakit K, Lektakul Y, Pongthanapisith V, Nimjaroenniyom N, Thanarattanakorn P, Wongchanchailert M, Komwilaisak P. The use of recombinant activated factor VII for controlling life-threatening bleeding in Dengue Shock Syndrome. Blood Coagul Fibrinolysis 2004;15:335-42. [PubMed]
29. Chuansumrit A, Wangruangsatid S, Lektrakul Y, Chua MN, Zeta Capeding MR, Bech OM; Dengue Study Group. Control of bleeding in children with Dengue hemorrhagic fever using recombinant activated factor VII: a randomized, double-blind, placebo-controlled study. Blood Coagul Fibrinolysis 2005;16:549-55. [PubMed]
30. Chungue E, Poli L, Roche C, Gestas P, Glaziou P, Markoff LJ. Correlation between detection of plasminogen cross-reactive antibodies and hemorrhage in dengue virus infection. J Infect Dis 1994;170:1304-7. [PubMed]
31. Colbert JA, Gordon A, Roxelin R, Silva S, Silva J, Rocha C, Harris E. Ultrasound measurement of gallbladder wall thickening as a diagnostic test and prognostic indicator for severe dengue in pediatric patients. Pediatric Infect Dis J 2007;26:850-852. [PubMed]
32. Dejnirattisai W, Wongwiwat W, Supasa S, Zhang X, Dai X, Rouvinsky A, Jumnainsong A, Edwards C, Quyen NT, Duangchinda T, Grimes JM, Tsai WY, Lai CY, Wang WK, Malasit P, Farrar J, Simmons CP, Zhou ZH, Rey FA, Mongkolsapaya J, Screaton GR. A new class of highly potent, broadly neutralizing antibodies isolated from viremic patients infected with dengue virus. class="jrnl" title="Nature immunology">Nat Immunol. 2015;16:170-7. [PubMed]
33. Dengue in Africa: emergence of DENV-3, Cote d'Ivoire, 2008. Releve epidemiologique hebdomadaire / Section d'hygiene du Secretariat de la Societe des Nations = Weekly epidemiological record / Health Section of the Secretariat of the League of Nations 2009, 84:85-88.
34. Dieng H, Boots M, Tuno N, Tsuda Y, Takagi M. A laboratory and field evaluation of Macrocyclopsdistinctus, Megacyclopsviridis and Mesocyclopspehpeiensis as control agents of the dengue vector Aedesalbopictus in a peridomestic area in Nagasaki, Japan. Med Vet Entomol 2002;16:285-91. [PubMed]
35. Dong T, Moran E, Vinh Chau N, Simmons C, Luhn K, Peng Y, Wills B, Phuong Dung N, Thi Thu Thao L, Hien TT, McMichael A, Farrar J, Rowland-Jones S. High pro-inflammatory cytokine secretion and loss of high avidity cross-reactive cytotoxic T-cells during the course of secondary dengue virus infection. PloS one 2007;2:e1192. [PubMed]
36. Duangchinda T, Dejnirattisai W, Vasanawathana S, Limpitikul W, Tangthawornchaikul N, Malasit P, Mongkolsapaya J, Screaton G. Immunodominant T-cell responses to dengue virus NS3 are associated with DHF. Proceedings of the National Academy of Sciences of the United States of America 2010;107:16922-16927.[PubMed]
37. Dung NM, Day NP, Tam DT, Loan HT, Chau HT, Minh LN, Diet TV, Bethell DB, Kneen R, Hien TT, White NJ, Farrar JJ. Fluid replacement in dengue shock syndrome: a randomized, double-blind comparison of four intravenous-fluid regimens. Clin Infect Dis 1999;29:787-94. [PubMed]
38. Duyen HT, Ngoc TV, Ha do T, Hang VT, Kieu NT, Young PR, Farrar JJ, Simmons CP, Wolbers M, Wills BA. Kinetics of plasma viremia and soluble nonstructural protein 1 concentrations in dengue: differential effects according to serotype and immune status. J Infect Dis 2011, 203:1292-1300. [PubMed]
39. Edeling MA, Diamond MS, Fremont DH. Structural basis of Flavivirus NS1 assembly and antibody recognition. Proceedings of the National Academy of Sciences of the United States of America>2014, 111:4285-4290. [PubMed]
40. Edelman R, Wasserman SS, Bodison SA, Putnak RJ, Eckels KH, Tang D, Kanesa-Thasan N, Vaughn DW, Innis BL, Sun W. Phase I trial of 16 formulations of a tetravalent live-attenuated dengue vaccine. Am J Trop Med Hyg 2003;69:48-60. [PubMed]
41. Falconar AK. The dengue virus nonstructural-1 protein (NS1) generates antibodies to common epitopes on human blood clotting, integrin/adhesin proteins and binds to human endothelial cells: potential implications in haemorrhagic fever pathogenesis. Arch Virol 1997;142:897-916. [PubMed]
42. Ferraboli R, Malheiro PS, Abdulkader RC, Yu L, Sabbaga E, Burdmann EA. Anuric acute renal failure caused by dextran 40 administration. Ren Fail 1997;19:303-6. [PubMed]
43. Fried JR, Gibbons RV, Kalayanarooj S, Thomas SJ, Srikiatkhachorn A, Yoon IK, Jarman RG, Green S, Rothman AL, Cummings DA. Serotype-specific differences in the risk of dengue hemorrhagic fever: an analysis of data collected in Bangkok, Thailand from 1994 to 2006. PLoS neglected tropical diseases 2010;4:e617. [PubMed]
44. Fry SR, Meyer M, Semple MG, Simmons CP, Sekaran SD, Huang JX, McElnea C, Huang CY, Valks A, Young PR. The diagnostic sensitivity of dengue rapid test assays is significantly enhanced by using a combined antigen and antibody testing approach. PLoS Neglected Tropical Diseases 2011;5:e1199. [PubMed]
