
Fever and Abdominal Pain - Causes and Diagnosis
(Set Margins at File -> Page SetUp at 0.0" for best result)
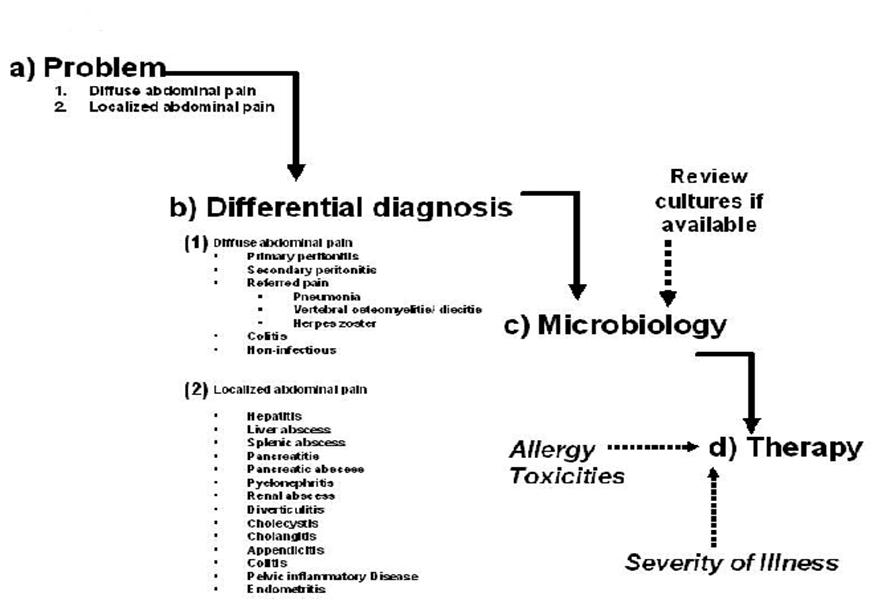
The patient presenting with fever and abdominal pain generates a broad differential diagnosis involving infections of the gastrointestinal tract, solid organs of the abdominal cavity, gynecologic organs and referred pain from infections outside of the abdominal cavity. The approach to diagnosis and antibiotic selection will follow that outlined in the chapter “Empiricism-Philosophy and Practice” (Figure 1). The first step is to develop a problem list. From that problem list, a clinical differential diagnosis can be generated. Based on the clinical diagnosis, a microbiologic differential diagnosis can then be created. The severity of illness, where the infection was acquired (community or hospital based setting), underlying co-morbidities and drug allergies also need to be accounted for. Finally, empiric antibiotic selection can occur only after careful consideration of the presumed microbiology, severity of illness, and site of infection acquisition.
Figure 1: Approach to the Patient with Fever and Abdominal Pain

RUQ, right upper quadrant; RLQ, right lower quadrant; LLQ, Left lower quadrant; LUQ, left upper quadrant; AAS. Acute abdominal series; CT, computerized tomography; PID, pelvic inflammatory disease
PROBLEM: Abdominal Pain
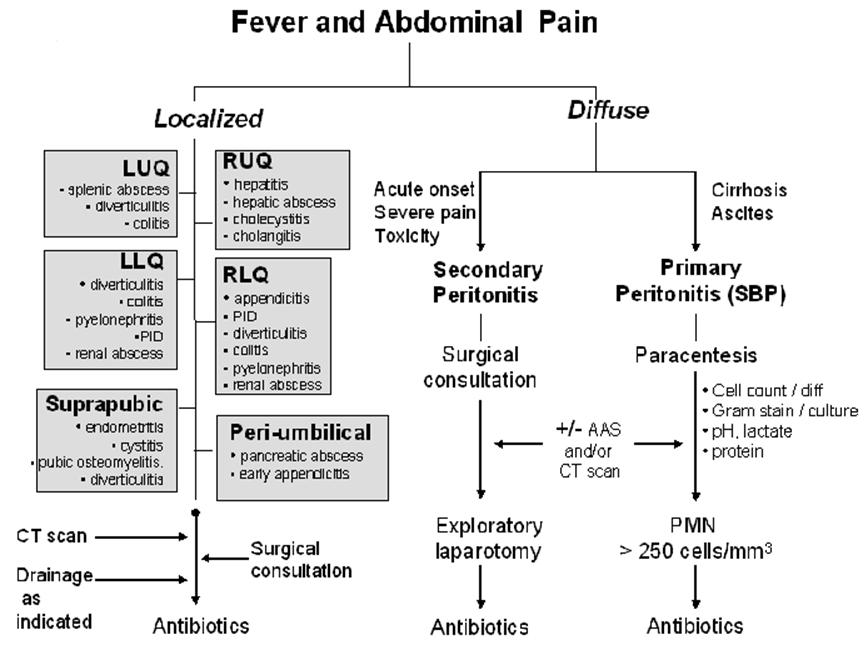
The first division point is the general nature of the pain being either diffuse or localized. See Figures 1 and 2. Diffuse abdominal pain raises the concern of a generalized peritonitis. Peritoneal irritation causes moderate to severe pain that is aggravated by motion, forcing the patient to lay still. Bowel sounds are hypoactive or absent. There is voluntary and involuntary guarding and rebound tenderness. Patients are often toxic appearing. Immune compromised patients, especially those on high dose corticosteroids, warrant heightened vigilance since the anti-inflammatory effects of the corticosteroids may mask both fever and the irritative signs of peritonitis.
Pain localized to a specific area or quadrant of the abdomen may incriminate a specific organ infection involving the liver, spleen, pancreas, kidney, bladder, colon, ovaries/salpinx or uterus (Table 2).
DIFFERENTIAL DIAGNOSIS
Diffuse Abdominal Pain (Figures 1 and 2, Table 1)
