Raltegravir
Authors: Joshua R. Sawyer, Pharm.D.
Introduction
As of December 2011, approximately 34 million people were living with HIV/AIDS worldwide (20). Despite significant advances in antiretroviral therapy (ART), which have led to dramatic decreases in patient morbidity and mortality, there remains no cure for HIV. In addition, the presence of naturally acquired and transmitted resistance to all currently available antiretroviral classes has limited available treatment options for patients living with the condition (25). It is estimated that as many as 18% of treatment naïve patients are resistant to at least one type of antiretroviral drug (22, 46), and as treatment experience progresses, as many as 76% of patients harbor viruses resistant to currently available medications (36). As such, there remains a need for the development of novel antiretroviral therapies that overcome current resistance patterns and treatment related adverse events, allowing patients to remain virologically suppressed.
Integrase is one of three essential HIV-1 specific enzymes encoded by the human immunodeficiency virus that catalyzes the insertion of viral double-stranded complementary DNA into the host cells genome (35, 48). Drugs that inhibit integrase block the incorporation of HIV-1 complementary DNA into the host cell, inhibiting the replication of HIV. The United States Food and Drug Association (FDA) have approved two integrase strand transfer inhibitors (ISTI), raltegravir and elvitegravir. As of February 2013, raltegravir is the only ISTI available as a single tablet medication, and data described below support its use in both treatment naïve and treatment experienced patients.
CLASS
Integrase inhibitor
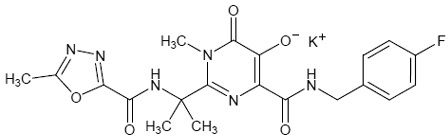
Chemical Structure
N-(2-(4-(4-fluorobenzylcarbamoyl)-5-hydroxy-1-methyl-6-oxo-1,6-dihydropyrimidin-2-yl) propan-2-yl)-5-methyl-1,3,4-oxadiazole-2-carboxamide.
Structure-Activity Relationship
Raltegravir is a 1-N-alkyl-5-hydroxypyrimidinone that is structurally different from diketo acids, but is a structural analog of this class of compounds (12). Raltegravir has a β-hydroxy-ketone structural motif with metal-chelating properties. This motif is thought to bind divalent metals within the active site of HIV-1 IN.
ANTIVIRAL ACTIVITY
The activity of HIV-1 reverse transcriptase, polymerase and human DNA polymerases alpha, beta, and gamma do not appear to be affected by raltegravir (14). Due to an alternative mechanism of action, synergistic activity is seen when the medication is used in combination with other antiretrovirals, including reverse transcriptase inhibitors or protease inhibitors. The drug has inhibitory effects on a wide range of clinical isolates of HIV-1 (including non-B subtypes) and HIV-2, which is resistant to non-nucleoside reverse transcriptase inhibitors and has reduced susceptibility to protease inhibitors. The IC95 values of raltegravir did not appear to change significantly against B and non-B subtypes of HIV-1 (14). The median IC50 and IC95 against HIV-2 strains were approximately 2.4 nmol/L and 12.5 nmol/L, respectively (38).![]()
MECHANISM OF ACTION
Raltegravir is an antiretroviral drug used in the treatment of HIV-1 infection. The drug inhibits the activity of HIV-1 integrase, which impedes the insertion of HIV-1 DNA into the host cell genome. Integrase is a vital enzyme that catalyzes two reactions, which must occur for viral DNA insertion into the host DNA. Primarily, it removes two deoxynucleotides from the 3’ end of the viral DNA. When the reaction is complete, the enzyme is also responsible for covalently binding viral DNA to that of the host cell. Raltegravir inhibits integration at an IC50 of ~10 nM and the results are consistent with inhibition of the third step of the integration process (i.e. strand transfer). This prevents the incorporation of linear HIV-1 cDNA into the host cell’s genome and disrupts the viral life cycle of HIV. Host cells do not express integrase activity; therefore, raltegravir appears unlikely to influence normal cell activities. Unlike maraviroc, which requires CCR5 viral tropism, raltegravir can be used in patients regardless of co-receptor subtype (CCR5, CXCR4, or dual tropic co-receptor).