45. Gamble J, Bethell D, Day NP, Loc PP, Phu NH, Gartside IB, Farrar JF, White NJ. Age-related changes in microvascular permeability: a significant factor in the susceptibility of children to shock? ClinSci (Lond) 2000;98:211-6. [PubMed]
46. Garcia-Bates TM, Cordeiro MT, Nascimento EJ, Smith AP, Soares de Melo KM, McBurney SP, Evans JD, Marques ET, Jr., Barratt-Boyes SM. Association between magnitude of the virus-specific plasmablast response and disease severity in dengue patients. J Immunol 2012;190:80-87. [PubMed]
47. Gonzalez D, Castro OE, Kouri G, Perez J, Martinez E, Vazquez S, Rosario D, Cancio R, Guzman MG. Classical dengue hemorrhagic fever resulting from two dengue infections spaced 20 years or more apart: Havana, Dengue 3 epidemic, 2001-2002. Int J Infect Dis 2005;9:280-5. [PubMed]
48. Green S, Vaughn DW, Kalayanarooj S, Nimmannitya S, Suntayakorn S, Nisalak A, Lew R, Innis BL, Kurane I, Rothman AL, Ennis FA. Early immune activation in acute dengue illness is related to development of plasma leakage and disease severity. J Infect Dis 1999;179:755-62. [PubMed]
49. Griffel MI, Kaufman BS. Pharmacology of colloids and crystalloids.Crit Care Clin 1992;8:235-53. [PubMed]
50.Gubler DJ, Suharyono W, Tan R, Abidin M, Sie A. Viremia in patients with naturally acquired dengue infection. Bull World Health Organ 1981;59:623-30. [PubMed]
51. Gubler DJ. Dengue and dengue hemorrhagic fever.ClinMicrobiol Rev 1998;11:480-96. [PubMed]
52. Gulati S, Maheshwari A. Atypical manifestations of dengue. Trop Med Int Health 2007;12:1087-1095. [PubMed]
53. Gutsche I, Coulibaly F, Voss JE, Salmon J, d'Alayer J, Ermonval M, Larquet E, Charneau P, Krey T, Megret F et al. Secreted dengue virus nonstructural protein NS1 is an atypical barrel-shaped high-density lipoprotein. Proc Natl Acad Sci USA. 2011;108:8003-8008. <[PubMed]
54. Guzman MG, Alvarez M, Rodriguez R, Rosario D, Vazquez S, Vald s L, Cabrera MV, Kouri G. Fatal dengue hemorrhagic fever in Cuba, 1997. Int J Infect Dis 1999;3:130-5. [PubMed]
55. Guzman MG, Kouri G, Bravo J, Valdes L, Vazquez S, Halstead SB. Effect of age on outcome of secondary dengue 2 infections. Int J Infect Dis 2002;6:118-24. [PubMed]
56. Halstead SB. Dengue and hemorrhagic fevers of Southeast Asia. Yale J Biol Med 1965;37:434-54. [PubMed]
57. Halstead SB. Observations related to pathogenesis of dengue hemorrhagic fever. VI. Hypotheses and discussion. Yale J Biol Med 1970;42:350-62. [PubMed]
58. Halstead SB, Nimmannitya S, Cohen SN. Observations related to pathogenesis of dengue hemorrhagic fever. IV. Relation of disease severity to antibody response and virus recovered. Yale J Biol Med 1970;42:311-28. [PubMed]
59. Halstead SB, O'Rourke EJ. Antibody-enhanced dengue virus infection in primate leukocytes. Nature 1977;265:739-41. [PubMed]
60. Hammon WM, Rudnick A, Sather G, Rogers KD, Morse LJ. New hemorrhagic fevers of children in the Phillipines and Thailand. Transactions of the Association of American Physicians 1960;73:140-155. [PubMed]
61. Hammond SN, Balmaseda A, Perez L, Tellez Y, Saborio SI, Mercado JC, Videa E, Rodriguez Y, Perez MA, Cuadra R, Solano S, Rocha J, Idiaquez W, Gonzalez A, Harris E. Differences In Dengue Severity In Infants, Children, And Adults In A 3-Year Hospital-Based Study In Nicaragua. Am J Trop Med Hyg 2005;73:1063-1070. [PubMed]
62. Hang VT, Nguyet NM, Trung DT, Tricou V, Yoksan S, Dung NM, Van Ngoc T, Hien TT, Farrar J, Wills B, Simmons CP. Diagnostic accuracy of NS1 ELISA and lateral flow rapid tests for dengue sensitivity, specificity and relationship to viraemia and antibody responses. PLoS Negl Trop Dis 2009;3:e360. [PubMed]
63. Harris AF, Nimmo D, McKemey AR, Kelly N, Scaife S, Donnelly CA, Beech C, Petrie WD, Alphey L. Field performance of engineered male mosquitoes. Nature Biotechnol 2011;29:1034-1037. [PubMed]
64. Harris E, Roberts TG, Smith L, Selle J, Kramer LD, Valle S, Sandoval E, Balmaseda A. Typing of dengue viruses in clinical specimens and mosquitoes by single- tube multiplex reverse transcriptase PCR. J ClinMicrobiol 1998;36:2634-9. [PubMed]
65. Harris E, Videa E, Perez L, Sandoval E, Tellez Y, Perez ML, Cuadra R, Rocha J, Idiaquez W, Alonso RE, Delgado MA, Campo LA, Acevedo F, Gonzalez A, Amador JJ, Balmaseda A. Clinical, epidemiologic, and virologic features of dengue in the 1998 epidemic in Nicaragua. Am J Trop Med Hyg 2000;63:5-11. [PubMed]
66. Hayes CG, Manaloto CR, Gonzales A, Ranoa CP. Dengue infections in the Philippines: clinical and virological findings in 517 hospitalized patients. Am J Trop Med Hyg 1988;39(1):110-6. [PubMed]
67. Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, Muzzi F, Greenfield M, Durkan M, Leong YS, Dong Y, Cook H, Axford J, Callahan AG, Kenny N, Omodei C, McGraw EA, Ryan PA, Ritchie SA, Turelli M, O'Neill SL. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 2011; 476:454-457. [PubMed]
