
Pulmonary Infiltrates in The ICU -
Management (Method of Antoni Torres MD)
In our intensive care unit, we
have developed an approach to diagnosis and treatment of ICU pneumonia (Figure 1). The major
differential
diagnosis of pulmonary infiltrates (print
separately) includes only two entities in which
antibiotics are clearly indicated: pneumonia and aspiration (Table
2). The
presence of an infiltrate on simple chest x-ray raises the possibility that a
patient may have pneumonia. Our suspicion of hospital-acquired pneumonia occurs
when two out of three clinical criteria are noted in a patient with pulmonary
infiltrate: fever or hypothermia, leukocytosis or leukopenia and purulent
respiratory secretions.
Table
2. Differential Diagnosis for Pulmonary Infiltrates in ICU Patients
-
Pneumonia
-
Aspiration
-
Atelectasis
-
Pulmonary edema
-
Cardiogenic
-
Noncardiogenic
-
Pleural effusion
-
Hemorrhage
-
Drug-induced
|
Figure 1: Clinical Suspicion of Hospital-Acquired
Pneumonia

APPROACH
We calculate the Clinical Pulmonary
Infection Score (CPIS) which allows objective analysis of the varying clinical
and radiologic variables (Table 1).
Cultures of the lower respiratory secretions are immediately taken (Table 3).
We emphasize the use of the Gram stain of the sample of respiratory secretions;
this improves diagnostic value of the score. Culturing should be performed prior to initiation of
antibiotic treatment, but it should not delay the administration of
antibiotics.
Collection may be
performed by sputum, tracheobronchial aspirate, bronchoalveolar lavage or
protected specimen brush. Blood
cultures should be obtained. Pleural
fluid cultures should be obtained, if the effusion is large and
the cause is uncertain or if etiologic diagnosis of pneumonia has eluded
identification. Legionella urinary antigen is performed routinely given its occurrence as an occult
cause of hospital-acquired pneumonia. If the patient has been only recently
hospitalized within 5 days, a urinary antigen for Streptococcus pneumoniae
is performed.
We obtain C-reactive protein (CRP) and procalcitonin (PCT).
Elevated levels
suggest more severe pneumonia.
Table
1. Clinical Pulmonary Infection Score
|
Score |
Day 0
|
|
Day 3 |
Score |
|
|
Temperature, ºC
³38.5º
- 38.9º
= 1 point
³39.0º
- 36.0º
= 2 points |
|
Temperature, ºC
38.5º
- 38.9º
= 1 point
39.0º
- 36.0º
= 2 points |
|
|
|
Blood
leucocytes, mm-3
<4.000 or >11.000 = 1
point
50% band
forms = add 1 point |
|
Blood
leucocytes, mm-3
<4.000 or >11.000=1
point
50% band forms = add 1 point |
|
|
|
Tracheal secretions
Presence of
non-purulent tracheal secretions = 1 point
Presence of purulent
tracheal secretions = 2 points |
|
Tracheal
secretions
Presence of
non-purulent tracheal secretions = 1 point
Presence of purulent
tracheal secretions = 2 points |
|
|
|
Oxygenation: PaO2/FIO2
>240 or ARDS = 0
point
< 240 and no
ARDS = 2 points |
|
Oxygenation: PaO2/FIO2
>240
or ARDS = 0 point
< 240 and no ARDS
= 2 points |
|
|
|
Pulmonary
radiography
No infiltrate = 0 point
Diffuse or patchy
infiltrate = 1 point
Localized infiltrate= 2
points |
|
Pulmonary
radiography
No infiltrate = 0 point
Diffuse or patchy
infiltrate = 1 point
Localized infiltrate= 2
points |
|
|
|
Microbiological Data
Pathogenic bacterial
cultured in rare or hight quantity or no growth = 0 point
Pathogenic bacterial
cultured in moderate or heavy quantity = 1 point
Same pathogenic bacterial seen
on Gram stain = add 1 point
|
|
Microbiological Data
Pathogenic bacterial
cultured in rare or hight quantity or no growth = 0 point
Pathogenic bacterial
cultured in moderate or heavy quantity = 1 point
Same pathogenic bacterial
seen on Gram stain = add 1
point
|
|
Total Day #0 = _________ Total Day
#3 = _________
Table 3. Diagnostic Tests in the Workup of Pulmonary
Infiltrates in the ICU
|
1.
Respiratory Cultures
-
Sputum
-
Endotracheal or
Tracheobronchial aspirate** [Toni: should this be transbronchial?]