PHARMACOKINETICS
Absorption
The absorption of raltegravir appears rapid, with a mean time to maximum concentration (Cmax) of 1.3-2 hours (27). The recommended oral dose of raltegravir for adult patients with HIV-1 is 400 mg twice daily. Administration of raltegravir after a high fat meal increases the area under the curve (AUC) by ~19%. High fat meals decrease the Cmax by 34% and cause an 8.5 fold increase in the 12-hr plasma concentration and a delay in tmax following a single 400- mg dose. Additional studies are required to define the effects of a variety of food types on the pharmacokinetics of raltegravir. However, since raltegravir was administered without regard to food in a number of Phase II studies, the FDA approved raltegravir use without regard to food. The raltegravir AUC and Cmax increase proportionally in relation to the dose over the range of 100 mg to 1600 mg (17, 27, 42). With twice daily dosing, steady-state is achieved by the second day of treatment. Considerable inter-patient pharmacokinetic variability has been reported during clinical trials of raltegravir.
Raltegravir at the end of the twelve hour dosing interval was 142 nmol/L (27).
Raltegravir appears to achieve clinically significant concentrations in the cerebralspinal fluid (CSF). In a study involving treatment experienced patients, 24 of 25 patients receiving raltegravir 400 mg twice daily obtained CSF levels that exceeded the EC95 for inhibition of HIV-1 (47). Similarly, the IC50 in treatment experienced AIDS patients reaches therapeutically significant levels within the central nervous system (7). Raltegravir high concentrations in seminal fluid and the genital tract of women (2, 5).
Raltegravir is a substrate of P-gp in humans.
Metabolism and Elimination
Clearance of raltegravir in humans occurs via UGT1A1 mediated glucuronidation (21). Following a single 400 mg dose of raltegravir, the concentrations of the drug were undetectable after twenty four hours. A glucuronide of raltegravir accounts for minimal amounts of plasma raltegravir concentrations. After an oral dose, the drug appears to be excreted both in the urine (32%, 23% - metabolite) and the feces (51%, 0% metabolite) (21).
Pharmacogenomic Considerations
Plasma concentrations for raltegravir appear slightly higher for patients with the UGT1A1*28/*28 genotype of UGT1A1, when compared to individuals with the typically seen UGT1A1*1/*1 (43). Further research is required to determine the clinical significance to this data.
Pharmacokinetics in Special Populations
Age, gender and race do not appear to influence the pharmacokinetics of raltegravir. The drug appears to be safe for use in HIV-1 positive patients with moderate hepatic dysfunction and severe renal dysfunction (18). Elevated liver enzymes were less likely in patients with hepatitis B or C co-infection than in patients treated with other classes of antiretroviral agents, and the drug appears a safe alternative for patients being treated for both HIV and hepatitis (37, 41). Further data is required to determine if the drug is safe and effective in patients with end stage renal disease, and data is conflicting regarding the significance of the amount removed from plasma by hemodialysis (8, 31).
Pediatric Pharmacokinetics
While data is limited, the pharmacokinetics of raltegravir in children aged six to eleven and weighing at least 25 kg appear similar to those seen in adults on the medication (32). A chewable formulation of the drug has less pharmacokinetic variability than the adult tablet formulation, and doses of 6mg/kg appear safe and efficacious in children (33).