68. Hombach J, Barrett AD, Cardosa MJ, Deubel V, Guzman M, Kurane I, Roehrig JT, Sabchareon A, Kieny MP. Review on flavivirus vaccine development. Proceedings of a meeting jointly organised by the World Health Organization and the Thai Ministry of Public Health, 26-27 April 2004, Bangkok, Thailand. Vaccine 2005;23:2689-95. [PubMed]
69. Hue KD, Tuan TV, Thi HT, Bich CT, Anh HH, Wills BA, Simmons CP. Validation of an internally controlled one-step real-time multiplex RT-PCR assay for the detection and quantitation of dengue virus RNA in plasma. J Virol Methods 2011;177:168-173. [PubMed]
70. Innis BL, Nisalak A, Nimmannitya S, Kusalerdchariya S, Chongswasdi V, Suntayakorn S, Puttisri P, Hoke CH. An enzyme-linked immunosorbent assay to characterize dengue infections where dengue and Japanese encephalitis co-circulate. Am J Trop Med Hyg 1989;40:418-27. [PubMed]
71. Isarangkura P, Tuchinda S. The behavior of transfused platelets in dengue hemorrhagic fever. Southeast Asian J Trop Med Public Health 1993;24:222-4. [PubMed]
72. Jeffery JA, Thi Yen N, Nam VS, Nghia le T, Hoffmann AA, Kay BH, Ryan PA. Characterizing the Aedes aegypti population in a Vietnamese village in preparation for a Wolbachia-based mosquito control strategy to eliminate dengue. PLoS NeglTrop Dis 2009;3:e552. [PubMed]
73. Kabra SK, Jain Y, Pandey RM, Madhulika, Singhal T, Tripathi P, Broor S, Seth P, Seth V. Dengue haemorrhagic fever in children in the 1996 Delhi epidemic. Trans R Soc Trop Med Hyg 1999;93:294-8. [PubMed]
74. Kalita J, Misra UK, Mahadevan A, Shankar SK. Acute pure motor quadriplegia: is it dengue myositis? Electromyogr Clin Neurophysiol 2005;45:357-61. [PubMed]
75. Kangwanpong D, Bhamarapravati N, Lucia HL. Diagnosing dengue virus infection in archived autopsy tissues by means of the in situ PCR method: a case report. ClinDiagnVirol 1995;3:165-72. [PubMed]
76. Kao CL, King CC, Chao DY, Wu HL, Chang GJ. Laboratory diagnosis of dengue virus infection: current and future perspectives in clinical diagnosis and public health. J MicrobiolImmunol Infect 2005;38:5-16. [PubMed]
77. Kay B, Vu SN. New strategy against Aedesaegypti in Vietnam. Lancet 2005;365:613-7. [PubMed]
78. Kay BH, Nam VS, Tien TV, Yen NT, Phong TV, Diep VT, Ninh TU, Bektas A, Aaskov JG. Control of aedes vectors of dengue in three provinces of Vietnam by use of Mesocyclops (Copepoda) and community-based methods validated by entomologic, clinical, and serological surveillance. Am J Trop Med Hyg 2002;66:40-8. [PubMed]
79. Khan Assir MZ, Kamran U, Ahmad HI, Bashir S, Mansoor H, Anees SB, Akram J. Effectiveness of platelet transfusion in dengue Fever: a randomized controlled trial. Transfusion medicine and Chemotherapy 2013:40:362-368.[PubMed]
80. Khor CC, Chau TN, Pang J, Davila S, Long HT, Ong RT, Dunstan SJ, Wills B, Farrar J, Van Tram T, Gan TT, Binh NT, Tri le T, Lien le B, Tuan NM, Tham NT, Lanh MN, Nguyet NM, Hieu NT, Van N Vinh Chau N, Thuy TT, Tan DE, Sakuntabhai A, Teo YY, Hibberd ML, Simmons CP.> Genome-wide association study identifies susceptibility loci for dengue shock syndrome at MICB and PLCE1. Nat Genet 2011;43:1139-1141. [PubMed]
81. Kliks S, Nimmanitya S, Nisalak A, Burke D. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am J Trop Med Hyg 1988;38:411-9. [PubMed]
82. Krishnamurti C, Kalayanarooj S, Cutting MA, Peat RA, Rothwell SW, Reid TJ, Green S, Nisalak A, Endy TP, Vaughn DW, Nimmannitya S, Innis BL. Mechanisms of hemorrhage in dengue without circulatory collapse. Am J Trop Med Hyg 2001;65:840-7. [PubMed]
83. Kuhn RJ, Zhang W, Rossmann MG, Pletnev SV, Corver J, Lenches E, Jones CT, Mukhopadhyay S, Chipman PR, Strauss EG, Baker TS, Strauss JH. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell 2002;108:717-25. [PubMed]
84. Kumar ND, Tomar V, Singh B, Kela K. Platelet transfusion practice during dengue fever epidemic. Indian J PatholMicrobiol 2000;43:55-60. [PubMed]
85. Kuniholm MH, Wolfe ND, Huang CY, Mpoudi-Ngole E, Tamoufe U, LeBreton M, Burke DS, Gubler DJ. Seroprevalence and distribution of Flaviviridae, Togaviridae, and Bunyaviridae arboviral infections in rural Cameroonian adults. The American journal of tropical medicine and hygiene 2006;74:1078-1083.[PubMed]
86. Lam PK, Tam DT, Diet TV, Tam CT, Tien NT, Kieu NT, Simmons C, Farrar J, Nga NT, Qui PT, Dung NM, Wolbers M, Wills B. Clinical characteristics of dengue shock syndrome in vietnamese children: a 10-year prospective study in a single hospital. Clin Infect Dis 2013;57:1577-1586. [PubMed]
87. Lambrechts L, Paaijmans KP, Fansiri T, Carrington LB, Kramer LD, Thomas MB, Scott TW. Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. Proc Natl Acad Sci U S A.2011;108:7460-7465. [PubMed]
88. Lanciotti RS, Calisher CH, Gubler DJ, Chang GJ, Vorndam AV. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J ClinMicrobiol 1992;30:545-51. PubMed]
