West Nile Virus in Transplant Recipients
Authors: Marilyn E. Levi, M.D. and Kenneth L. Tyler, M.D.
VIROLOGY AND EPIDEMIOLOGY
West Nile Virus (West Nile Virus) is an arthropod-borne virus of the Flaviviridae family that also includes dengue, St. Louis encephalitis, yellow fever, Murray Valley fever and Japanese encephalitis viruses. It is a small, enveloped, positive stranded RNA virus that is maintained in nature in an enzootic cycle between mosquitoes, particularly the Culex species as the predominant vectors, and birds including crows, blue jays and ravens who serve as the primary vertebrate host and are vulnerable to lethal infection. Humans, horses and other vertebraes are incidental dead-end hosts, becoming infected through mosquito bites primarily during the latter part of the summer and fall when the mosquitoes are maximally viremic. While humans are not part of the natural cycle of West Nile Virus infection, human to human transmission has been reported through breast milk, transplacental vertical transmission, blood product transfusions and solid organ transplantation.
West Nile Virus was first identified in 1937 in the West Nile province of Uganda and is indigenous to the Middle East, Europe and Africa. In 1999, the first cases of naturally occurring West Nile Virus encephalitis were diagnosed on the eastern seaboard of the United States in Queens, New York. Between 1999 and 2001, West Nile Virus infection remained confined to the eastern United States with a total of 149 cases reported. In 2002, a westward expansion of West Nile Virus infection in the United States began, with a peak in 2003 of nearly 3000 confirmed cases of neuroinvasive disease (encephalitis, meningitis, acute flaccid paralysis) and a total of 10,000 cases reported. This infection represents the largest epidemic of arboviral meningoencephalitis in US history. While the exact source of West Nile Virus infection in the United States is unclear, the initial New York isolates (NY1999) were found to be closely related to an Israeli strain, with closely related viruses also found in Romania and Eastern Europe. There is speculation that the virus was carried by infected migrating birds, mosquitoes or larvae that were inadvertently transported on an airplane. Due to the low-level viremia associated with human infection, it is unlikely that an infected international traveler was the source of infection.
While humans are not part of the natural cycle of West Nile Virus infection, human to human transmission has been reported through breast milk, transplacental vertical transmission, blood product transfusions and solid organ transplantation. The first report of donor organ transmission occurred in 2002 in Georgia, when the donor received a transfusion of West Nile Virus-infected blood products one day prior to harvesting her organs. A serum sample from the donor was subsequently found to be positive for West Nile Virus infection by polymerase chain reaction (PCR) and culture, with negative West Nile Virus IgM. All four organ recipients (liver, 2 kidneys, heart) became infected, with three of the four developing encephalitis with one death, and the fourth patient developing mild symptoms with full recovery. The second case of solid organ donor transmission occurred in 2005 when the donor was found to be serum West Nile Virus IgM and IgG positive with negative West Nile Virus RNA by PCR on a serum sample obtained on the day following declaration of brain death. Two of the four recipients developed acute flaccid paralysis and encephalitis. The absence of detectable West Nile Virus viremia with positive IgM in this case was unusual, as one would assume that donor-associated West Nile Virus infections would result from infectious virus present in blood or tissues and would therefore generally be associated with a positive serum PCR. The magnitude of viremia needed for transmission need not be substantial, as a review of 30 cases of blood transfusion-related West Nile Virus infection indicated that donor viremia was generally of low titer and occurred in patients who had not yet developed a West Nile Virus antibody response.
PATHOGENESIS
In mouse models, West Nile Virus inoculation of the skin is followed by initial viral replication in Langerhans dendritic cells. These infected cells migrate to the regional lymph nodes and spleen with subsequent primary viremia. The primary viremia spreads virus to the reticuloendothelial system where continued replication leads to a, secondary viremia that leads to more widespread organ distribution of viral infection. West Nile virus can be isolated from blood within 1-2 days of a mosquito bite and remains detectable for up to 7 days. Serum West Nile Virus IgM appears coincident with resolution of viremia. In immunocompromised individuals including transplant recipients, the increased incidence of West Nile neuroinvasive disease (WNND) may reflect a higher titer and more prolonged viremia that may be related to delayed production of neutralizing antibodies. This has been observed with B-cell depleting agents such as rituximab, highlighting the importance of humoral immunity in the control of West Nile Virus infection.