-
Bronchoalveolar
lavage (BAL) or mini-BAL **
-
Protected brush
specimen (PBS) **
2. Two blood cultures
3. Pleural fluid culture if parapneumonic effusion.
4. Legionella pneumophila urinary antigen and
Streptococcus pneumoniae
urinary antigen.
5. CBC, electrolytes, hepatic and renal function tests.
6. Arterial blood gases
7. C-reactive protein (CRP) and procalcitonin
|

Send samples to microbiology laboratory immediately (or if not possible,
refrigerate at 4ºC for a maximum of one hour) Gram staining, intracellular
organism counting (only in BAL and mini-BAL) and quantitative cultures ***
should be done.
*Collection of cultures should not delay the initiation of empiric treatment in
patients with severe sepsis.
**These techniques may be performed by bronchoscopy by blinded procedures.
***Quantitative cultures are performed with the respiratory secretions obtained
by transbronchial aspirate, BAL or PBS. The cut-off points for determining
infection colonization are: Transbronchial aspirate 105CFU/mL; BAL 104
CFU/mL and PSB 103 CFU/mL.
Assessment of CPIS, Clinical
Status, Gram Stain
If the CPIS < 6,
systemic inflammatory response syndromes (SIRS) (Table 9) is not present and the Gram stain
of respiratory secretions is negative or intracellular organisms < 2%, then pneumonia is not likely.
Patients fulfilling all of these criteria need not receive antibiotic treatment but be strictly monitored.
This is our unique addition to the protocol designed by Singh et al.; we have
found it to be reliable and clinically-plausible. These patients do not fulfill
classic criteria for pneumonia and they are clinically stable. Monitoring such
patients does not pose a substantial risk for poor outcome, since antibiotics
can be added if deterioration is observed. Keep in mind, it is probable that
noninfectious causes of pulmonary infiltrates will be diagnosed in such patients
within 3 days.
At the other extreme, if any of the
following are present: CPIS > 6, presence of
SIRS, and Gram stain of
respiratory secretions shows a predominant bacterium or intracellular bacteria,
these patients most likely have pneumonia.
Broad spectrum antibiotic treatment should be initiated (Figure 2).
If the
CPIS is > 6 and SIRS is not
present, pneumonia is still likely, and broad spectrum antibiotic therapy is
initiated immediately.
If the
CPIS is < 6 and the
Gram stain of the repiratory tract sample is difficult to interpret, then we
administer monotherapy. In the original protocol by Singh, et al., a quinolone
was used for monotherapy, and in an NIH proposed protocol, a carbapenem was
used.
Figure 2: Algorithm for the Management of Patients with
Pulmonary Infiltrates
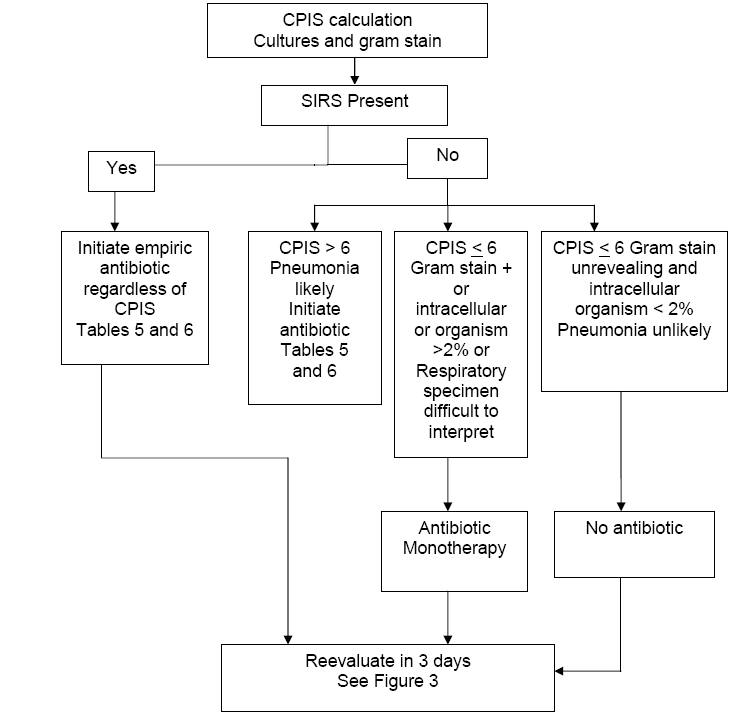
Table 8. Definitions
|
A. SIRS: 2 or more of the following variables:
1.
