Rilpivirine
Authors: Erica L. Dobson, PharmD, Amneris E. Luque, M.D.
CLASS
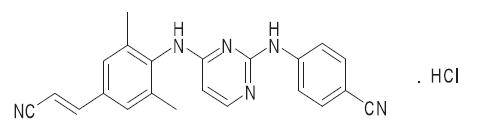
Chemical Structure
The molecular formula for rilpivirine is C22H18N6 ∙ HCl with a molecular weight of 402.88. The chemical name for rilpivirine is 4-[[4-[[4-[(E)-2-cyanoethenyl]-2,6-dimethylphenyl]amino]-2-pyrimidinyl]amino]benzonitrile monohydrochloride (17).
Common Brand Names and Manufactures
Rilpivirine hydrochloride (Edurant™), previously known as TMC278 or R278474 is manufactured by Janssen-Cilag S.p.A. for Tibotec Therapeutics, Division of Centocor Ortho Biotech Products, L.P. (17).
Structure-Activity Relationship
As a diarylpyrimidine compound, rilpivirine has a high affinity for wild-type and first-generation NNRTI-resistant HIV-1 strains (1, 8). The enhanced activity of rilpivirine is attributed to the cyanovinyl moiety (6). The cyano group likely interacts with Trp229, a key aromatic side chain in the nonnucleoside reverse transcriptase inhibitor binding pocket (NNIBP), resulting in increased potency (9). Additionally, a flexible dihedral angle between the aniline ring and the cyanovinyl moiety confers torsional flexibility. This conformational flexibility allows rilpivirine to bind to the NNIBP on the reverse transcriptase enzyme harboring resistance mutations (2, 6, 9, 11).
ANTIVIRAL ACTIVITY
Spectrum
Rilpivirine is a potent and highly selective HIV-1 reverse transcriptase inhibitor (17). While rilpivirine has demonstrated in vitro activity in the micromolar range against HIV-2, the clinical significance of this is not known, and rilpivirine is not indicated for the treatment of HIV-2 infection (1).
In Vitro Activity
The median in vitro 50% effective concentration (EC50) of rilpivirine for HIV-1IIIB is 0.73 nM (range 0.39-0.98 nM). The in vitro EC50 of rilpivirine for HIV-1 group M (subtype A, B, C, D, F, G, and H) primary isolates ranges from 0.07 to 1.01 nM. Rilpivirine is up to 15-fold less active against group O primary isolates compared to its activity against HIV-1 group M primary isolates. Rilpivirine demonstrated in vitro activity against HIV-2 with a median EC50 value of 5220 nM (range 2510 to 10830 nM) (1, 17).
Rilpivirine has a higher genetic barrier to resistance compared to the other currently approved first-generation NNRTIs (efavirenz, delavirdine and nevirapine). Rilpivirine provides enhanced activity against HIV-1 isolates harboring mutations that cause high level resistance to first generation NNRTI agents. These mutations include L100I, K103N, Y181C and Y181L as well as combinations of NNRTI resistant mutations such as K103N + Y181C and L100I + K103N (11). Of 4,786 HIV-1 recombinant clinical isolates resistant to at least one NNRTI, 62% retained in vitro sensitivity to rilpivirine (1).
Rilpivirine has demonstrated additive activity against HIV-1 when studied in vitro in combination with the nucleoside reverse transcriptase inhibitors (NRTIs) abacavir, stavudine, emtricitabine, and didanosine; the nucleotide reverse transcriptase inhibitor (N(t)RTI) tenofovir; the protease inhibitors (PIs) amprenavir, atazanavir, darunavir, indinavir, lopinavir, nelfinavir, ritonavir, saquinavir, and tipranavir; the nonnucleoside reverse transcriptase inhibitors (NNRTIs) efavirenz, etravirine, and nevirapine; the fusion inhibitor enfuvirtide; and the C-C chemokine receptor type 5 (CCR5) coreceptor antagonist maraviroc. Rilpivirine in combination with lamivudine, zidovudine and raltegravir demonstrated low levels of synergy against HIV-1 in vitro (1).
Animal Models
There are no published animal models evaluating the antiviral effects of rilpivirine.
Pharmacodynamic Effects
Bactericidal Effects: There is no information on potential bactericidal effects of rilpivirine.
