Febrile Neutropenia - Treatment
Treatment
The management of the febrile neutropenia syndrome has evolved progressively
over the years. In the 1960s, the initiation of broad-spectrum antibiotics at
the very onset of fever in neutropenic patients, decreased dramatically the
mortality. This concept of empiric therapy is still one of the major elements of
standard care of the febrile neutropenia nowadays. Until the 1980s, a combined
therapy of a ß-lactam and an aminoglycoside has prevailed as the regimen of
choice for empiric therapy, justified by a predominance of Gram-negative
bacteria causing severe infection in neutropenic patients. Moreover, the
available ß-lactam antibiotics at this period had a modest intrinsic activity
against P. aeruginosa. However, since the mid 1980s, and in parallel to
the shift from a predominance of Gram-negative to that of Gram-positive bacteria
causing bacteremia in neutropenic patients, several comparative studies were
able to show equivalent efficacy between a ß-lactam alone and its combination
with an aminoglycoside. In fact, the introduction of new ß-lactam drugs with
better intrinsic activity against Gram-negative bacteria in general, and against
P. aeruginosa in particular, contributed to decrease the role of
combination with aminoglycosides and its potential for nephrotoxicity and
ototoxicity. More recently, since the mid 1990s, the attitude consisting in
extra safety measures with hospitalization and intravenous antibiotic therapy
for all neutropenic patients began to wane in favor of a risk-adapted therapy
based on prediction rules of complication rates during the course of a febrile
neutropenic episode. Refinement of selection of patients at low-risk of
complications was achieved and oral antibiotic therapy with early discharge was
applied to this low-risk population. Thus, the risk categorization of the
febrile neutropenic episode allows to determine if the patient should be kept in
the hospital and treated intravenously, or if he can receive oral antibiotics
and be discharged early. Additional key elements are to be addressed for those
who receive intravenous empiric antibiotics in order to know which ß-lactam to
choose, in monotherapy or in combination with aminoglycosides or other
antibiotics (Figure 2).
Figure 2:
Factors that Influence the Choice of Empiric Therapy
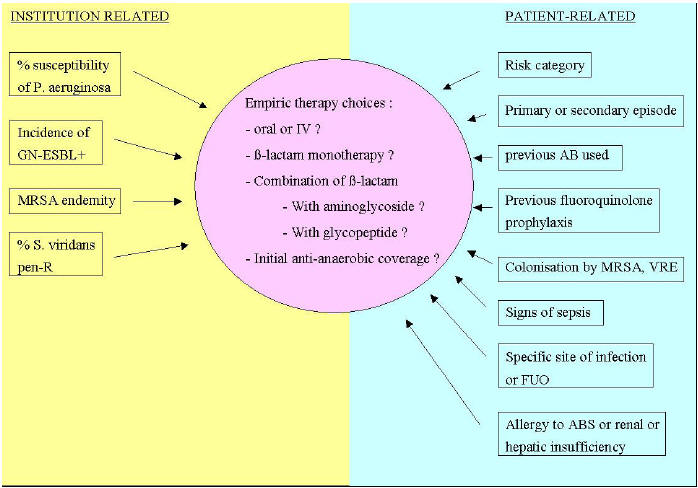
GN-ESBL
: Gram-negative bacilli producing extended-spectrum ß-lactamases
MRSA :
Methicillin-resistant Staphylococcus aureus
Pen-R :
Penicillin-resistant
AB(s) :
Antibiotic(s)
VRE : Vancomycin
resistant enterococci