The International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) P1066 trial is a raltegravir dose-finding study in treatment experienced HIV-1 infected children. Preliminary PK and safety data from the initial cohort I of adolescents (n=10) aged ≥12 to <19 years and cohort IIA aged ≥6 to <12 years have been presented. Raltegravir was added to a stable, failing ARV regimen, an intensive PK was performed between days 5 and 12, then background antiretrovirals were optimized. RAL therapy was well tolerated in these small cohorts of adolescents and younger children. Target parameters were a geometric mean (GM) area-under-the-curve (AUC)12 >14 μMxh (6.2 mgxh/L) and 12 hour concentrations (C12h) >33 nM (14.7 ng/mL); if not met the dose was adjusted with repeat pharmacokinetic assessments. Preliminary results suggest HIV-1 infected children (≥12 to <19 and ≥ 6 to <12 yrs) receiving 8 mg/kg bid achieved good trough concentrations but total systemic exposure was below the target AUC12. Due to the significant effect various meals can have on the variability in raltegravir exposure, this study was repeated in the same aged cohorts but under modified fasting conditions (34).
The ≥12 to <19 year old group receiving 8 mg/kg (mean dose 390 mg) bid achieved exposures similar to adults receiving 400 mg bid, and this is moving forward into a 48 week efficacy trial. Dosing in the fasted state on the day of pharmacokinetic analysis is preferred for a dose-finding study since food intake increases the variability of individual plasma pharmacokinetic profiles. The younger cohort (≥6 to <12 years, fasting) is currently under evaluation. Dose-finding studies for additional pediatric formulations are being evaluated in HIV-infected children < 12 years of age.
Wenning, et al described the pharmacokinetic and pharmacodynamic parameters of raltegravir as analyzed during phase II and phase III trials (44). The authors described the geometric mean of all concentrations (GM All) as the best measure of overall durable exposure to raltegravir during Phase III trials, linking the measure to AUC. The study also analyzed the geometric mean of observed C12h (GM C12h). There were no significant relationships identified between CM C12h and any pharmacodynamic endpoint in Phase II or Phase III trials, however there were a number of statistically significant relationships noted between pharmacodynamic end points and GM All at week 16 of treatment in Phase III trials. Changes from baseline HIV viral load, decreases in viral load to < 400 copies/ml, and decreases in viral load to < 50 copies/ml were significantly related to GM All, as was virologic failure.
DOSAGE
Adults
The standard oral dose of raltegravir in adults is 400 mg twice daily. No dose adjustments are necessary in renal dysfunction. There are also no clinically significant differences in the pharmacokinetic profile of raltegravir when administered to patients with severe renal insufficiency. No dosing adjustments are necessary for mild to moderate hepatic impairment, and there are no guidelines on the dosing of raltegravir in the presence of severe hepatic dysfunction. The 400 mg film-coated tablets are not bioequivalent with the 25 mg or 100 mg chewable tablets, and patients and providers are advised not to substitute the chewable tablets for the film-coated tablets, or vice versa (14).
Pediatrics
Children and Adolescents greater than twelve years of age are given the standard adult dose of raltegravir. For children between the ages of 6 and 11, but weighing greater than 25 kg, 400 mg by mouth twice daily is also recommended. Safety and efficacy data in children weighing less than 25 kg are not currently available. For infants and children less than six years of age, the safety and efficacy of raltegravir have not yet been established. However, the US Food and Drug Association recommends dosing the medication at 6 mg/kg and providing doses with the chewable tablets according to the protocol listed below (14).
Children 2 – 11 years weighing >= 40 kg: 300 mg (3 x 100 mg tablets) twice daily.
Children 2 – 11 years weighing 28 – 39 kg: 200 mg (2 x 100 mg tablets) twice daily.
Children 2 – 11 years weighing 20 – 27 kg: 150 mg (1.5 x 100 mg tablets) twice daily.
Children 2 – 11 years weighing 14 – 19 kg: 100 mg (1 x 100 mg tablets) twice daily.
Children 2 – 11 years weighing 10 – 13 kg: 75 mg (3 x 25 mg tablets) twice daily.
Children 2 – 11 years weighing < 10 kg: safety and efficacy have not been established.