Fever >38◦C or < 36◦C
2.
Heart rate >90 beats per minute
3.
Respiratory rate >20 breaths per minute or PaCO2 <32 mm Hg
4.
Abnormal white blood cell count (>12,000/mm3 or <4,000/ mm3
or >10% bands)
B. Bacteremia: bacteria within the blood stream (does
not always lead to SIRS or sepsis)
C. Sepsis: SIRS plus a documented or presumed
infection.
D. Severe sepsis: aforementioned sepsis criteria with
associated organ dysfunction, hypoperfusion or hypotension.
E. Sepsis induced hypotension: presence of a systolic
BP <90 mmHg or a reduction of > 40 mmHg from baseline in the absence of
other causes of hypotension.”
F. Septic shock: Persistent hypotension and perfusion
abnormalities despite adequate fluid resuscitation.
G. Multiorgan dysfunction syndrome: state of
physiological derangements in which organ function is not capable of
maintaining homeostasis. |
CPIS <
6
If the CPIS < 6, and the Gram stain of the respiratory tract specimen
is difficult to interpret, antibiotic therapy can be limited. Whether the CPIS
is a good predictor of pneumonia is controversial and numerous studies have
questioned its sensitivity and specificity. However, in our approach, the CPIS
is not used as a proven aid to diagnose ICU pneumonia; instead, its use is to
select those patients who do not need intensive and prolonged broad spectrum
antibiotic therapy. The usage for this purpose has been validated in one study. So, for these
patients, we administer antibiotic monotherapy initially. Since the classical
signs of pneumonia are largely absent and since the patient is clinically
stable, most clinicians would agree that limited antibiotic therapy (monotherapy)
of short duration (3 days) is prudent until results of cultures are known.
Studies estimate that for ICU patients with pulmonary infiltrates 70%-80% do not
have pneumonia, but currently most will receive combination broad spectrum
empiric antibiotic therapy with duration from 5-14 days. Receipt of unnecessary
antibiotics in patients without confirmed pneumonia is linked to higher
mortality.
The major reason contributing to “spiraling empiricism” in antibiotic use in the
ICU is that physicians are unwilling to risk missing a treatable infection.
Because ICU pneumonia carries substantial mortality, administration of
broad-spectrum antibiotics to most patients with pulmonary infiltrates has
emerged as the predominant practice in the ICU. The use of the CPIS shown in
Figure 2 allows the physician flexibility in initially managing patients with a
perceived treatable infection. However, the number of antibiotics to be given is
limited (monotherapy) as is the duration (3 days). Such an approach has led to
decreased costs for antibiotics and minimized the emergence of antimicrobial
resistance. As importantly, outcome was more favorable when compared to a
control group of patients with CPIS < 6 who received broad spectrum antibiotic
therapy for 5-21 days.
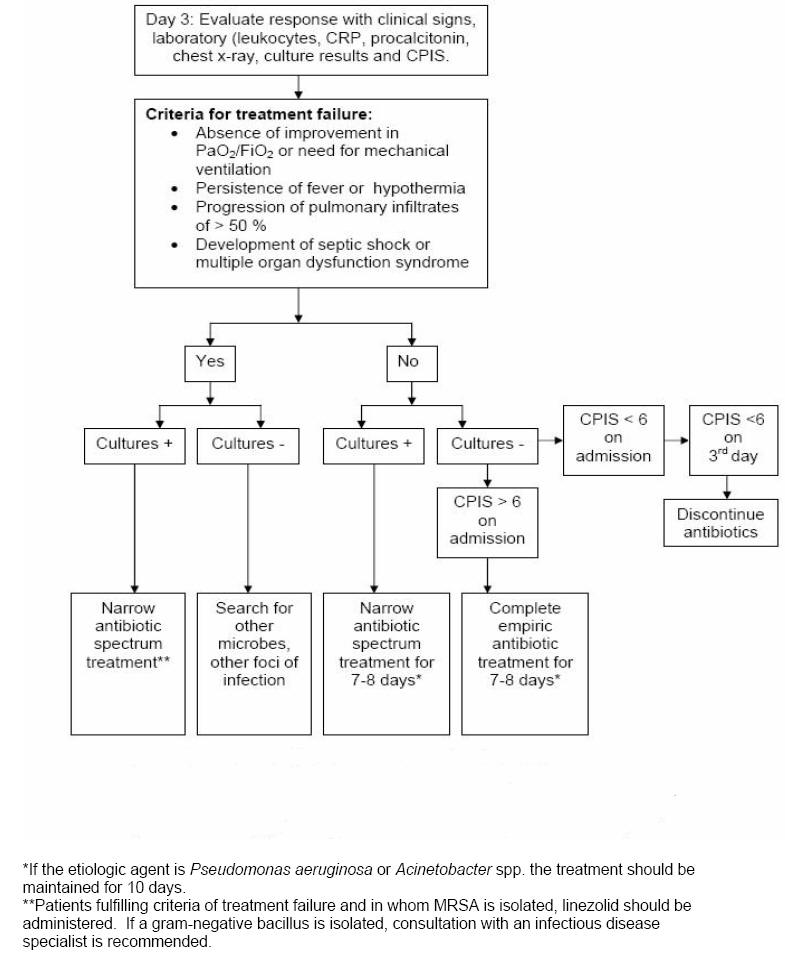
Re-Evaluation on Day 3
On the third day, response
to empiric antibiotics is observed using clinical, laboratory and radiologic parameters and the re-calculated CPIS
(Figure 3).