FUO : Fever of
unknown origin
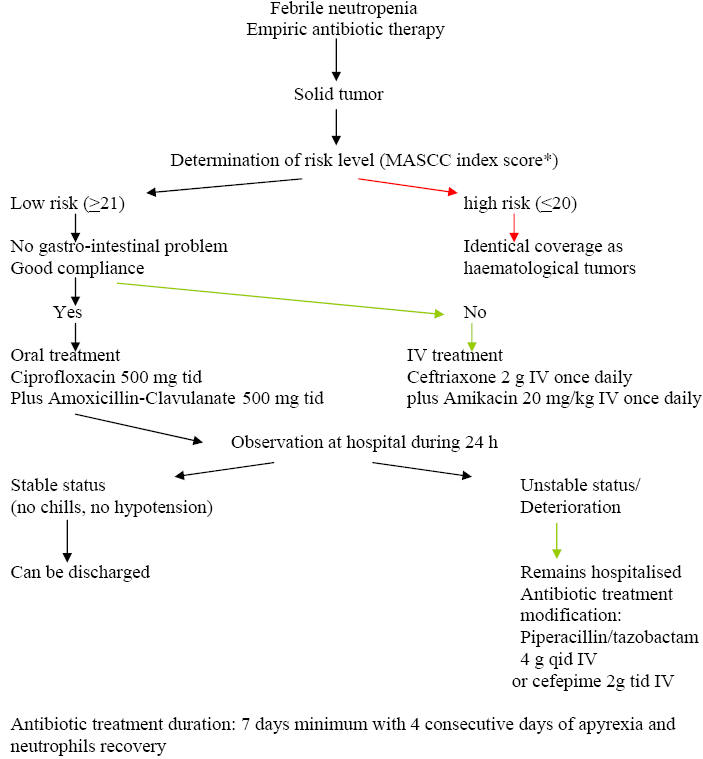
Treatment of Patients at Low-Risk
Once a
febrile neutropenic episode has occurred a risk-assessment of complications is
undertaken. For this purpose, the MASCC index scoring system is the most widely
used and allows selecting low-risk patients with good sensitivity and
specificity. Once the patient is categorized as being at low-risk, the next
question then is whether this patient is eligible for oral therapy. In fact,
several conditions should be present including the absence of severe oral mucositis, diarrhea and vomiting. Two large, prospective randomized trials
compared oral and intravenous antibiotics in patients with febrile neutropenia
at low risk of complications, both using amoxicillin-clavulanate plus
ciprofloxacin for oral therapy and both showed equivalent efficacy and safety
between the oral and the intravenous arms. Based on these results, the
association of amoxicillin-clavulanate plus ciprofloxacin has been recommended
for oral therapy providing that patients have not received fluoroquinolone
prophylaxis. Patients who are allergic to penicillin may benefit from an
alternative regimen associating ciprofloxacin and clindamycin although
creatinine elevation has been reported with this regimen in neutropenic
patients. Two small studies evaluated new-generation fluoroquinolone monotherapy
for oral empiric therapy of low risk febrile neutropenic patients. The first one
included 54 patients who received oral moxifloxacin 400 mg once daily with a
response rate of 91 %. The second one enrolled 43 patients treated with oral
gatifloxacin 400 mg once daily and showed a response rate of 95 %. Although
promising, these results must be confirmed by larger randomized trials. For
patients at low-risk who received intravenous antibiotics for temporary reasons,
a step-down strategy with a rapid shift to oral antibiotics may be applied.
Outpatient management for patients with febrile neutropenia at low-risk, with
oral antibiotic therapy has been evaluated recently in a randomized trial which
compared oral antibiotics, followed by early discharge after a 24 h observation
period, with in-patient intravenous antibiotics until resolution of the febrile
neutropenic episode. The efficacy with both strategies was equivalent and the
readmission rate in the outpatient arm was low. Whether some patients at
low-risk would benefit from immediate discharge, without a period of observation
is not yet established and the safety of such a procedure needs to be carefully
assessed before it could be generalized.