Use in Pregnancy
The pharmacokinetics of raltegravir in combination with other agents is currently being studied in women in the third trimester of pregnancy. Preliminary data from Best, et al reviewed the use of raltegravir in ten pregnant women. Target twelve hour drug concentrations were greater than 35 ng/ml, despite considerable interpatient variability in plasma levels (3). Similarly, trough concentrations did not appear to differ significantly between pregnant women and post partum women (6).
ADVERSE EFFECTS
In phase III trials combining raltegravir with optimized background therapy in treatment experienced patients with drug-resistant HIV (39), 96 week data suggest that raltegravir is well tolerated compared with placebo. Rates of clinically significant adverse events and laboratory abnormalities appeared similar in the raltegravir and placebo groups (Table 1). Grade 3 or 4 laboratory abnormalities occurred at similar rates between raltegravir and placebo, with the exception of increases in creatine phospokinase (CPK). While grade 4 elevations in CPK were more frequent in patients using raltegravir (3% versus 0.8% with placebo, respectively), this lab anomaly did not appear to be associated with clinical signs or symptoms of rhabdomyolysis or myositis. Discontinuation of therapy due to adverse events occurred in 3.7% of patients in the raltegravir arm versus 5.1% in the placebo arm, respectively.
In phase III trials combining raltegravir plus tenofovir/lamivudine versus efavirenz plus tenofovir/lamivudine in treatment naïve patients (28), 96 week data suggest that rates of serious adverse events and discontinuation of study protocol due to adverse events occurred at similar proportions in the raltegravir and efavirenz arms (Table 2). Discontinuation related to treatment related adverse events occurred in 1.3% of patients taking raltegravir versus 2.6% of patients taking efavirenz, though sample size was small. Grade 3 or 4 laboratory abnormalities varied dramatically between patients taking raltegravir versus those taking efavirenz (Table 3), most notably CPK and fasting lipid profiles. Whereas CPK increases were more common with raltegravir, elevations in LDL, triglycerides and total cholesterol were more frequent in patients taking efavirenz.
Phase III trials comparing raltegravir plus tenofovir/emtricitabine with efavirenz plus tenofovir/emtricitabine in treatment naïve patients (24), week 96 data suggests similar outcomes. Fewer adverse treatment related events were observed in patients taking raltegravir than in patients taking efavirenz (47% versus 78%, respectively; P<0.001). Table 4 describes the most common treatment related adverse events in this trial. Three patients taking raltegravir discontinued therapy due to treatment related adverse events, whereas twelve patients taking efavirenz discontinued therapy as a result of adverse drug events. In this trial, lipid abnormalities were monitored as well. Mean increases in total cholesterol, LDL and triglycerides were all statistically higher in patients taking efavirenz than in those taking raltegravir (P< 0.001).
MONITORING REQUIREMENTS
Therapeutic Drug Monitoring
Therapeutic drug monitoring for raltegravir is not currently recommended.
Other Lab Monitoring
Routine laboratory monitoring for HIV therapeutic response.![]()
DRUG INTERACTIONS
Effects of Raltegravir on the Pharmacokinetics of Other Agents
Raltegravir does not inhibit clinically significant CYP enzymes, including: 1A2, 2B6, 2C8, 2C9, 2C19, 2D6, 3A4 in vitro. Also, the drug does not induce CYP3A4 in vitro.
A drug interaction study involving raltegravir and a CYP3A4 substrate, midazolam, in vivo showed no effect of raltegravir on the pharmacokinetics of midazolam.
Raltegravir does not inhibit UDP-UGT or P-glycoprotein mediated transport systems. It isn’t expected that the pharmacokinetics of substrates of these enzymes or P-glycoprotein (ie, protease inhibitors, NNRTIs, methadone, opioid analgesics, statins, azole antifungals, oral contraceptives or drugs to treat erectile dysfunction) will be altered by concomitant use of raltegravir.