Diffuse abdominal pain may be due to bacterial infection of preexisting ascitic fluid, usually in the setting of hepatic cirrhosis, referred to as primary or spontaneous bacterial peritonitis (SBP). Organ perforation with spillage of gastrointestinal contents into the peritoneal cavity, referred to as secondary peritonitis, will also cause diffuse abdominal pain though the onset is often more acute and the patient more toxic than in those with SBP. In the cirrhotic patient with ascites presenting with diffuse abdominal pain, it is often challenging to differentiate primary from secondary peritonitis. Acute abdominal series or computerized tomography (CT) may reveal free peritoneal air in the latter condition. Secondary peritonitis is polymicrobic in nature while primary peritonitis is monomicrobic.
Table 1 outlines the clinical presentation, risk factors and diagnostic evaluation for diffuse abdominal pain (Problem # 1 from Figure 1). Patients suspected of having SBP should undergo paracentesis. Fluid should be sent for cell count and differential, protein content, lactate level, pH and Gram stain and culture. Some studies have suggested that the yield of ascitic fluid culture may be increased by injecting the fluid into blood culture bottles for incubation. SBP is diagnosed by the presence of bacteria on Gram stain or culture and/or a fluid white blood cell count > 250 cells/mm3. Supporting evidence includes a protein > 3g/dl and a lactate > 25 mg/dl. Less commonly, diffuse abdominal pain may be referred into the abdominal area from pneumonia, vertebral body infection or dermatomal Herpes zoster. Clostridium difficile associated colitis should be considered in those who present with diarrhea, diffuse (or local) abdominal pain (often with associated leukocytosis) and have recent exposure to antibiotics or have been recently hospitalized. Non-infectious syndromes such as inflammatory bowel diseases, ischemic colitis, pancreatitis, polyarteritis nodosa (PAN), familial Mediterranean fever (FMF), porphyria, sickle cell crisis and malignancies can present with fever and diffuse abdominal pain.
Some infectious processes such as secondary peritonitis from gastrointestinal perforation are immediately life threatening and require urgent diagnosis and surgical intervention. Others are more often subacute in nature (viral hepatitis) and workup can proceed along at a less critical pace.