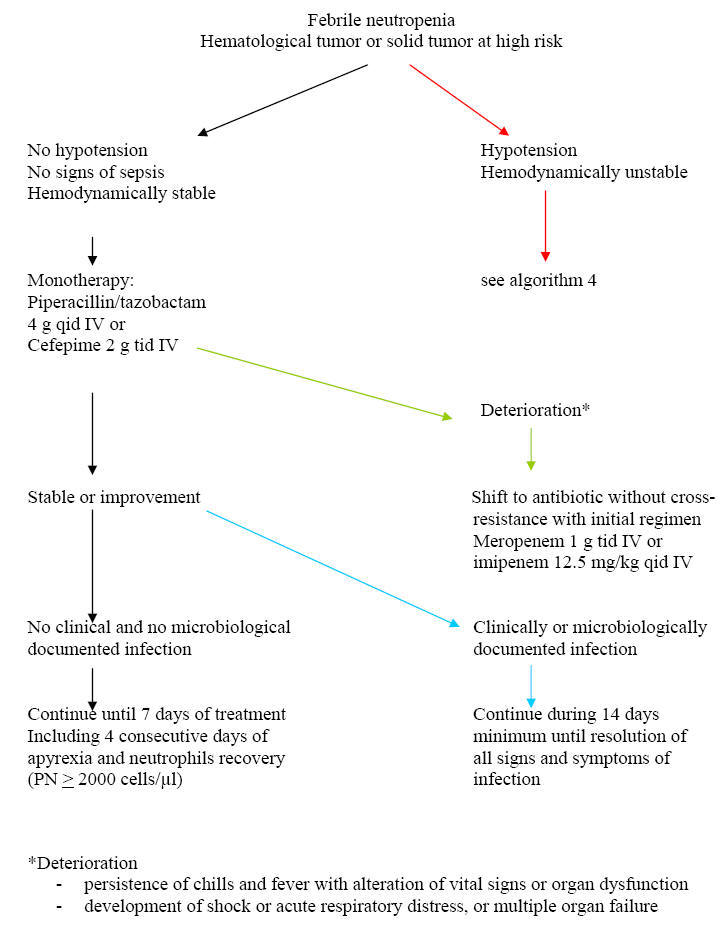
Treatment of Patients at High-Risk
The
standard care of febrile neutropenia at high-risk of complications includes
in-patient management with intravenous broad-spectrum antibiotics. A ß-lactam
agent active against Gram-negative bacteria including P. aeruginosa is
the corner stone around which the whole management strategy is build up.
Although detailed global and updated epidemiological data are lacking in the
neutropenic population, it is more likely that local institution epidemiology
would be more appropriate for the selection of initial ß-lactam agents for
empiric therapy. Among the different ß-lactams, the highest percentage of
susceptibility of P. aeruginosa is observed for piperacillin-tazobactam
and meropenem although no one single ß-lactam delineates 100 % activity against
P. aeruginosa anymore. Another epidemiologic feature to be taken
into account, is the rising incidence of extended spectrum ß-lactamase (ESBL)-producing
Gram-negative bacilli, especially E. coli and Klebsiella spp., in
the neutropenic patients. Among the few ß-lactam agents that are suitable
for empiric therapy of febrile neutropenia, it is important to make a
distinction between molecules such as ceftazidime, cefepime or
piperacillin-tazobactam among which the probability of cross-resistance is high,
and the carbapenems such as imipenem or meropenem which retain activity in case
of emergence of resistance to other ß-lactams. Based on these considerations, it
is recommended to use a strategy that differentiates between first-line therapy
based on molecules such as cefepime or piperacillin-tazobactam, and second-line
therapy constituted of the carbapenems. This allows keeping a valuable
alternative in case of emergence of resistance. However, such a strategy can be
implemented only if the baseline incidence of ESBL-producing Gram-negative
bacilli is low. In fact, carbapenems are the most active drugs against these
bacteria, and reduced the mortality in ESBL-producing K. pneumoniae
bacteremia. Among the first-line ß-lactam agents, there are some theoretical
advantages of piperacillin-tazobactam and cefepime over ceftazidime. These
include a better activity against streptococci and methicillin-susceptible S.
aureus with less need for the addition of a glycopeptide. Furthermore, less
induction and decreased emergence of ESBLs, are reported with cefepime and
piperacillin-tazobactam.