Effects of Subinhibitory Concentrations: Subinhibitory concentrations of rilpivirine may result in loss of virologic response and possible resistance to rilpivirine or cross-resistance to other drugs in the NNRTI class.
Post-Antiviral Effects: There is no information on potential post-antiviral effects of rilpivirine.
Effects on Host Immunity: In the pooled analysis of the ECHO and THRIVE trials the mean increase in the CD4 cell count from baseline was 192 cells/mm3 in the rilpivirine groups (17).
Pharmacodynamic Correlates With Outcome: A pharmacodynamic model correlating with outcomes has not been described.
MECHANISM OF ACTION
Rilpivirine is an antiretroviral agent belonging to the diarylpyrimidine subclass of NNRTIs, also referred to as “next-generation” or “second-generation” HIV-1 NNRTIs. Rilpivirine inhibits the replication of HIV-1 by binding in a non-competitive manner directly to the reverse transcriptase (RT) enzyme (17). Like all NNRTIs, rilpivirine binds to the NNRTI binding pocket, a flexible allosteric pocket located at a site adjacent to the DNA polymerizing processing site, resulting in conformational changes and altered function of reverse transcriptase (2).
MECHANISM OF RESISTANCE
Organisms Commonly Resistant
The following resistance-associated mutations (RAM) are likely to decrease the antiviral activity of rilpivirine based on in vivo and in vitro data when present at baseline in treatment-naïve patients: K101E, K101P, E138A, E138G, E138K, E138R, E138Q, V179L, Y181C, Y181I, Y181V, H221Y, F227C, M230I, M230L (17).
Single NNRTI substitutions K101P, Y181I, Y181V and E138K result in decreased susceptibility to rilpivirine by 52-fold, 15-fold, 12-fold, and 2.8-fold, respectively. E138K in combination with M184V reduces rilpivirine susceptibility 6.7 fold. K103N does not reduce rilpivirine susceptibility (17).
Mechanisms of Resistance
In patients who failed the rilpivirine containing regimen in the ECHO and THRIVE trials and developed one or more NNRTI RAM, the E138K substitution emerged most commonly (3). Of those with phenotypic resistance to rilpivirine, 90% exhibited cross-resistance to etravirine (3, 13); this potential for cross resistance should be considered if future therapy options are to be preserved. In addition, phenotypic testing in the pooled analysis revealed more cross-resistance to the other available NNRTIs (e.g. efavirenz, nevirapine) in patients who failed rilpivirine compared to those who failed on efavirenz (13). Considerable cross-resistance between rilpivirine and etravirine has also been demonstrated in vitro (1). Additional studies are needed to clarify if rilpivirine remains a treatment option following the development of etravirine resistance.
In the ECHO and THRIVE trials there were also more N(t)RTI-resistance-associated mutations that emerged in the rilpivirine group compared to the efavirenz group. N(t)RTI RAMs that most commonly emerged in the rilpivirine group were M184V/I (confers resistance to emtricitabine and lamivudine) and K65R/N (confers resistance to tenofovir) (3, 13, 17).
Methods to Overcome or Prevent Resistance
Rilpivirine must be used in combination with other antiretrovirals for the treatment of HIV-1 infection. Rilpivirine has been studied in a triple drug combination consisting of rilpivirine with two N(t)RTIs (tenofovir disoproxil fumarate plus emtricitabine, zidovudine plus lamivudine, or abacavir plus lamivudine) (3, 13). To date, no clinical trials of rilpivirine in treatment-experienced patients with NNRTI resistance mutations at study entry have been reported.
Treatment adherence remains of critical importance to the therapeutic success associated with antiretroviral therapy. Patients should be counseled to maintain adherence to rilpivirine and to administer the medication under optimal conditions (with a meal, avoidance of protein-rich drinks) to avoid virological failure. In addition, clinicians should be vigilant to avoid drug-drug interactions that may reduce rilpivirine exposure (e.g. drugs known to induce CYP3A or acid suppressive agents) resulting in subinhibitory concentrations.