Table 1: Diagnosis of patients with diffuse abdominal pain Risk Factors Presentation Evaluation Primary Peritonitis · Ascites due to cirrhosis, severe hypoalbuminemia from nephrotic syndrome, CHF, malignancy
· CAPD catheter
· VP shunts
· Diffuse abdominal pain, fever, nausea, vomiting1
· Leukocytosis3
· Other signs and symptoms of hepatic failure5
· Blood cultures4
· AAS
· Abdominal CT scan
· Paracentesis2
· Liver enzymes7
· Amylase, lipase
Secondary Peritonitis · Appendicitis
· Cholecystitis
· Diverticulitis
· Peptic ulcer disease
· Abdominal injury
· Bowel obstruction
· Mesenteric ischemia
· Surgical anastomotic leak
· GU infections6
· Acute onset of diffuse abdominal pain, fever, nausea, vomiting
· Abdomen rigid, hypoactive or absent bowel sounds, guarding and rebound tenderness
· Leukocytosis3
· AAS
· Abdominal CT scan
· Liver enzymes7
· Amylase, lipase
· Exploratory laparotomy
Lower Lobe Pneumonia · Aspiration
· Smoking
· COPD
· Upper abdominal pain
· Cough, hypoxia may be present
· Localized rales on chest examination
· Blood cultures
· Chest x-ray
CT, computerized tomography; CHF, congestive heart failure; COPD, chronic obstructive lung disease; GI, gastrointestinal; GU, genitourinary; CAPD, continuous peritoneal dialysis catheter; VP, ventriculoperitoneal; AAS, acute abdominal series
1 Patients with cirrhosis and primary peritonitis may occasionally present without fever or abdominal pain. Consideration should be given to perform paracentesis inpatients with ascites.
2 Ascitic fluid should be sent for white blood cell count and differential, protein, Gram stain and culture, lactate level and pH. Fluid may also be inserted into a blood culture bottle for culture. The yield of ascitic fluid Gram stain and culture is poor. A negative test result does not exclude spontaneous bacterial peritonitis (SBP). A fluid white blood cell count > 250 cells/mm3 is diagnostic of SBP.
3 Patients with overwhelming infections may have leukopenia and marked bandemia
4 Two sets should be obtained prior to the start of antibiotics. The yield of blood cultures in secondary peritonitis approaches 75%, while it is substantially poorer in patients with SBP.
5 Encephalopathy, variceal bleeding
6 septic abortion, salpingitis, post partum endometritis
7 Liver enzymes; aspartate amino transferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, bilirubin
Localized Abdominal Pain
Localized abdominal pain may be due to many causes including 1) organ perforation with localized containment such as diverticulitis, 2) infections of obstructed organs such as occurs in cholecystitis and appendicitis, 3) bacteremic spread resulting in abscess formation in the kidney, liver or spleen and 4) spread of bacteria into otherwise sterile organs such as the pancreas (infected pseudocyst or pancreatic abscess) or kidney (pyelonephritis). The differential diagnosis of localized abdominal pain can be approached by location of the pain: right upper quadrant (RUQ), left upper quadrant (LUQ), right lower quadrant (RLQ) and left lower quadrant (LLQ). See Figure 2.
Table 2 outlines the clinical presentation, risk factors and diagnostic evaluation for localized abdominal pain (Problem # 2 from Figure 1). As with diffuse abdominal pain non-infectious illnesses such as those summarized above in addition to acute alcoholic hepatitis, acute myocardial infarction, ectopic pregnancy and ruptured ovarian cyst may present with localized abdominal symptoms that may mimic infection.