The exact mechanism of West Nile Virus entry into the central nervous system is unclear, but blood brain permeability is increased by West Nile Virus-associated induction of a Toll-like-receptor (Tlr3)-dependent inflammatory response in mouse models. Furthermore, it has been suggested that inflammatory cytokines produced by the viremia will alter the blood-brain barrier. Direct invasion of neurons, most commonly involves the brainstem, deep nuclei and anterior horn of the spinal cord.
CLINICAL MANIFESTATIONS
Symptomatic West Nile Virus disease develops in approximately 20% of infected immunocompetent individuals. The viral incubation period ranges between 2-15 days (most commonly between 2-6 days). The most common manifestations include an abrupt onset of symptoms including headache, malaise, gastrointestinal disturbances (anorexia, vomiting, nausea and diarrhea), generalized adenopathy and occasional retroorbital pain. A transient maculopapular or roseolar truncal and facial rash lasting for up to one week can be seen. Rarely, nonneurologic complications have been described including myocarditis, pancreatitis and fulminant hepatitis. Following the acute illness, many patients report a prolonged convalescence complicated by fatigue, weakness and cognitive difficulties lasting a month or longer.
While a majority of symptomatic immunocompetent patients have relatively mild disease, an estimated 1 in 150 progress to develop West Nile neuroinvasive disease (WNND). In the transplant population and other immunocompromised hosts, the incidence of West Nile neuroinvasive disease is believed to be greater, with a small study placing the estimate at 1:40.
West Nile neuroinvasive disease includes a myriad of symptoms with significant overlap, including:
- West Nile encephalitis - considered the most severe form of West Nile neuroinvasive disease. Cases occur predominantly in the elderly (>60 years of age). The hallmark of encephalitis is an alteration in the state or quality of consciousness that may include confusion, lethargy, obtundation or even coma. Most patients have focal neurological deficits consistent with involvement of the basal ganglia, cerebellum and brainstem including parkinsonism with cogwheel rigidity, movement disorders (rigidity, tremors, myoclonus), and weakness. Patients may also develop cranial nerve palsies, optic neuritis or other ophthalmological complications and ataxia. Seizures are generally uncommon, reflecting the predilection for injury in deep gray matter and brainstem as opposed to cortex.
- West Nile meningitis - occurs in a smaller group of patients with West Nile neuroinvasive disease. Symptoms include headache, photo- and or phono-phobia, and nuchal rigidity. The presence of significant alteration in consciousness or focal neurological deficits other than cranial nerve palsies is more consistent with encephalitis.
- West Nile poliomyelitis-like syndrome – spinal anterior horn cell damage resulting in acute onset of asymmetric limb flaccid weakness or paralysis without sensory impairment, although pain may occur in involved limbs Weakness may involve a single limb, but more commonly produces para- or quadri-paresis and may be associated in the later cases with respiratory failure. Although most cases of West Nile Virus-associated acute flaccid paralysis are due to spinal cord involvement, occasional cases appear due to a Guillain-Barre like polyneuropathy. The distinction between West Nile Virus-associated myelitis and polyneuropathy may require neurologic consultation.
- A variety of isolated neurological syndromes have been associated with West Nile Virus infection including cranial nerve palsies (predominantly of the VIIth nerve resulting in peripheral facial weakness that may be unilateral or bilateral), ophthalmological complications (chorioretinitis, neuritis), and opsoclonus-myoclonus syndrome.
Studies of longterm outcome indicate that persistence of neurological defects such as headaches, cognitive impairment, emotional dysregulation with anxiety, irritability and depression, fatigue and weakness are common. Most patients show progressive improvement in symptoms, although this process may take six months or more. Prognosis of weakness resulting from myelitis is more guarded, with as few as 1/3rd of patients showing substantial clinical improvement, particularly in transplant recipients.
