Table 1. Use of the Galactomannan Antigenemia Test for Monitoring at Risk Patients
|
Population: Prolonged neutropenia, allogeneic SCT Frequency: Two or three times weekly during high level immunosuppression Criteria for positivity:
Considerations:
|
Table 2. Causes for False-Positivity or Cross Reactivity in the Galactomannan Antigenemia Test
|
False-positivity caused by galactomannan contamination |
Cross-reactivity caused by similar cell wall galactomannan |
|
Piperacillin-tazobactam Amoxacillin-clavulanate Other beta-lactam antibiotics Plasmalyte (sodium gluconate) Other intravenous hydration or nutrition fluids containing sodium gluconate Possibly cotton, cardboard, soybean protein
|
Penicillum spp. including P. marneffei Histoplasma capsulatum Geotrichum Neosartoria Possibly Paecilomyces, Alternaria, Trychophyton, Botrytis, Wallemia, Cladosporium, Bifidobacterium |
Table 3. Diagnosis of Invasive Pulmonary Aspergillosis using the Galactomannan Antigenemia EIA on BAL Specimens
|
Population
|
Cutoff 0.5 |
Cutoff 1.0 |
Reference |
||
|
Sensitivity-%
|
Specificity-% |
Sensitivity-%
|
Specificity-% |
|
|
|
Hematology |
Not stated |
Not stated |
100
|
100
|
(28) |
|
Bone marrow transplant |
76 |
94 |
61 |
98
|
(29) |
|
Solid organ transplant
|
100 |
84 |
100 |
91
|
(30) |
|
Solid organ transplant
|
67 |
95 |
67 |
98 |
(31) |
| Intensive Care Unit | 88 | 87 | Not Stated | Not Stated | (33) |
|
Nonimmunocompromised |
100 |
78 |
100 |
88 |
(32) |
Table 4. Use of the Galactomannan Antigenemia Test for Diagnosis of Invasive Aspergillosis
|
BAL and serum for evaluation of suspected pulmonary invasive aspergillosis Validate positive result by repeat testing before starting empiric therapy Search for other evidence of invasive aspergillosis ˇ CT scan of lungs and sinuses and ˇ Histopathology and culture Evaluate for causes for inaccurate results ˇ Galactomannan contamination ˇ Cross-reactive mycosis ˇ Mold-active antifungal therapy |
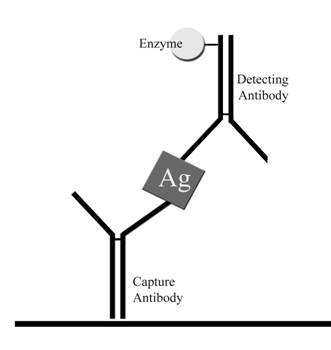
Figure 1. Galactomannan antigenemia detection in the PlateliaŽ Aspergillus EIA. The test specimen in heated at 100° C. for 3 minutes in the presence of 4% ethylenediaminetetraacetic acid (EDTA), after which the supernatant is removed and mixed with the enzyme labeled detector antibody. This mixture is incubated in the microplate wells precoated with the capture antibody. Then the plates are incubated with a chromogenic substrate, tetramethylbenzidine (TMB). If antigen is present color develops, which is recorded using a microplate reader.

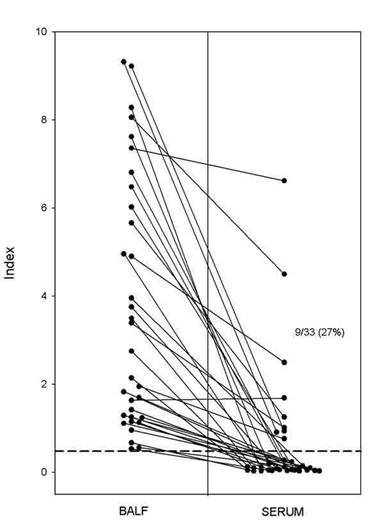
Figure 2. Galactomannan antigenemia EIA on BAL fluid (BALF) versus serum. These represent data for 33 cases in which either BAL or serum, obtained within one week of one another, were positive. All 33 BAL specimens but only 9 of 33 serums specimens (27%) were positive. In 29 of the 33 (88%) BAL specimens the result was >1.0.