PHARMACOKINETICS
Absorption
The absolute oral bioavailability of rilpivirine is unknown (17). The absorption of rilpivirine is affected by food administration and is pH-dependent. The Cmax and AUC of rilpivirine are approximately 46% and 43% lower, respectively, in a fasted state compared to a normal caloric meal (533 kcal). There is no appreciable difference in rilpivirine exposure when administered with a normal caloric (533 kcal) or high caloric meal (928 kcal). Administration with a protein-rich drink reduces the rilpivirine Cmax and AUC each by 50%, similar to administration under fasting conditions (4, 17).
Distribution
Rilpivirine is highly protein bound (99.7%) to plasma proteins, primarily albumin. Distribution into compartments other than plasma (e.g. genital tract, etc) has not yet been evaluated (17).
Routes of Elimination
Metabolism: Rilpivirine is primarily metabolized via oxidative metabolism mediated by CYP3A isoenzymes in the liver (17). In vitro experiments in human hepatocytes show a slow metabolic clearance of rilpivirine, likely contributing to its long terminal half-life (9).
Renal Excretion: Rilpivirine is primarily eliminated in the feces. Following oral administration of a single radiolabeled dose of rilpivirine 25 mg, an average of 85% of the dose was recovered in the feces (25% unchanged drug) and 6.1% in the urine (0.03% unchanged drug) (17).
Pharmacokinetic Parameters (Clearance, Vd steady state, half life, plasma binding, Bioavailability, therapeutic range)
The terminal half-life of rilpivirine is approximately 50 hours (17).
CNS/CSF Disposition: The CNS penetration and CSF disposition of rilpivirine are unknown.
Effect of Disease States (Hepatic Disease, Renal Disease, Shock, CHF, etc.): Rilpivirine is primarily metabolized and eliminated by the liver; therefore, it is anticipated that rilpivirine exposures will be increased in patients with hepatic impairment. Per product labeling, in a study of 8 subjects with mild hepatic impairment (Child-Pugh score A) compared to 8 matched controls and 8 subjects with moderate hepatic impairment (Child-Pugh score B) compared to 8 matched controls, multiple dose exposure of rilpivirine was 47% higher in subjects with mild hepatic impairment and 5% higher in subjects with moderate hepatic impairment (17). Rilpivirine has not been studied in patients with severe hepatic dysfunction. Hepatitis B and/or C virus co-infection does not alter the pharmacokinetics of rilpivirine (17).
Rilpivirine exposure in HIV-1 infected subjects with mild renal impairment is unchanged compared to subjects with normal renal function. There is little information regarding rilpivirine pharmacokinetics in patients with moderate or severe renal impairment or end-stage renal disease. Rilpivirine pharmacokinetics could be altered in patients with severe renal impairment or end-stage renal disease due to alterations in drug absorption, distribution and metabolism; however, this has not been studied (17).
DOSAGE
Adults and Children
The FDA approved dose of rilpivirine in adults is 25 mg administered by mouth once daily with a normal caloric meal (i.e. 533 kcal) (17).
The pharmacokinetics, dosing, and safety in pediatric patients (younger than 18 years of age) have not been established (7, 17). There is a pediatric granule formulation (11) under development, as well as a nanosuspension long-acting injectable formulation (20).
Renal Failure, Including CRRT
Dose adjustments are not necessary in patients with mild or moderate renal impairment. Rilpivirine should be used with caution and close monitoring for adverse drug events in patients with severe renal impairment or end-stage renal disease. Rilpivirine is highly protein bound, and it is therefore unlikely to be significantly removed by hemodialysis or peritoneal dialysis (17). There are no recommendations on dosing rilpivirine for the treatment of HIV-1 in patients receiving CRRT.
Hepatic Failure
Dose adjustments of rilpivirine are not required in patients with mild (Child-Pugh Class A) or moderate (Child-Pugh Class B) hepatic impairment. Rilpivirine has not been studied in patients with severe hepatic impairment and should be used with caution and close monitoring for adverse drug events (17).
Body Composition (Obesity, Wasting, Various Body Builds)
It is not known whether obesity or wasting alters the pharmacokinetics of rilpivirine.
Ascites/Edema
It is not known whether ascites or edema alters the pharmacokinetics of rilpivirine.
Chronic Diarrhea/Malabsorption
It is not know whether chronic diarrhea or malabsorption alters the pharmacokinetics of rilpivirine.