In further drug interaction studies, the pharmacokinetics of lamivudine and tenofovir were not significantly altered when used in combination with raltegravir.
Effects of Other Agents on the Pharmacokinetics of Raltegravir
Co-administration of rifampin, a strong inducer of UGT1A1, and raltegravir should be initiated cautiously, due to the fact that rifampin reduces plasma concentrations of raltegravir. Clinical guidelines suggest increasing the dose of raltegravir to 800 mg twice daily when used concomitantly with rifampin. However, a recent analysis by Wenning, et al (45), observed that this doubling of raltegravir dosing is effective in overcoming rifampin’s effect on raltegravir exposure, but does not have a similar effect on raltegravir trough concentrations. As such, further vigilance should be taken when administering raltegravir with other strong inducers of UGT1A1 including phenytoin or phenobarbital. Weaker inducers of UGT1A1, such as efavirenz, nevirapine, rifabutin, or St John’s wort may be used with the recommended dosage of raltegravir.
Atazanavir, which is a strong inhibitor of UGT1A1, as well as atazanavir combined with ritonavir, tend to increase plasma concentrations of raltegravir. A phase II study (10) evaluated the safety and efficacy of raltegravir administered with or without concomitant atazanavir. Two substudies of raltegravir HIV-infected patients were monitored, including those patients who received atazanavir as part of their optimized background therapy and those patients who did not. Response rates and adverse events were comparable between the substudies of this population. Also, raltegravir was used in combination with atazanavir/ritonavir in BENCHMRK studies and efficacy was again similar in both subsets of patients. Further trials by Iwamoto, et al, describe a slight increase in raltegravir levels when the medication is combined with atazanavir (15). In accordance with this information, no dose adjustment is required for raltegravir in patients who are also taking atazanavir/ritonavir.
A further pharmacokinetic study examined raltegravir dosed with either ritonavir or efavirenz in a double blind, randomized, placebo-controlled trial (19). Twelve healthy males received a single 400 mg oral dose of raltegravir or placebo. Following at least a four day wash out period, the same males received a single dose of raltegravir or placebo along with ritonavir 100 mg twice daily for fourteen days. In the efavirenz study arm, following four days of washout a single dose of raltegravir or placebo was given with efavirenz 600 mg once daily to another twelve healthy males. Patients receiving raltegravir tolerated it as well with ritonavir or efavirenz as without. Plasma concentrations of raltegravir were not substantially effected when co-dosed with ritonavir. While efavirenz modestly reduced plasma raltegravir concentrations there was no clinically significant effect.
In a phase I (1), three period open label trial the effects of etravirine on the pharmacokinetics of raltegravir were evaluated in healthy adults. In period one, patients received 400 mg of raltegravir twice daily for four days to determine the pharmacokinetic profile of this agent. After a washout period, patients in period two were administered etravirine 200 mg every twelve hours for eight days. During this period the pharmacokinetic profile of etravirine was determined. During the third period of this trial, which was preceded by no wash out period, patients received etravirine 200 mg twice daily in combination with raltegravir 400 mg twice daily. Minimal alterations in raltegravir pharmacokinetics occurred during this study, and suggest that no clinically significant drug no interaction occurs between raltegravir and etravirine.
Tipranavir/ritonavir also appeared to decrease plasma concentrations of raltegravir (11). However, in another pharmacokinetic study, healthy males and females received raltegravir 400 mg q12h over four days, followed by tipranavir 500 mg + ritonavir 200 mg q12h for seven days, and then tipranavir 500 mg q12h + ritonavir 200 mg q12h + raltegravir 400 mg q12h for seven doses. The authors concluded that multiple doses of raltegravir with or without boosted tipranavir were well tolerated. While tipranavir/ritonavir decreased the concentration of raltegravir at 12 hours it did not substantially affect the AUC or Cmax. Also, ~ one hundred patients received tipranavir/ritonavir with raltegravir in the BENCHMRK-1 and BENCHMRK-2 studies. Efficacy was similar in these patients as with those patients not receiving tipranavir/ritonavir.