89. Leitmeyer KC, Vaughn DW, Watts DM, Salas R, Villalobos I, de Chacon, Ramos C, Rico-Hesse R. Dengue virus structural differences that correlate with pathogenesis. J Virol 1999;73:4738-47. PubMed]
90. Leyssen P, De Clercq E, Neyts J. Perspectives for the treatment of infections with Flaviviridae. Clin Microbiol Rev 2000;13:67-82, table of contents. PubMed]
91. Libraty DH, Endy TP, Houng HS, Green S, Kalayanarooj S, Suntayakorn S, Chansiriwongs W, Vaughn DW, Nisalak A, Ennis FA, Rothman AL. Differing influences of virus burden and immune activation on disease severity in secondary dengue-3 virus infections. J Infect Dis 2002;185:1213-21. PubMed]
92. Libraty DH, Young PR, Pickering D, Endy TP, Kalayanarooj S, Green S, Vaughn DW, Nisalak A, Ennis FA, Rothman AL. High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J Infect Dis 2002;186:1165-8. PubMed]
93. Libraty DH, Young PR, Pickering D, Endy TP, Kalayanarooj S, Green S, Vaughn DW, Nisalak A, Ennis FA, Rothman AL. High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. The Journal of infectious diseases 2002;186:1165-1168. PubMed]
94. Loke H, Bethell DB, Phuong CX, Dung M, Schneider J, White NJ, Day NP, Farrar J, Hill AV. Strong HLA class I--restricted T cell responses in dengue hemorrhagic fever: a double-edged sword? J Infect Dis 2001;184:1369-73. PubMed]
95. Loke H, Bethell D, Phuong CX, Day N, White N, Farrar J, Hill A. Susceptibility to dengue hemorrhagic fever in vietnam: evidence of an association with variation in the vitamin d receptor and Fc gamma receptor IIa genes. Am J Trop Med Hyg 2002;67:102-6. PubMed]
96. Low JG, Sung C, Wijaya L, Wei Y, Rathore AP, Watanabe S, Tan BH, Toh L, Chua LT, Hou Y, Chow A, Howe S, Chan WK, Tan KH, Chung JS, Cherng BP, Lye DC, Tambayah PA, Ng LC, Connolly J, Hibberd ML, Leo YS, Cheung YB, Ooi EE, Vasudevan SG. Efficacy and safety of celgosivir in patients with dengue fever (CELADEN): a phase 1b, randomised, double-blind, placebo-controlled, proof-of-concept trial. Lancet Infect Dis 2014;14:706-715. PubMed]
97. Lum LC, Lam SK, Choy YS, George R, Harun F. Dengue encephalitis: a true entity? Am J Trop Med Hyg 1996;54:256-9. PubMed]
98. Lye DC, Lee VJ, Sun Y, Leo YS. Lack of efficacy of prophylactic platelet transfusion for severe thrombocytopenia in adults with acute uncomplicated dengue infection. Clin Infect Dis>2009;48:1262-1265.
99. Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med 2004;10:S98-109. PubMed]
100. Mairuhu AT, Mac Gillavry MR, Setiati TE, Soemantri A, ten Cate H, Brandjes DP, van Gorp EC. Is clinical outcome of dengue-virus infections influenced by coagulation and fibrinolysis? A critical review of the evidence. Lancet Infect Dis 2003;3:33-41. PubMed]
101. Malinoski FJ, Hasty SE, Ussery MA, Dalrymple JM. Prophylactic ribavirin treatment of dengue type 1 infection in rhesus monkeys. Antiviral Res 1990;13:139-49. PubMed]
102. Markoff LJ, Innis BL, Houghten R, Henchal LS. Development of cross-reactive antibodies to plasminogen during the immune response to dengue virus infection. J Infect Dis 1991;164:294-301. PubMed]
103. Martinez-Gutierrez M, Castellanos JE, Gallego-Gomez JC. Statins reduce dengue virus production via decreased virion assembly. Intervirology 2011;54:202-216. PubMed]
104. McMeniman CJ, Lane RV, Cass BN, Fong AW, Sidhu M, Wang YF, O'Neill SL. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science (New York, NY 2009:323:141-144.PubMed]
105. Michel CC, Curry FE. Microvascular permeability. Physiol Rev 1999;79:703-61. PubMed]
106. Miranda CH, Borges Mde C, Matsuno AK, Vilar FC, Gali LG, Volpe GJ, Schmidt A, Pazin-Filho A, Silva FM, Castro-Jorge LA, Evaluation of cardiac involvement during dengue viral infection. Clin Infect Dis 2013;57:812-819. PubMed]
107. Mitrakul C, Poshyachinda M, Futrakul P, Sangkawibha N, Ahandrik S. Hemostatic and platelet kinetic studies in dengue hemorrhagic fever. Am J Trop Med Hyg 1977;26:975-84. PubMed]
108. Modis Y, Ogata S, Clements D, Harrison SC. Structure of the dengue virus envelope protein after membrane fusion. Nature 2004;427:313-9. PubMed]
109. Mongkolsapaya J, Dejnirattisai W, Xu XN, Vasanawathana S, Tangthawornchaikul N, Chairunsri A, Sawasdivorn S, Duangchinda T, Dong T, Rowland-Jones S, Yenchitsomanus PT, McMichael A, Malasit P, Screaton G. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat Med 2003;9:921-7. PubMed]
110. Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu G, Pyke AT, Hedges LM, Rocha BC, Hall-Mendelin S, Day A, Riegler M, Hugo LE, Johnson KN, Kay BH, McGraw EA, van den Hurk AF, Ryan PA, O'Neill SL.>A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 2009;139:1268-1278. PubMed]
111. Muller DA, Landsberg MJ, Bletchly C, Rothnagel R, Waddington L, Hankamer B, Young PR. Structure of the dengue virus glycoprotein non-structural protein 1 by electron microscopy and single-particle analysis. The Journal of general virology 2012;93:771-779. PubMed]