The criteria of treatment failure are: 1) absence of improvement in the PaO2/FiO2
or the need for mechanical ventilation, 2) persistence of fever or hypothermia,
3) 50 % progression in the pulmonary infiltrates, and 4) development of septic
shock or multiple organ dysfunction syndrome.
For patients whose original
CPIS < 6, if the patient appears stable and
the cultures are negative, all antibiotic treatment should be discontinued on day
3.
For those patients in whom cultures
are revealing, antibiotic therapy should be focused on the specific pathogen and
the spectrum narrowed. In patients with increasing level of biomarkers (RCP and
procalcitonin) on day 3-5, an extensive microbiological and radiological
re-evaluation is warranted and broadening the spectrum of the antimicrobial
therapy is done.
Figure 3: Follow up of Patients with Pulmonary
Infiltrates
Antibiotic Selection
Empiric antibiotic therapy
(print
separately)
should be based on local
in vitro susceptibility
patterns and patient risk factors for infection by multiresistant microorganisms (Table 4).
For patients with CPIS > 6, and risk factors for infection by multiresistant
microorganisms or duration of mechanical ventilation more than 5 days, we use a combination of an anti-pseudomonal
beta-lactam antibiotic with the addition of a quinolone or an aminoglycoside (Table
5, Figure
6).
This is a reasonable choice for both
Pseudomonas aeruginosa
or multi-drug resistant microorganisms.
Table 4. Risk Factors for Infection by Multiresistant
Microorganisms
|
Risk factors for Multi-Resistant Microorganisms |
-
Antibiotic treatment within the last 90 days (> 5 days)
-
Current hospital admission or within the last 90 days (> 5 days)
-
Immunosuppressive disease and/or treatment
-
Chronic dialysis within the last 30 days
-
Epidemic outbreak in the unit by multiresistant organism
|
Table 5: Initial Empiric Antibiotic Treatment For
Hospital-Acquired Pneumonia or VAP Of Late Onset Or In Patients With Risk
Factors For Resistance
|
Microbial Etiology |
Combination antibiotic treatment |
|
Microorganisms from Table 3 plus:
Pseudomonas aeruginosa
Klebsiella pneumoniae (ESBL+)
Serratia marcescens
Acinetobacter spp.
Other nonfermentative GNB
MRSA
Legionella pneumophila
|
Antipseudomonal cephalosporin
(ceftazidime or
cefepime)*
or
Carbapenem (imipenem, meropenem)*
or
Beta-lactam / betalactamase inhibitor (piperacillin / tazobactam)*
+
Antipseudomonal quinolone (ciprofloxacin,
levofloxacin)**
or
Aminoglycoside ** (amikacin)
±
Linezolid or
vancomycin***
|
GNB = Gram negative bacilli
ESBL = Extended-spectrum beta-lactamase
MRSA = Methicillin-resistant Staphylococcus aureus
*The choice of beta-lactam antibiotic is made as follows: patients who have not
received any antipseudomonal beta-lactam antibiotic within the last 30 days are
administered piperacillin/tazobactam or antipseudomonal beta-lactam
cephalosporin. Patients who have received these prior antipseudomonal drugs are
given a carbapenem. Patients with infection by ESBL-producing microorganisms are
treated with carbapenem regardless of the results of the antibiogram.
**For combination empiric therapy for multiresistant gram-negative bacilli, an
antipseudomonal quinolone is used in cases of renal failure or concomitant use
of vancomycin. In other settings combined empiric therapy is initiated with
amikacin and is maintained for a 5 day period.