Acid suppressing agents increase plasma concentrations of raltegravir. A single blind randomized, two-period crossover study was recently described that compared the pharmacokinetics of a 400 mg dose with 20 mg of omeprazole or placebo in HIV-negative patients (16). In one treatment period patients were given a placebo at the same time that they were given 400 mg of raltegravir once daily for four days. On the fifth day, they were given a single dose of raltegravir without placebo or omeprazole in the fasted state. The second treatment arm consisted of dosing 20 mg of omeprazole or placebo to match 400 mg of raltegravir once daily for four days. On the fifth day, each patient was given 20 mg of omeprazole with the raltegravir dose. The geometric mean ratio (GMR) of treatments and 90% confidence interval for area under the curve (AUC) was 3.12 (2.13, 4.56). The GMR for Cmax was 4.15 (2.82, 6.10) and for C12hr was 1.46 (1.1, 1.93). The authors concluded that plasma concentrations of raltegravir appeared to increase with co-administration of omeprazole in healthy subjects.
CLINICAL INDICATIONS
A Phase II dose-ranging trial in treatment naïve patients compared ten days of raltegravir monotherapy in twenty eight patients with seven patients who were given placebo (29). Patients received 100 mg, 200 mg, 400 mg or 600 mg of raltegravir twice daily or placebo twice daily. Adherence was assessed by use of diary card and pill counts. Plasma samples were collected for HIV viral load at screening, prior to dose one, six and twelve hours after dose one, and on days 2,3,4,5, 8,10, and 24. The primary end point of the study was change from baseline HIV RNA after ten days of monotherapy. Mean decreases in viral load from baseline to day ten were 1.7 to 2.2 log10 at all doses studied, and when compared to placebo, these decreases were statistically significant for all treatment groups. Results are shown in Table 7.
In the second part of the study (30), 198 treatment naïve patients were randomized to receive the same doses of raltegravir as described above or 600 mg of efavirenz once daily, both in combination with tenofovir and lamivudine. One hundred and sixty patients received raltegravir and thirty eight received efavirenz. The primary end point for this portion of the study was the proportion of patients with HIV RNA < 400 copies / ml at week twenty four. At 24 weeks, 85%-95% of patients in the raltegravir based regimens achieved viral loads of < 50 copies/ml. In the efavirenz regimen, 92% of patients achieved a viral load < 50 copies/ml. By week 48, viral reductions were maintained in 85%-98% of patients on raltegravir regimens versus 83%-88% of efavirenz receiving patients. Results are summarized in Table 8.
Virologic failure by week 48 was comparable between raltegravir-based regimens (3%) and efavirenz based regimens (3%). A second Phase II randomized double blind, placebo controlled study (10), compared raltegravir at 200 mg, 400 mg, and 600 mg twice daily with placebo. All patients also received optimized background therapies, which were based on resistance testing. Early pharmacokinetic studies suggested that co-administration of raltegravir with atazanavir increased overall exposure to raltegravir. As such, two sub-studies were conducted. Substudy A involved patients who did not receive atazanavir in their OBT and substudy B involved patients who did receive atazanavir. All 178 patients enrolled had viral loads of greater than 5000 copies /ml and were failing Highly Active Antiretroviral Therapy (HAART). Resistance testing showed resistance to a minimum of one drug in the NRTI, NNRTI and PI classes. Primary end points in this study involved safety and a change in HIV RNA from baseline to week twenty-four. (Data regarding atazanavir can be seen below in the Drug Interactions Section)
At week 16, interim results showed that the percentage of patients in raltegravir groups + OBT who had a viral load of < 400 copies / ml ranged from 64-84% versus only 22% of patients taking placebo + OBT. Patients in the raltegravir + OBT arms who had viral loads of < 50 copies/ml ranged from 56%-72% as compared to only 19% of patients in the placebo + OBT arm.