112. Murgue B, Deparis X, Chungue E, Cassar O, Roche C. Dengue: an evaluation of dengue severity in French Polynesia based on an analysis of 403 laboratory-confirmed cases. Trop Med Int Health 1999;4:765-73. [PubMed]
113. Ngo NT, Cao XT, Kneen R, Wills B, Nguyen VM, Nguyen TQ, Chu VT, Nguyen TT, Simpson JA, Solomon T, White NJ, Farrar J. Acute management of dengue shock syndrome: a randomized double-blind comparison of 4 intravenous fluid regimens in the first hour. Clin Infect Dis 2001;32:204-13. [PubMed]
114. Nguyen TH, Nguyen TL, Lei HY, Lin YS, Le BL, Huang KJ, Lin CF, Do QH, Vu TQ, Lam TM, Yeh TM, Huang JH, Liu CC, Halstead SB. Association between sex, nutritional status, severity of dengue hemorrhagic fever, and immune status in infants with dengue hemorrhagic fever. Am J Trop Med Hyg 2005;72:370-4. [PubMed]
115. Nguyen NM, Tran CN, Phung LK, Duong KT, Huynh Hle A, Farrar J, Nguyen QT, Tran HT, Nguyen CV, Merson L, Hoang LT, Hibberd ML, Aw PP, Wilm A, Nagarajan N, Nguyen DT, Pham MP, Nguyen TT, Javanbakht H, Klumpp K, Hammond J, Petric R, Wolbers M, Nguyen CT, Simmons CP. A randomized, double-blind placebo controlled trial of balapiravir, a polymerase inhibitor, in adult dengue patients. The Journal of infectious diseases 2013;207:1442-1450.[PubMed]
116. Nguyet MN, Duong TH, Trung VT, Nguyen TH, Tran CN, Long VT, Dui le T, Nguyen HL, Farrar JJ, Holmes EC, Rabaa MA, Bryant JE, Nguyen TT, Nguyen HT, Nguyen LT, Pham MP, Nguyen HT, Luong TT, Wills B, Nguyen CV, Wolbers M, Simmons CP. Host and viral features of human dengue cases shape the population of infected and infectious Aedes aegypti mosquitoes. Proceedings of the National Academy of Sciences of the United States of America 2013:110:9072-9077.[PubMed]
117. Nisalak A, Endy TP, Nimmannitya S, Kalayanarooj S, Thisayakorn U, Scott RM, Burke DS, Hoke CH, Innis BL, Vaughn DW. Serotype-specific dengue virus circulation and dengue disease in Bangkok, Thailand from 1973 to 1999. Am J Trop Med Hyg 2003;68:191-202. [PubMed]
118. Osorio JE, Velez ID, Thomson C, Lopez L, Jimenez A, Haller AA, Silengo S, Scott J, Boroughs KL, Stovall JL, Luy BE, Arguello J, Beatty ME, Santangelo J, Gordon GS, Huang CY, Stinchcomb DT. Safety and immunogenicity of a recombinant live attenuated tetravalent dengue vaccine (DENVax) in flavivirus-naive healthy adults in Colombia: a randomised, placebo-controlled, phase 1 study. Lancet Infect Dis 2014;14:830-838. [PubMed]
119. Pang J, Salim A, Lee VJ, Hibberd ML, Chia KS, Leo YS, Lye DC. Diabetes with hypertension as risk factors for adult dengue hemorrhagic fever in a predominantly dengue serotype 2 epidemic: a case control study. PLoS neglected tropical diseases 2012;6:e1641. [PubMed]
120. Paton NI, Aboulhab J. Hydroxychloroquine, hydroxyurea and didanosine as initial therapy for HIV-infected patients with low viral load: safety, efficacy and resistance profile after 144 weeks. HIV Med 2005;6:13-20. [PubMed]
121. Petchclai B, Saelim P. Circulating immune complexes in dengue haemorrhagic fever. Lancet 1978;2:638-9. [PubMed]
122. Phuong CX, Nhan NT, Kneen R, Thuy PT, van Thien C, Nga NT, Thuy TT, Solomon T, Stepniewska K, Wills B; Dong Nai Study Group. Clinical diagnosis and assessment of severity of confirmed dengue infections in Vietnamese children: is the world health organization classification system helpful? Am J Trop Med Hyg 2004;70:172-9. [PubMed]
123. Reinert JF, Harbach RE, Kitching IJ. Phylogeny and classification of Aedini (Diptera: Culicidae), based on morphological characters of all life stages. Zool. J. LinneanSoc 2004;142:289-368. [PubMed]
124. Rico-Hesse R. Dengue virus virulence and transmission determinants. Curr Top Microbiol Immunol 2010;338:45-55. [PubMed]
125. Rico-Hesse R, Harrison LM, Salas RA, Tovar D, Nisalak A, Ramos C, Boshell J, de Mesa MT, Nogueira RM, da Rosa AT. Origins of dengue type 2 viruses associated with increased pathogenicity in the Americas. Virology 1997;230:244-51. [PubMed]
126. Rico-Hesse R. Microevolution and virulence of dengue viruses. Adv Virus Res 2003;59:315-41. [PubMed]
127. Roberts CH, Mongkolsapaya J, Screaton G. New opportunities for control of dengue virus. Curr Opin Infect Dis 2013;26:567-574. [PubMed]
128. Rosen L, Gubler D. The use of mosquitoes to detect and propagate dengue viruses. Am J Trop Med Hyg 1974;23:1153-60. [PubMed]
129. Rosen L, Drouet MT, Deubel V. Detection of dengue virus RNA by reverse transcription-polymerase chain reaction in the liver and lymphoid organs but not in the brain in fatal human infection. Am J Trop Med Hyg 1999;61:720-4. [PubMed]
130. Rush AB. An account of the bilious remitting fever as it appeared in Philadelphia in the summer and autumn of the year 1780. In: Medical Enquiries and Observations. Philadelphia: Prichard and Hall; 1789. p. 104-117.