LABORATORY DIAGNOSIS
- Basic laboratory studies: may reveal a mild peripheral leukocytosis or leukopenia with lymphocytosis. Patients with encephalitis may develop an associated syndrome of inappropriate antidiuretic hormone (SIADH), resulting in hyponatremia. Electrolytes, liver and renal functions are otherwise normal.
- Cerebrospinal fluid examination: West Nile Virus meningitis, encephalitis and neuroinvasive disease are associated with an average CSF pleocytosis of >100 leukocytes/mm3. Persistent neutrophilic pleocytosis is more characteristic of West Nile neuroinvasive disease than most other commonly encountered forms of viral encephalitis or meningitis. CSF may also contain significant numbers of reactive lymphocytes or monocytes, including plasma cells and Mollaret-like cells. Elevated CSF protein is common, with the highest levels generally seen in patients with myelitis. CSF glucose is commonly within normal limits.
- West Nile Virus serologies: The diagnosis of West Nile Virus infection relies on serologic and PCR testing of the serum and/or spinal fluid (Figure 1). The primary method for the diagnosis of acute West Nile Virus infection is the detection of West Nile Virus IgM in serum and/or CSF. By the day prior to the onset of symptoms, over 90% of patients develop detectable West Nile Virus IgM antibody in serum or CSF as measured by antibody-capture, enzyme-linked immunosorbent assay (MAC-ELISA). The presence of CSF West Nile Virus IgM is considered pathognomonic of West Nile neuroinvasive disease, as IgM antibody crosses the blood-brain barrier poorly, and as a result its presence in CSF is generally diagnostic of intrathecal synthesis in response to an antigen present in the CNS. CSF West Nile Virus IgM does not develop with systemic West Nile Virus infections that spare the CNS or as a result of vaccinations against other flaviviruses (yellow fever, Japanese encephalitis). In fact, killed vaccinations (eg Japanese encephalitis) do not induce an IgM response in either the serum or CSF.
West Nile Virus IgG antibodies commonly appear by day 7 of illness, persist for life and are considered protective against future infection.
One should be aware of potential causes of false-positive West Nile Virus serologies:- Serum West Nile Virus IgM may persist for as long as 500 days so that the clinical picture may be related to an alternate diagnosis
- Serum West Nile Virus serologies may cross-react with infections or vaccinations with other flaviviridae. In many parts of the United States, St. Louis encephalitis is the most common flavivirus infection, and in underdeveloped areas in the world, dengue fever may be common. It is also important to obtain a travel history including vaccinations against Yellow Fever and Japanese encephalitis, which may result in false-positive serologies for West Nile Virus.
- One report suggested a 25% increase in false positive West Nile Virus IgG rates with higher cyclosporine levels in liver transplant recipients
The time of presentation is also important, as one would expect infection to occur during mosquito season, peaking in the late summer and early fall. Other important environmental factors that may vary from year to year include heavy rains resulting in standing water and growth of mosquito larvae and the predominance of specific species of mosquitoes, in particular Culex spp. Therefore, interpretation of West Nile Virus IgM and IgG antibodies should be made in the context of the history and clinical picture.
- West Nile Virus Polymerase chain reaction: In most cases, viremia, as manifested by PCR or nucleic acid amplification test (NAAT) develops approximately 5-6 days prior to the onset of symptoms and antibody production. The viremia is transient, often undetectable at the time of presentation and therefore may not be useful for the diagnosis of acute infection. CSF PCR is less sensitive (50-70%) than detection of CSF IgM antibody for diagnosis of West Nile neuroinvasive disease, although a positive CSF PCR is highly specific (~100%) and generally considered diagnostic of infection. PCR studies may be particularly useful in the diagnosis of West Nile Virus in immunocompromised patients who may fail to seroconvert or show delayed antibody responses to infection.