Malnutrition
It is not known whether malnutrition alters the pharmacokinetics of rilpivirine.
Pregnancy
Rilpivirine is pregnancy category B. To date no adequate, well-controlled safety or pharmacokinetic studies of the use of rilpivirine in pregnant women have been conducted. Studies in animals have not shown evidence of embryonic or fetal toxicity or an effect on reproductive function (17).
ADVERSE EFFECTS
In the two international phase 3, randomized, placebo controlled clinical trials (ECHO and THRIVE) most adverse events associated with the use of rilpivirine were described as mild to moderate (grade 1 or 2). Grade 2 or greater adverse events possibly related to rilpivirine were dizziness, abnormal dreams, nightmares, insomnia, nausea and rash. Neurological events were described in 15-18% of the participants and included cluster headache, disturbance in attention, dizziness, headache, memory impairment, mononeuropathy, circumoral paresthesias, photophobia, restlessness, sensation of pressure in the ear, somnolence, uveitis, vertigo, or blurred vision. Psychiatric events reported in > 2% of patients were abnormal dreams, nightmares, insomnia, depression, anxiety, and sleep disorder. Most of the neurological and psychiatric adverse events were grade 1 or 2. The prevalence of adverse events declined after the first 4-8 weeks of treatment. It is worth noting that the prevalence of adverse events in both trials was significantly lower for rilpivirine compared to efavirenz.
Rash was reported in 3% of the participants. Most rashes observed were grade 1-2 and resolved with continued dosing. Treatment discontinuation due to rash was uncommon.
Laboratory abnormalities occurred in 3% or less of the patients and included increase in total cholesterol (1%), LDL cholesterol (1%) and triglycerides (< 1%); increases in amylase (3%) and lipase (1%); Increases transaminases (2%) and hypophosphatemia (2%). A small increase in mean serum creatinine was also observed in patients taking rilpivirine. The increase in creatinine remained stable over the 48 weeks of treatment and the estimated GFR remained below baseline but within normal limits. It is postulated that the increase in creatinine might be related to a rilpivirine effect on the disposition of creatinine, rather than renal toxicity, since the use of cystatin C rilpivirine did not decrease GFR in the THRIVE trial (3).
Both ECHO and Thrive reported an increase in the QT interval corrected according to Fridericia’s formula (QTcF) with a mean change from baseline of 10.9 to 12 ms. There were few adverse events potentially related to conduction abnormalities or to rate and rhythm disturbances (3, 13).
Mechanism
The specific mechanisms of the adverse events seen with rilpivirine have not been elucidated. It is plausible that inter-individual differences in metabolism may, in part, explain susceptibility to central nervous system side effects as has been described with other NNRTIs. Although the CNS adverse effects might wane with time, the long-term consequences of these events are currently unknown.
The cause of lipid changes induced by rilpivirine is not completely understood. In general lipid changes with NNRTIs are less atherogenic than those seen with the protease inhibitors; genetic and other risk factors may be involved.
The mechanism for QT prolongation associated with rilpivirine is likely related to inhibition of potassium channels encoded by hERG based on in vitro studies (15). Risk factors for the development of QT prolongation may be multifactorial including heart rate, electrolyte imbalances, older age and delay in repolarization caused by medications leading to electrical instability (14).
Risk Factors
Risk factors for adverse effects include concurrent diseases, immunologic function, and treatment adherence.
Treatment and Avoidance
Patients must be instructed to contact the prescribing provider should they experience any adverse events. This is important to maintain treatment adherence and avoid erratic dosing that could lead to antiretroviral resistance. Drug-drug interactions that may result in supratherapeutic concentrations of rilpivirine may result in an increased risk of adverse effects of rilpivirine and should be avoided.
Overdoses (Manifestations and Management)
No reports of overdose with rilpivirine have been filed to date and no symptoms of overdose have been observed. Supratherapeutic doses in healthy volunteers (75 mg and 300 mg once daily) have been shown to prolong the QTc interval of the electrocardiogram (11, 17).
Supportive care should be provided along with the optional removal of unabsorbed drug by emesis, gastric lavage, or activated charcoal and cardiac monitoring (i.e. ECG). There is no specific antidote for use in rilpivirine overdose (17).