***Empiric therapy aimed at MRSA is initiated in patients with established
colonization, previous infection by MRSA, or implementation of mechanical
ventilation for more than 6 days. Our antibiotic of choice is vancomycin.
However, linezolid is used in patients allergic to vancomycin, creatinine values
≥ 1.6 mg/dL or in patients presenting signs of infection after 48 hours of
vancomycin therapy and in those in whom MRSA has been isolated.
Empiric therapy for
methicillin-resistant Staphylococcus aureus (MRSA) is
implemented only in patients who demonstrate colonization or previous infection by MRSA
or who have received mechanical ventilation for more than 6 days. In many hospitals, linezolid is preferred to
vancomycin.
In the absence of risk factors for infection by multiresistant microorganisms or in
patients who have been hospitalized or have received mechanical ventilation for
less than 5 days, pathogens of
community-acquired pneumonia
should be covered; Ceftriaxone
or levofloxacin can be added for these pathogens (Streptococcus
pneumoniae, Legionella pneumophila) (Table 6,
Figure 5,
6).
Table 6. Initial Empiric Antibiotic Treatment In
Hospital-Acquired Pneumonia or VAP of
Early Onset In Patients Without Risk Factors For Resistance
|
Probable Microorganism |
Empiric Antibiotic |
|
Streptococcus pneumonia
Haemophilus influenzae
Enteric Gram-negative bacilli
Escherichia coli
Klebsiella pneumoniae
Enterobacter spp.
Proteus spp. |
Ceftriaxone
or
Levofloxacin
|
Figure 5:
Empiric antibiotic treatment of VAP in patients without factors of infection by
P. aeruginosa

Figure
6: Empiric antibiotic treatment of VAP in setting of risk of infection by P. aeruginosa or
by multiresistant microorganisms
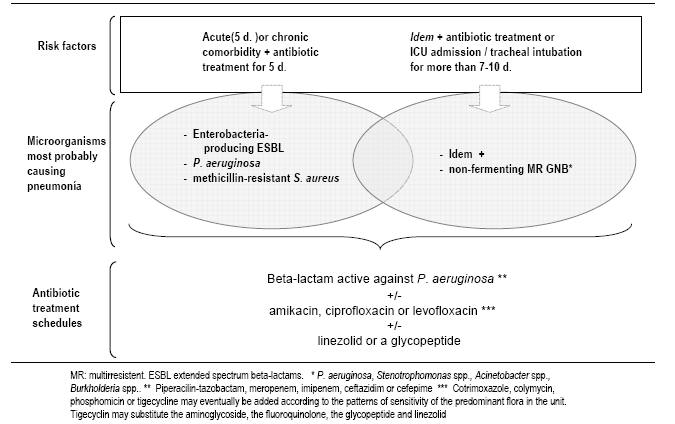
Table 7
shows the antibiotics, the doses, treatment schedule and the length of infusion.
If beta-lactam agents are selected, administration by continuous
infusion should be considered.
Table 7. Antibiotic Dosages and Timing
|
Antibiotic |
Doses
|
Interval of administration |
Infusion time
|
|
Ceftriaxone |
1 g |
12 hours |
1 / 2 - 1 hour† |
|
Levofloxacin |
750 mg |
12 hours* |
1 / 2 hour |
|
Ceftazidime |
2 g |
8 hours |
2 - 3 hours† |
|
Cefepime |
2 g |
8 hours |
2 - 3 hours† |
|
Imipenem |
0.5 g |
6 hours |
1 hour† |
|
Meropenem |
0.5 – 1 g |
6 hours |
2 - 3 hours† |
|
Piperacillin/Tazobactam |
4 / 0.5 g |
6 hours |
2 - 3 hours† |
|
Ciprofloxacin |
400 mg |
8 hours |
1 / 2 hour |
|
Amikacin |
15 mg / Kg |
24 hours
** |
1 / 2 - 1 hour |
|
Vancomycin |
1 g |
8-12 hours*** |
1- 3 hours* |
|
Linezolid |
600 mg |
12 hours |
1 hour |
*Administer this dose for 3 days and after continue with 500 mg / 24 hours
**Adjust the dosage according to PK / PD parameters
***Initiate this dose with 24 hours,
measure trough blood levels prior to the following dosage and adjust the levels
according to values.
†For beta-lactam agents and
vancomycin, continuous infusion should be considered.