NAAT screening of blood donations began in 2003, initially on an investigational basis. As a result of this testing strategy, the transmission of West Nile Virus via blood donations has been markedly reduced. During mosquito season, NAAT screening of blood, tissue (e.g., cornea, bone) and organs is performed. A logistical difficulty for some organ transplant centers is the inability to obtain NAAT results prior to transplant, resulting in the potential transmission from viremic organs which has been reported. In addition, NAAT testing may be associated with false positive and false negative results that may require confirmatory testing which also may not be available prior to transplantation. As a result, there is the potential loss of organs associated with false-positive NAAT testing. This is not an issue for blood and tissue samples, as they do not require the rapid turnaround time needed for organ transplantation, allowing for confirmatory testing. - Plaque reduction neutralization test (PRNT): This test distinguishes between the different flaviviridae and serologic cross-reactivity by IgM ELISA. Neutralizing antibody titers are higher against the infecting virus as compared to potentially cross-reactive species. There are some limitations related to the availability of testing, as this may not be available in real-time, and can be performed through the Centers for Disease Control.
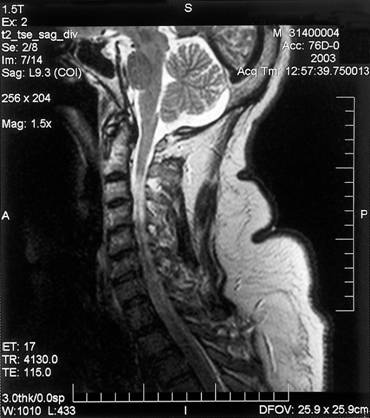
- Radiologic findings: CT is less sensitive than MRI in identifying West Nile Virus-associated CNS injury. The sensitivity of MRI in turn depends on the imaging protocol performed with abnormalities most commonly evident on T2, fluid attenuated inversion recovery (FLAIR), fast spin echo (FSE) and diffusion-weighted imaging (DWI) sequences. Even with detailed testing, up to 50% of patients may lack acute abnormalities. Sequential studies may be needed to demonstrate presence or progression of disease. Patients with West Nile Virus meningitis may show acute meningeal enhancement while those with encephalitis often have abnormalities involving deep structures including basal ganglia, thalamus and the upper brainstem and cerebellum (Figure 1). Patients with acute flaccid paralysis, may demonstrate areas of increased T2 or FLAIR signal in the anterior horns of the spinal cord. Parkinsonian signs and symptoms may be associated with abnormalities in the basal ganglia and midbrain. (Figure 2 and Figure 3)
- Electroencephalogram (EEG): Although seizures are uncommon, , EEG abnormalities may be present in 57-86% of patients with West Nile Virus encephalitis, although they are not specific. In a study of 13 hospitalized patients with West Nile neuroinvasive disease, the most common abnormalities included generalized slowing with anterior or temporal prominence and intermittent temporal slowing.
- Histopathology: In case of death, postmortem examination of the encephalitic brain can show glial nodules, diffuse inflammation of the brain and spinal cord with associated neuronal degeneration, hemorrhages and perivascular cuffing. Individuals with flaccid paralysis have involvement of the lower motor neurons or anterior horn cells of the spinal cord. Immunohistochemical staining for West Nile Virus antigen and amplification of viral nucleic acid may be useful for postmortem or biopsy diagnosis.
TREATMENT
To date, supportive care has been the mainstay of therapy. There are case reports supporting the use of intravenous West Nile Virus-specific immunoglobulin (IVIG). Since the 1980’s, West Nile Virus antibody titers as high as 1:1600 have been present in standard Israeli IVIG, with descriptions in the literature of use of this product in successful treatment of acutely ill patients. Until the appearance of West Nile Virus in the United States in 1999, antibody titers against West Nile Virus were undetectable in North American IVIG. Subsequently, West Nile Virus has become endemic in the U.S., resulting in a progressive increase in specific neutralizing antibodies in US IVIG. It is important to emphasize that the efficacy of any form of passive immunotherapy in human West Nile Virus infection remains anecdotal and unproven. Animal studies suggest that the benefits of passive immunotherapy are dose-dependent and markedly enhanced by early administration.