MONITORING REQUIREMENTS
Given the limited clinical experience with rilpivirine, close clinical monitoring for neuropsychiatric symptoms is in order. Such monitoring includes depression and anxiety screens, as well as direct questioning regarding sleep disturbances, dizziness, abnormal dreams, decrease in concentration, and significant mood changes in an attempt to determine the impact of these potential side effects on quality of life.
Rilpivirine should be used with caution in individuals with prolonged QT syndrome and in those patients taking medications which are known to prolong the QT interval such as methadone, certain antibiotics, antidepressants, antihistamines, diuretics, heart medications, cholesterol-lowering drugs, diabetes medications, as well as some antifungal and antipsychotic drugs. If rilpivirine is to be coadministered with one or more QT-prolonging agents, a baseline and follow-up ECG to monitor the QT interval may be warranted.
Therapeutic Drug Monitoring
Rilpivirine plasma concentrations in vivo necessary to achieve the desired clinical response have not been clinically evaluated. At this time therapeutic drug monitoring for rilpivirine is not recommended.
Other Laboratory Monitoring
Laboratory monitoring for rilpivirine safety should include quarterly liver function tests and a basic metabolic profile. A lipid panel should be monitored at baseline, 6 months after starting rilpivirine and then annually.
DRUG INTERACTIONS
Rilpivirine is primarily metabolized by CYP3A. Thus, drugs that induce or inhibit CYP3A may result in sub- or supratherapeutic concentrations of rilpivirine respectively. Rilpivirine exhibits mild and dose-related induction of CYP3A activity; however, at the approved dose of 25 mg once daily, it is not expected that rilpivirine will alter the pharmacokinetics of concomitant CYP3A substrates (5). Rilpivirine is unlikely to alter the exposure of medications metabolized via CYP2EI (18). Because it is not a p-glycoprotein substrate, p-glycoprotein inhibitors and inducers are unlikely to alter the pharmacokinetics of rilpivirine.
Based on pharmacokinetic studies that have been conducted, the product manufacturer does not suggest that dose adjustment, dose separation, or increased clinical monitoring is necessary when rilpivirine is co-administered with the following medications: acetaminophen, atorvastatin, chlorzoxazone, ethinyl estradiol/norethindrone, sildenafil, or tenofovir (17).
Rilpivirine is the first NNRTI to enter the antiretroviral armamentarium with clinically significant drug-drug interactions with acid suppressive agents. Coadministration of rilpivirine with drugs that increase gastric pH may reduce rilpivirine plasma concentrations, potentially leading to virologic failure and NNRTI resistance. Antacids (e.g. aluminum, magnesium hydroxide, or calcium carbonate) and H2-receptor antagonists (e.g. famotidine, cimetidine, nizatidine, and ranitidine) should be used with caution in patient on rilpivirine (17). It is advised that antacids be administered at least 2 hours before or at least 4 hours after rilpivirine-containing drugs, while H2-receptor antagonists should be administered at least 12 hours before or at least 4 hours after rilpivirine-containing drugs (17, 19). Proton pump inhibitors (e.g. esomeprazole, lansoprazole, pantoprazole and rabeprazole) are contraindicated with rilpivirine therapy (17).
Several agents are contraindicated with rilpivirine due to the potential for CYP3A induction or increases in gastric pH which may result in loss of effective concentrations of rilpivirine including the anticonvulsants carbamazepine, oxcarbazepine, phenobarbital, and phenytoin; all available proton pump inhibitors; rifamycins such as rifabutin, rifampin, and rifapentine; more than a single dose of systemic dexamethasone; and St. John’s Wort. Agents that are known to prolong the QTc interval or are associated with a risk of Torsade de Pointes should be used with caution in patients receiving rilpivirine (17). Other clinically relevant drug-drug interactions are listed in Table 1.
In clinical practice, providers may be considering rilpivirine as a potential option in patients who are unable to tolerate the CNS effects or lipid abnormalities associated with efavirenz. However, a pharmacokinetic study evaluating such a switch in healthy volunteers resulted in a reduced rilpivirine Cmin by up to 25% for up to 4 weeks; this interaction is likely due to induction of the CYP3A enzyme by efavirenz. A pilot study was conducted to evaluate the efficacy and safety of a switch from efavirenz/tenofovir/emtricitabine to rilpivirine/tenofovir/emtricitabine in virologically suppressed patients who were intolerant to efavirenz (n=50). All of the study participants remained virologically suppressed at 12 weeks, indicating that this drug-drug interaction may not be clinically relevant, though a 48 week analysis is also planned (12). A clinical trial evaluating the efficacy and safety of switching from protease-inhibitor based therapy to rilpivirine in virologically suppressed patients is ongoing (NCT01252940).