Several randomized comparative trials have assessed the potential of each the
ß-lactams, namely ceftazidime, cefepime, piperacillin-tazobactam, imipenem,
meropenem and aztreonam, for empiric therapy of primary episodes of febrile
neutropenia at high-risk. No significant differences in response rates or
mortality were observed in these individual trials. So, the clinical
evidence-based medicine assessment of these different ß-lactams will result in a
strong recommendation with good evidence to support their use. But, the
continuously changing microbial distribution and the emergence of new mechanisms
of resistance that occurred after the completion of many of these studies,
contribute to obscure the interpretation of results and their relevance to the
actual epidemiology. Nevertheless, a recent meta-analysis focusing on response
rate in the different comparative trials of empiric therapy of febrile
neutropenia, showed superiority of the carbapenems and piperacillin-tazobactam
over ceftazidime. In some situations, specific anti-anaerobic coverage is
indicated. These include severe mucositis or gingivitis, typhlitis, peri-anal
and allogeneic HSCT where up to 17 % of bloodstream infections were anaerobes. Piperacillin-tazobactam and carbapenems cover the majority of anaerobes,
otherwise, for cephalosporins or aztreonam, metronidazole should be added.
Although this is not a current first choice, in case of penicillin-allergy
mediated by IgE where the risk of anaphylaxis is important, aztreonam combined
with a glycopeptide is an acceptable alternative.
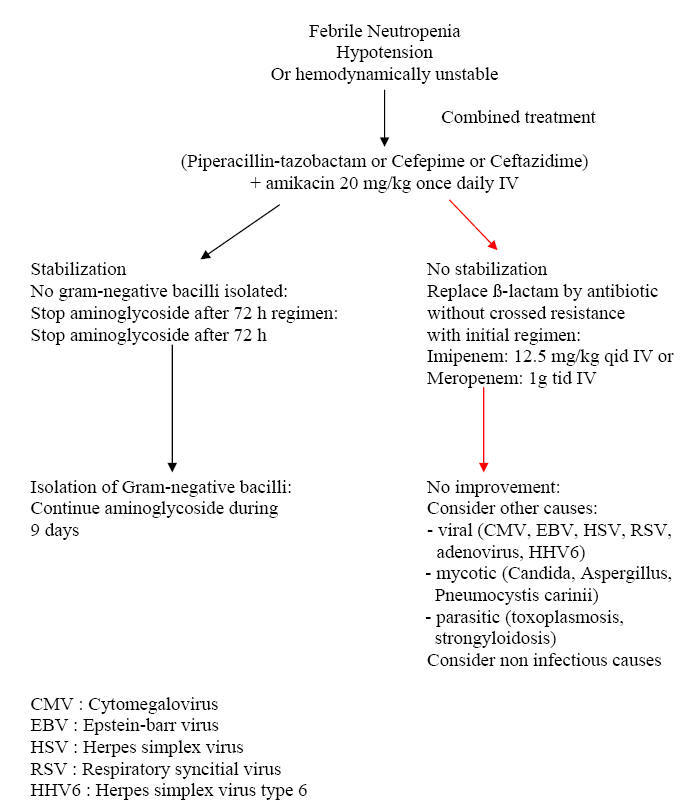
Is There Still a Role for Combination Therapy of
a ß-lactam Plus an Aminoglycoside for Empiric Therapy?