131. Sabchareon A, Lang J, Chanthavanich P, Yoksan S, Forrat R, Attanath P, Sirivichayakul C, Pengsaa K, Pojjaroen-Anant C, Chokejindachai W, Jagsudee A, Saluzzo JF, Bhamarapravati N. Safety and immunogenicity of tetravalent live-attenuated dengue vaccines in Thai adult volunteers: role of serotype concentration, ratio, and multiple doses. Am J Trop Med Hyg 2002;66:264-72. [PubMed]
132. Sabchareon A, Lang J, Chanthavanich P, Yoksan S, Forrat R, Attanath P, Sirivichayakul C, Pengsaa K, Pojjaroen-Anant C, Chambonneau L, Saluzzo JF, Bhamarapravati N. Safety and immunogenicity of a three dose regimen of two tetravalent live-attenuated dengue vaccines in five- to twelve-year-old Thai children. Pediatr Infect Dis J 2004;23:99-109. [PubMed]
133. Sakuntabhai A, Turbpaiboon C, Casademont I, Chuansumrit A, Lowhnoo T, Kajaste-Rudnitski A, Kalayanarooj SM, Tangnararatchakit K, Tangthawornchaikul N, Vasanawathana S, Chaiyaratana W, Yenchitsomanus PT, Suriyaphol P, Avirutnan P, Chokephaibulkit K, Matsuda F, Yoksan S, Jacob Y, Lathrop GM, Malasit P, Desprès P, Julier C.A variant in the CD209 promoter is associated with severity of dengue disease. Nat Genet 2005;37:507-513. [PubMed]
134. Sangkawibha N, Rojanasuphot S, Ahandrik S, Viriyapongse S, Jatanasen S, Salitul V, Phanthumachinda B, Halstead SB. Risk factors in dengue shock syndrome: a prospective epidemiologic study in Rayong, Thailand. I. The 1980 outbreak. Am J Epidemiol 1984;120:653-69. [PubMed]
135. Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: an old drug against today's diseases? Lancet Infect Dis 2003;3:722-7. [PubMed]
136. Seet RC, Lim EC, Wilder-Smith EP. Acute transverse myelitis following dengue virus infection. J ClinVirol 2006;35:310-2. [PubMed]
137. Seligman SJ, Gould EA. Live flavivirus vaccines: reasons for caution. Lancet 2004;363:2073-5. [PubMed]
138. Serpa Neto A, Veelo DP, Peireira VG, de Assuncao MS, Manetta JA, Esposito DC, Schultz MJ. Fluid resuscitation with hydroxyethyl starches in patients with sepsis is associated with an increased incidence of acute kidney injury and use of renal replacement therapy: a systematic review and meta-analysis of the literature. J Crit Care 2014;29:185 e181-187. [PubMed]
139. Setiawan MW, Samsi TK, Wulur H, Sugianto D, Pool TN. Dengue haemorrhagic fever: ultrasound as an aid to predict the severity of the disease. Pediatr Radiol 1998;28:1-4. [PubMed]
140. Shaio MF, Chang FY, Hou SC. Complement pathway activity in serum from patients with classical dengue fever. Trans R Soc Trop Med Hyg 1992;86:672-5. [PubMed]
141. Sierra B, Perez AB, Alvarez M, Garcia G, Vogt K, Aguirre E, Schmolke K, Volk HD, Guzman MG. Variation in inflammatory/regulatory cytokines in secondary, tertiary, and quaternary challenges with dengue virus. Am J Trop Med Hyg 2012;87:538-547. [PubMed]
142. Simmons CP, Farrar JJ, Nguyen VV, Wills B: Dengue. New Engl J Med 2012;366:1423-1432. [PubMed]
143. Simmons CP, Chau TN, Thuy TT, Tuan NM, Hoang DM, Thien NT, Lien le B, Quy NT, Hieu NT, Hien TT, McElnea C, Young P, Whitehead S, Hung NT, Farrar J.>Maternal antibody and viral factors in the pathogenesis of dengue virus in infants. J Infect Dis>2007;196:416-424. [PubMed]
144. Solomon T, Dung NM, Vaughn DW, Kneen R, Thao LT, Raengsakulrach B, Loan HT, Day NP, Farrar J, Myint KS, Warrell MJ, James WS, Nisalak A, White NJ. Neurological manifestations of dengue infection. Lancet 2000;355:1053-9. [PubMed]
145. Srichaikul T, Nimmannitya S, Sripaisarn T, Kamolsilpa M, Pulgate C. Platelet function during the acute phase of dengue hemorrhagic fever. Southeast Asian J Trop Med Public Health 1989;20:19-25. [PubMed]
146. Srikiatkhachorn A, Krautrachue A, Ratanaprakarn W, Wongtapradit L, Nithipanya N, Kalayanarooj S, Nisalak A, Thomas SJ, Gibbons RV, Mammen MP, Libraty DH, Ennis FA, Rothman AL, Green S. Natural history of plasma leakage in dengue hemorrhagic fever: a serial ultrasonographic study. Pediatr Infect Dis 2007;26:283-290. [PubMed]
147. Stephens HA, Klaythong R, Sirikong M, Vaughn DW, Green S, Kalayanarooj S, Endy TP, Libraty DH, Nisalak A, Innis BL, Rothman AL, Ennis FA, Chandanayingyong D. HLA-A and -B allele associations with secondary dengue virus infections correlate with disease severity and the infecting viral serotype in ethnic Thais. Tissue Antigens 2002;60:309-18. [PubMed]
148. Sumarmo, Talogo W, Asrin A, Isnuhandojo B, Sahudi A. Failure of hydrocortisone to affect outcome in dengue shock syndrome. Pediatrics 1982;69:45-9. [PubMed]
149. Tai DY, Chee YC, Chan KW. The natural history of dengue illness based on a study of hospitalised patients in Singapore. Singapore Med J 1999;40:238-242. [PubMed]
150. Talarico LB, Pujol CA, Zibetti RG, Faria PC, Noseda MD, Duarte ME, Damonte EB. The antiviral activity of sulfated polysaccharides against dengue virus is dependent on virus serotype and host cell. Antiviral Res 2005;66:103-10. [PubMed]
151. Tam DT, Ngoc TV, Tien NT, Kieu NT, Thuy TT, Thanh LT, Tam CT, Truong NT, Dung NT, Qui PT, Hien TT, Farrar JJ, Simmons CP, Wolbers M, Wills BA. Effects of short-course oral corticosteroid therapy in early dengue infection in Vietnamese patients: a randomized, placebo-controlled trial. Clin Infect Dis 2012;55:1216-1224. [PubMed]
152. Tan GK, Ng JK, Trasti SL, Schul W, Yip G, Alonso S. A non mouse-adapted dengue virus strain as a new model of severe dengue infection in AG129 mice. PLoS Negl Trop Dis 2010;4:e672. [PubMed]