CLINICAL INDICATIONS
Rilpivirine (25 mg by mouth daily) was approved by the US Food and Drug Administration (FDA) as part of a combination antiretroviral therapy for treatment-naïve HIV-1 infected patients. The FDA also approved a fixed-dose combination tablet of rilpivirine plus tenofovir plus emtricitabine as a one tablet once a day regimen (7). The approval of rilpivirine was based on the results of the ECHO and THRIVE studies, two large international, randomized, double-blind, double dummy, phase 3 clinical trials that compared efavirenz with rilpivirine in combination with two nucleoside or nucleotide reverse transcriptase inhibitors in treatment naïve adults with HIV-1 infection (3, 13). Analysis of the proportion of patients with plasma HIV viral load lower than the limit of detection at 48 weeks demonstrated that rilpivirine was not inferior to efavirenz.
However, the virologic response to rilpivirine appears to be influenced by the baseline HIV-1 RNA with a higher rate of virologic failure among those individuals with baseline viral loads of over 100,000 copies/ml (20% failure) compared to those treated with efavirenz (11%). For individuals with baseline viral loads of over 500,000 copies/mL, the rate of virologic failure was reported as 30% compared to 18% in those with similar HIV-1 RNA plasma levels who received efavirenz. Furthermore, subjects receiving RPV who experienced virologic failure were more likely to have failure with genotypic resistance to other NNRTIs (efavirenz, etravirine, and nevirapine) and to have resistance to their prescribed NRTIs (3, 13).
Based on the limited data on durability of treatment responses (48 weeks), the lower virologic response compared with efavirenz in subjects with pretreatment HIV RNA >100,000 copies/mL, and the greater likelihood of NNRTI resistance with failure,rilpivirine is considered an alternative NNRTI as part of the initial regimen for the treatment of HIV-1 infection in treatment-naïve adult patients (7). The variable response rate may be related to drug exposure; however, therapeutic drug monitoring or dose adjustment is not recommended given the narrow therapeutic range of rilpivirine and the risk of prolonged QT at higher rilpivirine doses (10). Conditions that may result in decreased rilpivirine exposure (e.g. intake without food, co-administration with exposure-lowering drugs including drugs that increase gastric pH) should be considered as important factors that could influence drug exposure and should be avoided (3,11,13).
A open-label study evaluating the safety and efficacy of single tablet emtricitabine/rilpivirine/tenofovir disoproxil fumarate compared to a single tablet efavirenz/emtricitabine/tenofovir disoproxil fumarate in treatment-naïve HIV-1 infected adults is ongoing (NCT01309243).
REFERENCES
1. Azijn H, Tirry, I, Vingerhoets J, de Bethune MP, Kraus G, Boven K, Jochmans D, Van Craenenbroeck E, Picchio G, Rimsky L. TMC278, a next-generation nonnucleoside reverse transcriptase inhibitor (NNRTI), active against wild-type and NNRTI-resistant HIV-1. Antimicrob Agents Chemother, 2010; 54(2):718-727. [PubMed]
2. Chen X, Zhan P, Li D, De Clercq E, Liu X. Recent advances in DAPYs and related analogues as HIV-1 NNRTIs. Current Medicinal Chemistry, 2011;18:359-376. [PubMed]
3. Cohen C , Andrade-Villanueva J, Clotet B, Fourie J, Johnson M A, Ruxrungtham K, Wu H, Zorrilla C, Crauwels H, Rimsky LT, Vanveggel S, Boven K and on behalf of the THRIVE study group. Rilpivirine versus efavirenz with two background nucleoside or nucleotide reverse transcriptase inhibitors in treatment-naive adults infected with HIV-1 (THRIVE): a phase 3, randomised, non-inferiority trial. The Lancet, 2011; 378(9787):229-23. [PubMed]
4. Crauwels HM, van Heeswijk R, Bollen A, Stevens M, Buelens A, Boven K, Hoetelmans RMW. The effect of different types of food on the bioavailability of TMC278, an investigational NNRTI. Ninth International Workshop on Pharmacology of HIV Therapy (April 7–9, New Orleans) 2008, Poster P_32.