This question has
been addressed in two recent meta-analyses which reviewed the studies that
compared monotherapy with combination therapy. The conclusion was that of no
advantage of the combination with an excess of toxicity. However, it should be
mentioned that mortality is highest in the subgroup of febrile neutropenic
patients with sepsis, varying between 18 and 40 % as compared with 2.8 % for
non-septic febrile neutropenic patients. If any benefit is to be expected from
combination therapy, it is within this subgroup that would occur. But, no single
study targeted specifically such patients. Thus, if for the majority of patients
with febrile neutropenia, a combination therapy is not justified by the existing
data, in the subgroup of patients with sepsis and high mortality, the benefits
of combining a ß-lactam with an aminoglycoside may outweigh the risk of
toxicity. Another important question has been the addition of a glycopeptide to
the initial regimen. In fact, among the Gram-positive pathogens that cause
infection or bacteremia during neutropenia, very few cause a fulminant infection
course with significant morbidity and mortality. These include viridans
streptococci, S. pneumoniae and S. aureus, which need optimal
coverage upfront. Again, the local epidemiology and the penicillin or
methicillin-resistance of these organisms, which is highly variable between
institutions, plays an important role. A recent Cochrane review of 13 randomized
trials comparing the addition of an anti-Gram-positive antibiotic to the initial
empiric regimen, did not show any benefit in reducing treatment failure, all
cause mortality or Gram-positive superinfections. However, there are
circumstances in which a glycopeptide or another antibiotic active against
resistant Gram-positive bacteria, should be added up-front. These include
patients who are already known to be colonized by MRSA, if MRSA is endemic in
the institution and in several skin infections that could be caused by resistant
staphylococci as folliculitis, furonculosis and periporth cellulitis and also if
penicillin-resistant viridans streptococci are prevalent in the institution.
When and How to Modify Initial Empiric Therapy
in Persistently Febrile Neutropenic Patients?
Few studies have specifically
addressed this question. According to the study by Cometta et al conducted
by the anti-infection group of the EORTC, the mean time for defervesence on
piperacillin-tazobactam monotherapy was 5 days. Therefore, for patients without
hypotension or sepsis and in whom no resistant pathogen is isolated, it seems
reasonable to wait until day 5 before modification. On the contrary, for those
who develop signs of sepsis, hypotension or other early findings of
deterioration, at any time, a shift to non-cross resistant antibiotic is
recommended. After day 5, those who remain febrile and had no non-infectious
cause of fever, may benefit from a shift in ß-lactam coverage i.e. from a
first-line treatment with piperacillin-tazobactam or cefepime to a carbapenem.
Simultaneously, a thorough investigation including chest and sinus CT scan,
galactomannan test and other diagnostic tests for viral or parasitic infections,
should be undertaken. Algorithms 1,
2, 3 and
4 provide general lines of management
according to specific situations of febrile neutropenia.
Algorithm 1: Approach to the
Presence of Clinical Signs of Febrile Neutropenia
Febrile neutropenia Presence of specific clinical sign
↓ ↓
Yes No
(see algorithms 2-4)
↓
Adapted empirical treatment
Always
|
ß-lactam active against P. aeruginosa
|
Cellulitis around a
catheter
|
Add a glycopeptide
|
|
Perianal abscess,
necrotic gingivitis or typhilitis |
Add anaerobic coverage
(piperacillin, tazobactam, imipenem, 3rd of 4th
generation
cephalosporin plus metronidazole)
|
Severe mucositis
|
Optimal coverage of
streptococci + acyclovir
|
Cutaneous cellulitis
|
Optimal coverage of
S. aureus and Pseudomonas aeruginosa. If important
resistance rate to
oxacillin in institution : add glycopeptide
|
Diarrhea
|
Toxin detection of
Clostridium difficile and add oral metronidazole
|
Algorithm 2: Approach of Empiric Antibiotic
Therapy for Febrile Neutropenia by MASCC score
Algorithm 3: Approach to Febrile Neutropenia with
Stable Hemodynamic Status
Algorithm 4: Approach to Febrile Neutropenia with
Unstable Hemodynamic Status
Antifungal Empiric Therapy
The rationale
for empiric antifungal therapy in persistently febrile neutropenic patients, is
based on the fact that early diagnosis of invasive fungal infections is
difficult to establish and the mortality is increased by delay in adequate
therapy. The concept of empiric antifungal therapy in neutropenic patients with
persistent fever despite broad-spectrum antibiotics, has been introduced