Table 2: Diagnosis of Patients With Localized Abdominal Pain Risk Factors Presentation Evaluation Hepatitis · Alcohol ingestion
· IDU
· Ingestion of contaminated food
· RUQ pain
· Fever, nausea, vomiting
· Jaundice
· Liver enzymes7
· Serologic tests for viral hepatitis3
· Serologic tests for less common causes as indicated4
Hepatic abscess · Appendicitis, Diverticulitis, Cholecystitis
· Bacteremia
· RUQ and epigastric pain
· Fever, nausea, vomiting
· leukocytosis
· Blood cultures
· Liver enzymes7
· RUQ ultrasound
· CT scan
Cholecystitis1 · Gallstones
· Trauma, burns
· Postprandial RUQ and epigastric pain
· Fever, nausea, vomiting
· (+) Murphy’s sign2
· Leukocytosis
· Blood cultures
· RUQ ultrasound5
· Liver enzymes7
· Amylase, lipase
· HIDA scan
Cholangitis · Obstruction of the biliary tree from gallstones, malignancy, surgery
· RUQ pain6
· Fever, nausea, vomiting
· Jaundice
· Leukocytosis
· Blood cultures
· RUQ ultrasound
· Liver enzymes7
· Amylase, lipase
Appendicitis · Generally none
· Foreign bodies
· Tumor
· Strictures
· Parasitic infection8
· Periumbilical pain migrating to RLQ
· Fever, nausea, vomiting
· Leukocytosis
· CT scan
Diverticulitis · Diverticulosis
· LLQ pain9
· Fever, nausea, vomiting
· Leukocytosis
· Blood cultures
· CT scan
Splenic abscess · Bacteremia
· Endocarditis
· Sickle cell disease
· IDU
· LUQ pain referred to left shoulder
· Fever, nausea, vomiting
· Leukocytosis
· CT scan
· CXR11
Colitis10 · Contaminated food and water
· Antibiotics
· Diarrhea, hematochezia
· RLQ, LLQ pain
· Fever
· Leukocytosis
· Stool culture10
· Fecal leukocytes
· Clostridium difficile toxin assay
Pelvic Inflammatory Disease · Young age and sexual active12
· New sexual partner
· Bacterial vaginosis
· IUD
· RLQ, LLQ pain
· Fever
· Leukocytosis
· Bimanual pelvic examination
· Pelvic ultrasound
· CT scan
· Cervical culture for Neisseria gonorrhea and Chlamydia trachomatis
Endometritis · Pregnancy13
· Suprapubic pain
· Fever
· Leukocytosis
· Bimanual pelvic examination
Pancreatic abscess · Pancreatitis
· Periumbilical and back pain
· Fever
· Leukocytosis
· Blood cultures
· Liver enzymes7
· Amylase, lipase
· CT scan
Renal abscess · Kidney stones
· Ureteral obstruction
· DM
· Bacteremia
· Flank and back pain
· Fever
· Leukocytosis
· Blood cultures
· Urine culture
· Renal ultrasound
· CT scan
Pyelonephritis · Kidney stones
· Ureteral obstruction
· DM
· Flank and back pain
· Fever, nausea, vomiting
· Leukocytosis
· Blood cultures
· Urine culture
· Renal ultrasound
IDU, injection drug use; RUQ, right upper quadrant; RLQ, right lower quadrant; LLQ, Left lower quadrant; LUQ, left upper quadrant; CT, computerized tomography; DM, diabetes mellitus; CXR, chest x-ray; HIDA = hepatobiliary iminodiacetic acid, IUD, Intrauterine contraceptive devices
1 95% due to gallstones. Acalculous cholecystitis can be seen after trauma, surgery, burns and in those with HIV infection, immune suppression and DM
2 Murphy’s sign: inspiratory arrest during palpation of the RUQ. Named after John B. Murphy (1857- 1918), a Chicago, Illinois surgeon.
3 Hepatitis A virus IgG and IgM antibody, Hepatitis B surface antigen (HBsAg), Hepatitis B surface antibody (HBsAb), Hepatitis B core antibody (HBcAb), Hepatitis C virus antibody.
4 IgM, IgG antibody for cytomegalovirus (CMV), monospot for Epstein Barr Virus (EBV) infection, antibody for human immunodeficiency virus (HIV) infection
5 Thickened gallbladder wall, pericholecystic fluid, (+) sonographic Murphy’s Sign
6 The classic Charcot’s triad of RUQ pain, fever and jaundice is seen in less than 20% of patients.
7 Liver enzymes: aspartate amino transferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, bilirubin
8 Enterobius vermicularis, Ascaris lumbricoides, Strongyloides stercoralis
9 Pain may be RLQ or suprapubic depending on the position of the colon and location of the inflamed diverticula.
10 Including food borne bacterial colitis from Campylobacter, Salmonella, Shigella, Escherichia. Coli 015H7 and antibiotic related Clostridium difficile.
11 May reveal left lower lobe atelectasis, effusion, elevated left hemidiaphragm
12 Pelvic inflammatory Diseases (PID) are often due to sexually transmitted pathogens such as Neisseria gonorrhoeae or Chlamydia trachomatis.