5. Crauwels HM, van Heeswijk R, Stevens T, Stevens M, Buelens A, Boven K, Hoetelmans R. The effect of TMC278, a next-generation NNRTI, on CYP3A activity in vivo. Tenth International Workshop on Pharmacology of HIV Therapy (April 15-17, Amsterdam) 2009, Poster P_28.
6. Das K, Bauman JD, Clark AD, Frenkel YV, Lewi PJ, Shatkin AJ, Hughes SH, Arnold E. High-resolution structures of HIV-1 reverse transcriptase/TMC278 complexes: Strategic flexibility explains potency against resistance mutations. PNAS 2008, 105(5): 1466-1471. [PubMed]
7. DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents. Recommendations for NNRTI use in Antiretroviral Treatment-Naïve Patients with HIV-1 Infection. Supplemental Information Regarding the Role of Rilpivirine (RPV) as Initial Therapy - August 16, 2011. Accessed on line on October 1, 2011 http://aidsinfo.nih.gov/contentfiles/NNRTI_One_Page_Info-RPV.pdf
8. de Bethune M-P, Andries K, Azijn H, Guillemont J, Heeres J, Vingerhoets J, Lewi P, Lee E, Timmerman P, Williams P. TMC278, a new potent NNRTI, with an increased barrier to resistance and favourable pharmacokinetic profile. 12th Conference on Retroviruses and Opportunistic Infections (Feb 22-25, Boston) 2005, Poster 556.
9. Janssen PA, Lew PJ, Arnold E, Daeyaert F, de Jonge M, Heeres J, Koymans L, Vinkers M, Guillemont J, Pasquier E, Kukla M, Ludovici D, Andries K, de Bethune MP, Pauwels R, Das K, Clark AD, Frenkel YV, Hughes SH, Medaer B, De Knaep F, Bohets H, De Clerck F, Lampo A, Williams P, Stoffels P. In search of a novel anti-HIV drug: multidisciplinary coordination in the discovery of 4-[[4-[[4-[(1E-2-cyanoethenyl]-2,6-dimethylphenyl]amino]-2-pyrimidinyl]amino]=benzonitrile (R278474, rilpivirine). J Med. Chem, 2005; 48:1901-1909. [PubMed]
10. Medical Review. Rilpivirine, Edurant. FDA Approval package for NDA 202022. May 20, 2011. Accessed on line on September 15, 2011 http://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/202022Orig1s000MedR.pdf
11. Miller CD, Crain J, Tran B, Patel N. Rilpivirine : a new addition to the HIV-1 anti-HIV-1 armamentarium. Drugs of Today, 2011; 47(1):5-15. [PubMed]
12. Mills A, Cohen C, DeJesus E, Rashbaum B, Brinson C, Yale K, Ramanathan S, Ebrahim R, Jandourek A, Cheng A. Switching from Efavirenz/Emtricitabine/Tenofovir Disoproxil Fumarate (EFV/FTC/TDF) single tablet regimen (STR) to Emtricitabine/Rilpivirine/Tenofovir Disoproxil Fumarate (FTC/RPV/TDF) STR
in virologically suppressed, HIV-1 infected subjects. 51st Interscience Conferene on Antimicrobial Agents and Chemotherapy (September 17-20, Chicago) 2011, Poster H2-794c.