following two studies with a limited number of patients, comparing amphotericin
B with placebo and showing a decrease in fungal infection-related mortality in
patients receiving amphotericin-B deoxycholate. These studies were accomplished
at a period where antifungal prophylaxis was ineffective and CT-scans and ELISA
galactomannan tests on PCR for Aspergillus detection were inexistent. Is this
concept still true today? We just don’t know, because all the large studies that
have been done thereafter addressing the question of empiric antifungal therapy
were not placebo controlled. With this in mind, we see in the latest trials that
the true rate of failure of empiric antifungal therapy which is the development
of a breakthrough fungal infection and the absence of cure of base line fungal
infection is 6.9 % for liposomal amphotericin-B, 3.6 % for voriconazole and 7.7
% for caspofungin. The need for empirical antifungal therapy during neutropenia
has decreased secondarily to the implementation of more effective antifungal
prophylaxis. In one of the most recent trials using posaconazole prophylaxis in
neutropenic patients, the need for empiric therapy was only of 27 %. The next
question is whether our new imaging techniques and new laboratory methods are able
to detect invasive fungal infections early enough and whether they can serve to
build-up a pre-emptive or a diagnostic-driven strategy in order to save
unnecessary costs and drug exposure, without increase in mortality. One such a
pre-emptive study by Maertens et al, based on positive ELISA galactomannan
test or suggestive infiltrate on chest CT-scan, showed a reduction of 78 % in
the use of empiric antifungal drugs. Another study by Cordonnier et al
comparing prospectively the empiric with the pre-emptive strategy in high-risk
febrile neutropenic patients showed again a decrease of 20 % in the use of
antifungal drugs with the pre-emptive strategy, a similar mortality rate but
surprisingly similar median medication costs. Thus, the pre-emptive or
diagnostic-driven strategy is able to reduce the rate of antifungal
overtreatment but the safety and cost effectiveness should be confirmed by large
comparative prospective trials.
Adjunctive Therapy
The recovery of neutrophils in patients with severe infection and hematological
malignancy plays a major role in resolution and survival. In order to palliate a
temporary deficit in neutrophils, a logical approach was the development of
transfusion of donor neutrophils as adjunctive therapy to antibiotics and
antifungals, in neutropenic patients with refractory infections. Although
initial clinical successes were reported between 1970s and beginning 1980s,
ganulocyte transfusion adjunctive therapy declined progressively due to several
reasons. In fact, adverse effects such as allo-immunization, CMV transmission,
GVHD and pulmonary reactions, have been reported. Besides, the availability of
more advanced antimicrobials, the time and cost consuming procedure and a
marginal effect demonstrated in randomized trials, all together contributed to
this decline. Moreover, the lack of standard optimal granulocyte dose required
daily (>1010 cells) and storage conditions and duration (8-24h) add to the
uncertainty of study results. More recently, a revival in granulocyte
transfusion use has occurred as donors were stimulated more efficiently by G-CSF
at 5 to 10 µg/kg. Higher yields and improved phagocytosis and killing of
neutrophils with prolonged intravascular survival are obtained after
stimulation. However, the clinical efficacy of this new generation of
granulocyte transfusion is still limited to case reports and small non
randomized series. A recent meta-analysis of 8 randomized controlled trials
involving granulocyte transfusions, given therapeutically to neutropenic
patients showed inconclusive evidence to support or refute the generalized use
granulocyte transfusion therapy. Thus, in the absence of definitive data, it is
reasonable to provide granulocyte transfusions for neutropenic patients with
hematological malignancy and documented bacterial or invasive fungal infection,
not controlled by adequate antimicrobial therapy.
The role of G-CSF and GM-CSF as adjunct therapy to antimicrobials in febrile neutropenic patients is controversial. Individual small studies have generated
conflicting results. A recent meta-analysis on 13 controlled trials comparing
CSFs plus antibiotics versus antibiotics alone for the treatment of established
febrile neutropenia, showed a benefit in terms of length of hospitalization and
time to neutrophil recovery, in favor of CSFs, but no advantage on overall
mortality and a trend toward a decrease in infection-related mortality. This
latter effect needs further investigation.