Clinical Trials
- Humanized monoclonal to West Nile virus, also known as MGAWN1 (Macrogenics, Inc.) is currently available in the Paradigm clinical trial, “A Phase 2, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Safety and Efficacy of MGAWN1 in Subjects With Suspected Central Nervous System Infection Due to West Nile Virus”, ClinicalTrials.gov identifier: NCT00927953. Patients do not require documented West Nile virus infection, and can be enrolled based on a syndrome compatible with West Nile neuroinvasive disease. Contact information: Lara Blake, 301-354-3819, http://www.macrogenics.com/products-MGAWN1.html Study director: John Beigel, MD
- Alpha-Interferon – Double blinded placebo controlled trial of Alpha-Interferon (Alferon) for West Nile Meningoencephalitis (Protocol WN-102). Patients must be enrolled within five days of hospitalization and do not require prior serologic confirmation of West Nile virus infection.
Antiviral Prophylaxis
Patient education for the prevention of mosquito bites is key to the prevention of West Nile Virus infection, as vaccines are not available. As mosquitoes do not survive temperatures < 50F, these recommendations are to be implemented primarily during mosquito season, usually during the summer and early fall:
- Use of insect repellents containing DEET (N, N-diethyl-mtoluamide), picaridin (KBR 3023), or oil of lemon eucalyptus (p-menthane 3,8-diol), which typically provide longer-lasting protection than other products.
- Permethrin application to bednets, clothing and camping gear. This agent is not intended for direct application to skin.
- Use of protective clothing, ie. long sleeved shirts, long pants. As mosquitoes can bite through thin fabric, permethrin application is appropriate.
- Avoid activities between dusk to dawn, during peak mosquito biting periods.
REFERENCES
1. Davis LE, DeBiasi R, Goade DE, Haaland KY, Harrington JA, Harnar JB, Pergam SA, King MK, DeMasters BK, Tyler KL. West Nile virus neuroinvasive disease. Ann Neurol 2006;60: 286-300. [PubMed]
2. Hamdan A, Green P, Mendelson E, Kramer MR, Pitlik S, Weinberger M. Possible benefit of intravenous immunoglobulin therapy in a lung transplant recipient with West Nile virus encephalitis. Transpl Infect Dis 2002;4:160-162. [PubMed]
3. Kiberd BA, Forward K. Screening for West Nile virus in organ transplantation: a medical decision analysis. Am J Transpl 2004;4:1296-1301. [PubMed]
4. Kleinschmidt-DeMasters BK, Marder BA, Levi ME, Laird SP, McNutt JT, Escott EJ, Tyler KL. Naturally acquired West Nile virus encephalomyelitis in transplant recipients:clinical, laboratory, diagnostic and neuropathological features. Arch Neurol.2004;61:1210-1220. [PubMed]
5. Kumar D, Prasad GV Ramesh, Zaltzman J, Levy GA, Humar A. Community-acquired West Nile Virus infection in solid-organ transplant recipients. Transplantation 2004;77:399-402. [PubMed]