13. Molina JM, Cahn P, Grinsztejn B, Lazzarin A, Mills A, Saag M, Supparatpinyo K, Walmsley S, Crauwels H, Rimsky LT, Vanveggel S, Boven K and on behalf of the ECHO study group. Rilpivirine versus efavirenz with tenofovir and emtricitabine in treatment-naive adults infected with HIV-1 (ECHO): a phase 3 randomised double-blind active-controlled trial. The Lancet, 2011; 378(9787):238-246. [PubMed]
14. Owens RC, Nolin TD. Antimicrobial-Associated QT Interval Prolongation: Points of Interest. Clinical Infectious Diseases, 2006:43:1603-1. [PubMed]
15. Pharmacology Review(s). Rilpivirine, Edurant. FDA Approval package for NDA 202022. May 20, 2011. Accessed on line on September 15, 2011 http://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/202022Orig1s000PharmR.pdf
16. Schrijvers R, Desimmie BA, Debyser Z. Rilpivirine: a step forward in tailored HIV treatment. The Lancet, 2011;378:201-203. [PubMed]
17. Tibotec, Inc.: Edurant (rilpivirine) package insert. May 2011.
18. van Heeswijk R, Hoetelmans R, Kestens D, Stevens M, Peeters M, Williams P, Woodfall B, Boven K. The effects of TMC278, a next-generation NNRTI, on the pharmacokinetics of acetaminophen and CYP2EI activity in HIV-negative volunteers. Eighth International Workshop on Clinical Pharmacology of HIV Therapy (April, Budapest) 2007, Poster P67.
19. van Heeswijk R, Hoetelmans R, Kestens D, Stevens M, Peeters M, Williams P, Woodfall B, Boven K. The pharmacokinetic interaction between famotidine and TMC278, a next-generation NNRTI, in HIV-negative volunteers. Fourth IAS Conference on HIV Pathogenesis, Treatment and Prevention (April 22-25, Sydney) 2007, Poster TUPDB01.
20. van ‘t Klooster G, Hoeben E, Borghys H, Looszaova, Bouche M-P, van Velsen F, Baert L. Pharmacokinetis and disposition of rilpivirine (TMC278) nanosuspension as a long-acting injectable antiretroviral formulation. Antimicrobal Agents Chemother, 2010;54(5):2042-2050). [PubMed]
Tables
Table 1: (Adapted from Edurant package insert (17)): Clinically Relevant Interactions with Rilpivirine
| Drug Class | Co-administered Drug(s) | Dosing Recommendation |
|---|---|---|
| Antacids | Aluminum, magnesium, calcium carbonate | Co-administration should be used with caution; may significantly reduce rilpivirine concentrations. Administer antacids at least 2 hours before or at least 4 hours after rilpivirine. |
| Azole Antifungal | Fluconazole, itraconazole, ketoconazole*, posaconazole, voriconazole | Co-administration may result in increased rilpivirine concentrations and decreased azole antifungal concentrations. No dose adjustment is necessary. Monitor for breakthrough fungal infections. |
| H2-Receptor Antagonists (H2RAs) | Cimetidine, famotidine*, nizatidine, ranitidine | Co-administration should be used with caution; may significantly reduce rilpivirine concentrations. Administer H2RAs at least 12 hours before or at least 4 hours after rilpivirine. |
| Macrolide Antibiotics | Clarithromycin, erythromycin | Co-administration may increase rilpivirine concentrations. When possible alternatives should be considered (e.g. azithromycin). |
| Narcotics | Methadone* | No dose adjustment is necessary. Monitor for signs and symptoms of methadone withdrawal. |
| NRTI | Didanosine* | No dose adjustment is required. Didanosine must be administered on an empty stomach and at least 2 hours before or at least 4 hours after rilpivirine. |
| NNRTIs | Efavirenz, delavirdine, etravirine, nevirapine | Do not co-administer. |
| PIs | Darunavir/ritonavir*, Lopinavir/ritonavir*, Atazanavir/ritonavir, atazanavir, fosamprenavir, fosamprenavir/ritonavir, indinavir, nelfinavir, saquinavir/ritonavir, tipranavir/ritonavir | Co-administration may result in increased rilpivirine concentrations. No dose adjustment necessary. |
*Drug-drug interactions were evaluated between the drug and rilpivirine. All others are predicted drug-drug interactions.
Sharma M, et al. Rilpivirine: a new non-nucleoside reverse transcriptase inhibitor. J Antimicrob Chemother 2013;68:250-256.
Nelson MR, Elion RA, Cohen CJ, Mills A, Hodder SL, Segal-Maurer S, et al. Rilpivirine versus efavirenz in HIV-1-infected subjects receiving emtricitabine/tenofovir DF: Pooled 96-week data from ECHO and THRIVE studies. HIV Clin Trials. 2013 May-Jun;14(3):81-91.