153. Tassniyom S, Vasanawathana S, Chirawatkul A, Rojanasuphot S. Failure of high-dose methylprednisolone in established dengue shock syndrome: a placebo-controlled, double-blind study. Pediatrics 1993;92:111-5. [PubMed]
154. Tassniyom S, Vasanawathana S, Dhiensiri T, Nisalak A, Chirawatkul A. Failure of carbazochrome sodium sulfonate (AC-17) to prevent dengue vascular permeability or shock: a randomized, controlled trial. J Pediatr 1997;131:525-8. [PubMed]
155. Tomasello D, Schlagenhauf P. Chikungunya and dengue autochthonous cases in Europe, 2007-2012. Travel medicine and infectious disease 2013;11:274-284. [PubMed]
156. Tricou V, Minh NN, Van TP, Lee SJ, Farrar J, Wills B, Tran HT, Simmons CP. A randomized controlled trial of chloroquine for the treatment of dengue in Vietnamese adults. PLoS neglected tropical diseases 2010; 4:e785. [PubMed]
157. Tricou V, Vu HT, Quynh NV, Nguyen CV, Tran HT, Farrar J, Wills B, Simmons CP. Comparison of two dengue NS1 rapid tests for sensitivity, specificity and relationship to viraemia and antibody responses. BMC Infect Dis 2010;10:142. [PubMed]
158. Trung DT, Thao le TT, Dung NM, Ngoc TV, Hien TT, Chau NV, Wolbers M, Tam DT, Farrar J, Simmons C, Wills B. Clinical features of dengue in a large Vietnamese cohort: intrinsically lower platelet counts and greater risk for bleeding in adults than children. PLoS neglected tropical diseases 2012;6:e1679. [PubMed]
159. Trung DT, Thao le TT, Hien TT, Hung NT, Vinh NN, Hien PT, Chinh NT, Simmons C, Wills B. Liver involvement associated with dengue infection in adults in Vietnam. Am J Trop Med Hyg. 2010;83:774-780. [PubMed]
160. Tsai CJ, Kuo CH, Chen PC, Changcheng CS. Upper gastrointestinal bleeding in dengue fever. Am J Gastroenterol 1991;86:33-5. [PubMed]
161. Vairo F, Nicastri E, Meschi S, Schepisi MS, Paglia MG, Bevilacqua N, Mangi S, Sciarrone MR, Chiappini R, Mohamed J, Racalbuto V, Di Caro A, Capobianchi MR, Ippolito G. Seroprevalence of dengue infection: a cross-sectional survey in mainland Tanzania and on Pemba Island, Zanzibar. Int J Infect 2012;16:e44-46. [PubMed]
162. Vaughn DW, Nisalak A, Solomon T, Kalayanarooj S, Nguyen MD, Kneen R, Cuzzubbo A, Devine PL. Rapid serologic diagnosis of dengue virus infection using a commercial capture ELISA that distinguishes primary and secondary infections. Am J Trop Med Hyg 1999;60:693-8. [PubMed]
163. Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, Endy TP, Raengsakulrach B, Rothman AL, Ennis FA, Nisalak A. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis 2000;181:2-9. [PubMed]
164. Vazeille M, Moutailler S, Pages F, Jarjaval F, Failloux AB. Introduction of Aedes albopictus in Gabon: what consequences for dengue and chikungunya transmission? Troop Med Int Health 2008;13:1176-1179. [PubMed]
165. Vercueil A, Grocott MP, Mythen MG. Physiology, pharmacology, and rationale for colloid administration for the maintenance of effective hemodynamic stability in critically ill patients. Transfus Med Rev 2005;19:93-109. [PubMed]
166. Villar L, Dayan GH, Arredondo-Garcia JL, Rivera DM, Cunha R, Deseda C, Reynales H, Costa MS, Morales-Ramirez JO, Carrasquilla G, Rey LC, Dietze R, Luz K, Rivas E, Miranda Montoya MC, Cortés Supelano M, Zambrano B, Langevin E, Boaz M, Tornieporth N, Saville M, Noriega F; CYD15 Study Group. Efficacy of a Tetravalent Dengue Vaccine in Children in Latin America. New Engl J Med 2015;372:113-23 [PubMed]
167. Wang WK, Chao DY, Kao CL, Wu HC, Liu YC, Li CM, Lin SC, Ho ST, Huang JH, King CC. High levels of plasma dengue viral load during defervescence in patients with dengue hemorrhagic fever: implications for pathogenesis. Virology 2003;305:330-8. [PubMed]
168. Wang WK, Chen HL, Yang CF, Hsieh SC, Juan CC, Chang SM, Yu CC, Lin LH, Huang JH, King CC. Slower rates of clearance of viral load and virus-containing immune complexes in patients with dengue hemorrhagic fever. Clin Infect Dis 2006;43:1023-1030. [PubMed]
169. Ward MA, Burgess NR. Aedes albopictus--a new disease vector for Europe? J R Army Med Corps. 1993;139:109-111. [PubMed]
170. Watts DM, Porter KR, Putvatana P, Vasquez B, Calampa C, Hayes CG, Halstead SB. Failure of secondary infection with American genotype dengue 2 to cause dengue haemorrhagic fever. Lancet 1999;354:1431-4. [PubMed]
171. Whitby K, Pierson TC, Geiss B, Lane K, Engle M, Zhou Y, Doms RW, Diamond MS. Castanospermine, a potent inhibitor of dengue virus infection in vitro and in vivo. J Virol 2005;79:8698-706. [PubMed]
172. Whitehorn J, Yacoub S, Anders KL, Macareo LR, Cassetti MC, Nguyen Van VC, Shi PY, Wills B, Simmons CP. Dengue therapeutics, chemoprophylaxis, and allied tools: state of the art and future directions. PLoS Negl Trop Dis 2014;8:e3025. [PubMed]
173. Whitehorn J, Van Vinh Chau N, Truong NT, Tai LT, Van Hao N, Hien TT, Wolbers M, Merson L, Dung NT, Peeling R, Simmons C, Wills B, Farrar J. Lovastatin for adult patients with dengue: protocol for a randomised controlled trial. Trials 2012;13:203.[PubMed]
174. Whitehorn J, Chau TN, Nguyet NM, Kien DT, Quyen NT, Trung DT, Pang J, Wills B, Van Vinh Chau N, Farrar J, Hibberd ML, Khor CC, Simmons CP. Genetic variants of MICB and PLCE1 and associations with non-severe dengue. PloS one 2013;8:e59067.[PubMed]
175. WHO: Dengue: Guidelines for treatment, prevention and control. Geneva: World Health Organization 2009.
176. WHO. Dengue haemorrhagic fever: Diagnosis, treatment, prevention and control. Geneva: World Health Organisation; 1997. Report No.: Second edition.