13 Seen more often with cesarian section, ruptured membranes for > 6 hours, multiple cervical examinations and chorioamnionitis.
Figure 2: Sequential Process for Thinking Through the Diagnosis, Microbiology and Therapy of a Patient with Fever and Abdominal Pain
MICROBIOLOGY
Decisions regarding antibiotic selection most often have to be made prior to the identification of the offending pathogen(s). In many instances, microbial pathogens are never isolated or cultures are performed after antibiotics have been initiated causing false negative results. In addition, an isolated pathogen may not fully represent the full microbiology of an infection. For example, Escherichia coli may be the only isolate from a blood culture in a patient with a diverticular abscess despite the polymicrobic nature of the infection. Therefore, the microbiology of an infection must be based on the clinical diagnosis.
Another important factor in thinking through the microbiology includes careful consideration of where the patient acquired the infection. Infections acquired in the community are more often caused by less resistant aerobic gram negative rods (GNR) such as E. coli, Klebsiella and Proteus spp.. Hospital acquired infections are more often caused by more resistant GNR Enterobacter spp, Serratia spp, Morganella spp, Pseudomonas aeruginosa and vancomycin resistant enterococci (VRE).
Table 3 summarizes the microbiology of peritonitis causing diffuse abdominal pain. Primary bacterial peritonitis is classically mono-bacterial. The bacteriology of secondary peritonitis depends on the site of the ruptured organ. Gastric and upper small bowel flora often includes salivary organisms such as streptococci, lactobacillus and candida spp. The distal jejunum, ileum and colon are polymicrobic including aerobic GNR, anaerobes such as bacteroides and clostridia spp., and enterococci. . In the acutely ill patient with secondary peritonitis, it is often difficult to determine the origin of the perforation prior to laparotomy. Therefore it is best to assume the worst microbial case scenario and assume the perforation involves colonic bacteria.
Table 4 summarizes the microbiology of localized intraabdominal infections. Those infections related to biliary or gastrointestinal sources (diverticulitis, cholecystitis, appendicitis, pancreatic abscess) are polymicrobic. Pelvic inflammatory disease (PID) is usually a community acquired sexually transmitted illness. Pyelonephritis and renal abscesses are most often monobacterial. Abscesses in the liver can be polymicrobic (if originating from the gallbladder, appendix or intestine) or monomicrobic (if due to bacteremic seeding) depending on the source, while splenic abscesses are most often of bloodstream origin and monomicrobial.
1Primary peritonitis is most often monomicrobial. One third of patients have negative cultures from paracentesis. Anaerobic bacteria are uncommon and isolation should raise the concern of secondary peritonitis.
Table 3: Microbiology of diffuse peritonitis
Clinical Diagnosis
Community acquired
Hospital acquired
Primary Peritonitis1
· E. coli, Klebsiella pneumoniae, Proteus spp, Enterobacter spp
OR
· S. pneumoniae
OR
· Streptococci, enterococci
· Resistant E. coli, Klebsiella spp, Proteus spp, Enterobacter spp
OR
· P. aeruginosa
Secondary Peritonitis2 · E. coli, Klebsiella spp, Proteus spp, Enterobacter spp
AND
· Enterococci
AND
· Anaerobes including Bacteroides, Clostridium, Prevotella
· Resistant E. coli, Klebsiella spp, Proteus spp, Enterobacter spp, P. aeruginosa, Serratia, Acinetobacter
AND
· Enterococci including VRE
AND
· Anaerobes including Bacteroides, Clostridium, Prevotella
AND
· Candida spp.