6. Levi ME, Quan D, Ho JT, Kleinschmidt-DeMasters BK, Tyler KL, Grazia TJ. Impact of rituximab-associated B-cell defects on West Nile virus meningoencephalitis in solid organ transplant recipients. Clinical Transplantation 2009, accepted for publication. [PubMed]
7. West Nile virus infections in organ transplant recipients – New York and Pennsylvania, August – September, Morbidity and Mortality Weekly Report 2005;54(40): 1021-1023. [PubMed]
8. Nicolle LE, Gutkin A, Smart G, Dawood M, Drebot M, Van Caeseele P, Giulivi A, Minuk GY. Serological studies of West Nile virus in a liver transplant population. Can J Infect Dis 2004;15(5): 271-274. [PubMed]
9. Planitzer CB, Modrof J, Kreil TR. West Nile Virus neutralization by US plasma-derived immunoglobulin products. Journal of Infect Dis 2007;196:435-40. [PubMed]
10. Tilley PAG, Fox JD, Lee B, Chui L, Preiksaitis J. Screening of organ and tissue donors for West Nile virus by nucleic acid amplification – a three year experience in Alberta. Am J Transpl 2008;8:2119-2125. [PubMed]
Figure 1. Laboratory diagnosis of West Nile Virus using PCR and serology. ELISA = enzyme-linked immunosorbent assay; pfu = plaque-forming units. (Figure reproduced with permission from )
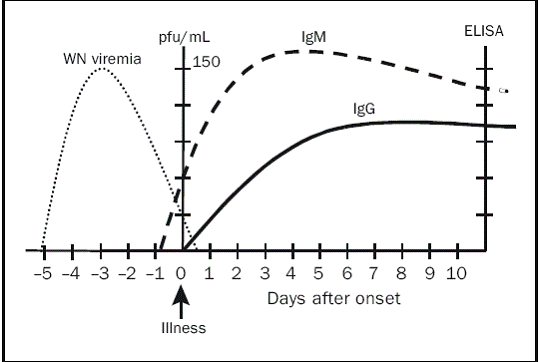
Figure 2: Coronal fluid-attenuated inversion recovery (FLAIR) image shows an area of abnormally increased signal in the thalami, substantia nigras (extending superiorly toward the subthalamic nuclei) and white matter (Figure reproduced with permission from reference 4)
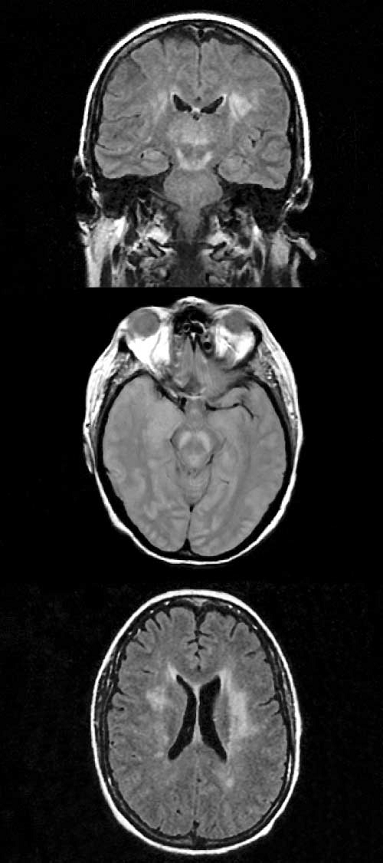
Figure 3: MRI of a patient with WNV encephalitis, acute flaccid paralysis of the upper extremities and respiratory failure (Courtesy of Dr. Lyle Peterson, Centers for Diseases Control, Fort Collins, Colorado)
Specter M. The Mosquito Solution. Can genetic modification eliminate a deadly tropical disease? The New Yorker July 9 & 16, 2012.
Reisen et al. Subprime Lending and West Nile Virus Infection. Clin Infect Dis. 2009;48:v–vi
Jean CM, Honarmand S, et al. Risk Factors for West Nile Virus Neuroinvasive Disease, California, 2005. Emerg Infect Dis. 2007 Dec;13:1918-20.
A downloadable patient brochure for transplant recipients is available at http://www.cdc.gov/ncidod/dvbid/westnile/resources/West Nile Virus_transplant%20brochure6_12_07.pdf erties of nitazox
GUIDED MEDLINE SEARCH FOR
Wehbeh WA., Rahal JJ. West Nile Virus
CDC. Epidemiology and Transmission Dynamics of West Nile Virus Disease. Emerg Infect Dis, Aug 2005.
CDC. Virology, Pathology, and Clinical Manifestations of West Nile Virus Disease. Emerg Infect Dis, Aug 2005
GUIDED MEDLINE SEARCH FOR RECENT REVIEWS
Berger S. Emergence of Infectious Diseases into the 21st Century, 2008.
West Nile Virus and Its Appearance in the U.S., 2013