177. WHO. Guidelines for treatment of dengue fever / dengue haemorrhagic fever in small hospitals. New Delhi: WHO Regional office for South- East Asia; 1999.
178. Wichmann O, Gascon J, Schunk M, Puente S, Siikamaki H, Gjorup I, Lopez-Velez R, Clerinx J, Peyerl-Hoffmann G, Sundoy A, Genton B, Kern P, Calleri G, de Górgolas M, Mühlberger N, Jelinek T; European Network on Surveillance of Imported Infectious Diseases. Severe dengue virus infection in travelers: risk factors and laboratory indicators. J Infect Dis 2007;195:1089-1096. [PubMed]
179. Wills BA, Oragui EE, Stephens AC, Daramola OA, Dung NM, Loan HT, Chau NV, Chambers M, Stepniewska K, Farrar JJ, Levin M. Coagulation abnormalities in dengue hemorrhagic Fever: serial investigations in 167 Vietnamese children with Dengue shock syndrome. Clin Infect Dis 2002;35:277-85. [PubMed]
180. Wills BA, Oragui EE, Dung NM, Loan HT, Chau NV, Farrar JJ, Levin M. Size and charge characteristics of the protein leak in dengue shock syndrome. J Infect Dis 2004;190(4):810-8. [PubMed]
181. Wills BA, Nguyen MD, Ha TL, Dong TH, Tran TN, Le TT, Tran VD, Nguyen TH, Nguyen VC, Stepniewska K, White NJ, Farrar JJ. Comparison of three fluid solutions for resuscitation in dengue shock syndrome. N Engl J Med 2005;353:877-89. [PubMed]
182. Wills B, Tran VN, Nguyen TH, Truong TT, Tran TN, Nguyen MD, Tran VD, Nguyen VV, Dinh TT, Farrar J. Hemostatic changes in Vietnamese children with mild dengue correlate with the severity of vascular leakage rather than bleeding. The American journal of tropical medicine and hygiene 2009;81:638-644. [PubMed]
183. Wu SJ, Grouard-Vogel G, Sun W, Mascola JR, Brachtel E, Putvatana R, Louder MK, Filgueira L, Marovich MA, Wong HK, Blauvelt A, Murphy GS, Robb ML, Innes BL, Birx DL, Hayes CG, Frankel SS. Human skin Langerhans cells are targets of dengue virus infection. Nat Med 2000;6:816-20. [PubMed]
184. Wu SJ, Paxton H, Hanson B, Kung CG, Chen TB, Rossi C, Vaughn DW, Murphy GS, Hayes CG. Comparison of two rapid diagnostic assays for detection of immunoglobulin M antibodies to dengue virus. ClinDiagn Lab Immunol 2000;7:106-10. [PubMed]
185. Yacoub S, Griffiths A, Chau TT, Simmons CP, Wills B, Hien TT, Henein M, Farrar J. Cardiac function in Vietnamese patients with different dengue severity grades. Critical care medicine 2012;40:477-483. [PubMed]
186. Yacoub S, Wertheim H, Simmons CP, Screaton G, Wills B. Cardiovascular manifestations of the emerging dengue pandemic. Nat Rev Cardiol 2014;11:335-45. [PubMed]
187. Young PR, Hilditch PA, Bletchly C, Halloran W. An antigen capture enzyme-linked immunosorbent assay reveals high levels of the dengue virus protein NS1 in the sera of infected patients. J ClinMicrobiol 2000;38:1053-7. [PubMed]
188. Yu IM, Zhang W, Holdaway HA, Li L, Kostyuchenko VA, Chipman PR, Kuhn RJ, Rossmann MG, Chen J. Structure of the immature d dengue virus at low pH primes proteolytic maturation. Science 2008;319:1834-1837. [PubMed]
189. Zhang X, Ge P, Yu X, Brannan JM, Bi G, Zhang Q, Schein S, Zhou ZH. Cryo-EM structure of the mature dengue virus at 3.5-A resolution. Nature structural & molecular biology 2013;20:105-110. [PubMed]
190. Zhang X, Sheng J, Plevka P, Kuhn RJ, Diamond MS, Rossmann MG. Dengue structure differs at the temperatures of its human and mosquito hosts. Proceedings of the National Academy of Sciences of the United States of America 2013;110:6795-6799. [PubMed]
191. Zompi S, Harris E. Animal models of dengue virus infection. Viruses 2012;4:62-82. [PubMed]
Tables
Table 1. Criteria for Dengue Warning Signs, Taken from the 2009 WHO Classification
| Criteria for Dengue |
|---|
- Abdominal Pain or tenderness - Persistent vomiting - Clinical fluid accumulation - Mucosal bleeding - Lethargy/restlessness - Liver enlargement >2 cm - Laboratory increase in HCT concurrent with rapid decrease in platelet count |
| Criteria for Severe Dengue |
- Shock (DSS) - Fluid accumulation with respiratory distress - Severe bleeding - Severe organ involvement: ---● Liver: AST or ALT > = 1000 ---● CNS: Impaired consciousness ---● Heart and other organs |
Figure 1. Global Evidence Consensus, Risk and Burden of Dengue in 2010
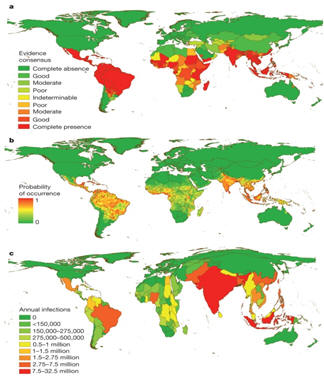
Figure 2. Dengue Disease Phases and Potential Complications
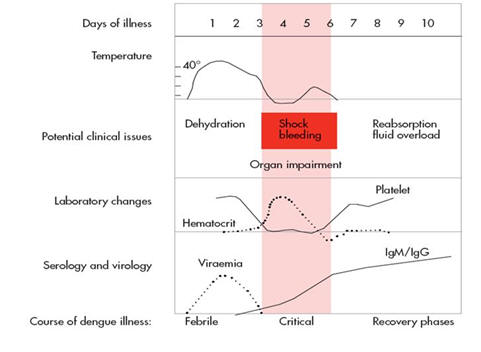
What's New
None
Guided Medline Search For:
Reviews
None