2 Secondary peritonitis is polymicrobic involving aerobic gram negative rods (GNR), enterococci and anaerobes.
Table 4: Microbiology of localized intra-abdominal infections
Clinical Diagnosis
Community Acquired
Hospital Acquired
Diverticulitis
· Enteric GNR1
AND
· Enterococci
AND
· Anaerobes3
· Resistant Enteric GNR2
AND
· Enterococci including VRE
AND
· Anaerobes3
Appendicitis
Same as diverticulitis
Same as diverticulitis
Pancreatic abscess
Same as diverticulitis
Same as diverticulitis
Cholecystitis
Cholangitis
· Enteric GNR1
· Anaerobes3,10
· Resistant Enteric GNR2
· Anaerobes3,10
Hepatic abscess4
· Enteric GNR1
AND/OR
· Enterococci
AND/OR
· Anaerobes3
OR
· S. aureus, Streptococci, Candida, Yersinia5 OR
· Entamoeba histolytica6
· Resistant Enteric GNR2
AND/OR
· Enterococci including VRE
AND/OR
· Anaerobes3
OR
· S. aureus, Streptococci, Candida,
Splenic abscess7
· S. aureus, Streptococci
· E. coli, Klebsiella spp, Proteus spp, Enterobacter spp
· MRSA, VRE
· Resistant E. coli, Klebsiella spp, Proteus spp, Enterobacter spp, P. aeruginosa, Serratia, Acinetobacter
Colitis
· Campylobacter jejuni, Salmonella spp, Shigella spp, E. coli 0157:H7, Vibrio parahaemolyticus, Yersinia enterocolitica 8
· Entamoeba histolytica6
· Clostridium difficile9
· Clostridium difficile9
Pelvic Inflammatory Disease
· Neisseria gonorrhoeae
· Chlamydia trachomatis
· Enteric GNR1 and Anaerobes3
· Mycoplasma
Not applicable
Endometritis
· Enteric GNR1 and Anaerobes3
· Streptococcus agalactiae
· Gardnerella vaginalis
· Resistant Enteric GNR2 and Anaerobes3
· Streptococcus agalactiae
Renal abscess
· E. coli, Proteus mirabilis, Klebsiella pneumoniae
· S. aureus, streptococci
· Resistant Enteric GNR2
· Enterococci including VRE
· Candida spp
Pyelonephritis
· E. coli, Proteus mirabilis, Klebsiella pneumoniae
· Resistant Enteric GNR2
· Enterococci including VRE
· Candida spp
Hepatitis
· Viral hepatitis A, B,C
· CMV
GNR, Gram negative rod; CMV, cytomegalovirus; VRE, vancomycin resistant enterococci; MRSA, methicillin resistant Staphylococcus aureus
1 E. coli, Klebsiella spp, Proteus spp, Enterobacter spp
2 Resistant E. coli, Klebsiella spp, Proteus spp, Enterobacter spp, P. aeruginosa, Serratia, Acinetobacter
3 Bacteroides, Clostridium, Prevotella, anaerobic Streptococcus
4 Most often polymicrobic originating from infections of the hepatobiliary tree, appendicitis, diverticulitis.
5 May be monobacterial due to endocarditis or bacteremia.
6 Related to travel outside of the United States
7 Most often monobacterial related to bacteremia and endocarditis. 25% are polymicrobic.
8 Foodborne
9 Related to antibiotic usage.
10 Less often isolated except in patients with biliary-intestinal anastomosis.
DIAGNOSIS
In the absence of physical findings of diffuse peritonitis, diagnostic imaging with either computed tomography (CT) or ultrasound should be routinely performed in seriously ill patients with intra-abdominal infection. The urgency of investigation is dictated by the degree of hemodynamic instability present. Most patients should be evaluated within hours of clinical diagnosis. This initial imaging study has become central to therapeutic decision-making since interventional radiology has replaced operative treatment for many localized processes, including diverticular abscesses. Double contrast CT is the single best modality for fully evaluating the extent of disease in most situations. Ultrasound is also quite versatile and has the added advantage of being portable, thus allowing certain procedures to be performed in the ICU. However, ultrasonography is limited by bowel gas, body habitus, and a lower sensitivity for retroperitoneal processes or parenchymal infection. Usually the choice of modality is based on the experience and preference of the interventional radiologist.
The Gram Stain
The Gram stain is an empirical method of differentiating bacterial species into two large groups (Gram-positive and Gram-negative) based on the chemical and physical properties of their cell walls. The method is named after its inventor, the Danish scientist Hans Christian Joachim Gram (1853 – 1938), who formatted the technique in 1884.
Gram-positive bacteria have a thick mesh-like cell wall made of peptidoglycan (50-90% of cell wall), which stain purple and Gram-negative bacteria have a thinner layer (10% of cell wall), which stain pink.
Gram-negative bacteria also have an additional outer membrane which contains lipids, and is separated from the cell wall by the periplasmic space. There are four basic steps of the Gram stain, which include applying a primary stain crystal violet to a heat-fixed smear of a bacterial culture or specimen, followed by the addition of a mordant (Gram's iodine), rapid decolorization with alcohol or acetone and counterstaining with safranin. The Gram stain is the most common staining procedure in clinical microbiology. This tool can aid the physician in determining if infection is present, a possible etiology and a guide to choosing the appropriate antibiotic in just 15 minutes.
The Gram Positive Cell Wall
The Gram positive cell wall The Gram positive cell wall is characterized by the presence of a very thick peptidoglycan layer, which is responsible for the retention of the crystal violet dyes during the Gram staining procedure. Imbedded in the Gram positive cell wall are polyalcohols called teichoic acids which are lipid-linked to form lipoteichoic acids. Because lipoteichoic acids are covalently linked to lipids within the cytoplasmic membrane they are responsible for linking the peptidoglycan to the cytoplasmic membrane. Teichoic acids give the Gram positive cell wall an overall negative charge due to the presence of phosphodiester bonds between teichoic acids monomers.
The Gram Negative Cell Wall
The Gram negative cell wall Unlike the Gram positive cell wall, the Gram negative cell wall contains a thin peptidoglycan layer adjacent to the cytoplasmic membrane, which is responsible for the cell wall's inability to retain the crystal violet stain upon decolorization with ethanol during Gram staining. In addition to the peptidoglycan layer, the Gram negative cell wall also contains an additional outer membrane composed by phospholipids and lipopolysaccharides.
Gram Staining Tips
By Jack D. Rihs
1. Slide preparation
Purulent material should be selected whenever possible.
Apply the sample evenly and thinly to the slide. Smears that are too thick will be difficult to decolorize and imposable to read.
Do not cover the entire slide with the sample. This will make handling difficult and areas may be missed during decolorization. An area the size of a nickel usually is adequate.
2. Slide fixation
The material must be fixed to the slide to prevent it from washing off during staining. This can be done by quickly passing the slide over a gentle flame (the slide should not become to hot to touch) or on a slide warmer. Overheating may alter cell morphology or cause organisms to decolorize more quickly.
An alternative and superior method of fixation is to flood the slide with methanol for 1 minute. Methanol fixation prevents liquid specimens from washing off the slide better than heat fixing, preserves blood cell morphology and results in a clearer background.
3. Staining
Flood the entire slide when crystal violet, iodine and safranin are applied. This will ensure that all areas are stained evenly.
4. Decolorization
The critical step of the Gram staining procedure is the decolorization step. Hold the slide in a tilted downward position and allow the decolorizer to flow over the smear. Be careful not to miss any portion of the smear. Usually a few seconds will suffice.
95% ethanol will decolorize slower than acetone/alcohol, than does acetone.
5. Reading the Gram stain
Begin reviewing the slide using the 10x objective. This will allow you to focus quickly and to look for areas of purulence. The 100x oil immersion lens is essential for viewing individual bacteria.
When reviewing a Gram stain, look at many microscopic fields and in different areas of the slide.
The presence of many squamous epithelial cells in the smear usually indicates a poorly collected sample that will contain normal flora, whereas, the presence of polymorphonuclear leukocytes and no squamous epithelial cells indicates a good sample and an inflammatory process.
Some specimens on preparation will have areas on the slide that are thicker than others. This can result in areas that are under decolorized, thus organisms that are truly Gram-negative will appear Gram-positive. Microscopic fields that contain crystal violet precipitant, or PMNs, macrophages or epithelial cells that are staining purple are areas that are under decolorized.
Gram positive bacteria will usually stand out easily against the pinkish background. Gram negative organisms because of their lower contrast can be missed, particularly in thicker smears. Haemophilus, Bacteroides and Fusobacterium are Gram-negative bacteria that are often overlooked due to their size, pleomorphic morphology or faintly staining qualities.