At 24 weeks mean viral load had decreased in 99% of raltegravir treated patients and 50% of patients treated with placebo. Similar virologic outcomes were maintained in a majority of the patients through the forty-eighth week of the study. Results are summarized in Table 9.
Steigbigel, et al compared the efficacy and safety of raltegravir in combination with optimized background therapy in treatment experienced patients with drug resistant HIV infection. The BENCHMRK 1 and BENCHMRK 2 trials (39) were randomized, placebo-controlled, double-blind trials to evaluate the efficacy, safety and tolerability of raltegravir in patients who are failing OBT. In each of these studies the patients are randomized to receive raltegravir 400 mg twice daily plus OBT or placebo + OBT. Both trials include over two hundred patients in the raltegravir arms and over one hundred patients in the placebo arms of the studies. Primary end points include CD4 count increases, viral load suppression to less than 400 copies/ml and less than 50 copies/ml. Combined data from week ninety-six for both BENCHMRK studies describes the durability of this agent in combination with other antiretroviral agents for the treatment of HIV in treatment experienced patients, and is described in Table 5.
The STARTMRK trial (23), compared the safety and efficacy of raltegravir versus efavirenz both in combination with tenofovir/emtricitabine in treatment naïve patients infected with HIV/AIDS. In this double-blind study, patients were randomly allocated in a 1:1 ratio to receive 400 mg oral raltegravir twice daily or 600 mg oral efavirenz once daily, in combination with tenofovir and emtricitabine. The primary efficacy endpoint was achievement of a vRNA concentration of less than 50 copies per mL. Week 96 data (24) is described in Table 6.
VIROLOGIC RESISTANCE
Mechanisms of Resistance
Mutations in the integrase gene that are associated with resistance to raltegravir include Q148 and N155. A third pathway to resistance includes an amino acid substitution at Y143. Secondary mutations include L74, E92, T97, E138, G140, V151, G163, H183, V226, S230, or D232 (29). In a recent study of 462 patients, 105 patients had virologic failure (viral load > 400 copies/ml) during the BENCHMRK trials, and of those 105, only 64 showed genotypic differences between baseline and at the time of virologic failure. In these patients the N155 mutation was the most common mutation (45%), but over time, the Q148 mutation emerged as the most dominant mutation, regardless of which was seen first. The N155 mutation reduced sensitivity ten fold, when present alone. Secondary mutations nearly always emerged, which further reduced sensitivity by nearly 100 fold (13).
Raltegravir’s different mechanism of action compared to other FDA approved antiretroviral agents is consistent with the lack of cross-resistance to other classes of antiretrovirals. The use of raltegravir without other medications that have activity against HIV is associated with a rapid loss of activity secondary to a low genetic barrier to resistance, and emergence of resistant subspecies. Substantial cross-resistance exists between raltegravir and elvitgravir, another first generation integrase inhibitor (9, 26) currently FDA approved as part of the coformulated single tablet regimen consisting of elvitegravir/cobicistat/tenofovir/ emtricitabine) (40).
Second generation inhibitor, dolutegravir (S/GSK1349572), is currently in phase III trials. Data suggest that this agent has a slightly different resistance profile than raltegravir or elvitegravir (4). In raltegravir resistant patients with mutations at codons 143 or 155, dolutegravir appears to have continued susceptibility. However, in raltegravir resistant mutations at codon 148, HIV has reduced susceptibility to dolutegravir.
Methods to Overcome or Prevent Resistance
As with other components of combined antiretroviral therapeutic regimens, providing an optimal raltegravir regimen includes sustaining therapeutic plasma concentrations while also including other active antiretrovirals in the combination approach. Adherence training and patient education, as well as avoidance of negative pharmacokinetic interactions are recommended to reduce the emergence of resistance.
REFERENCES
1. Anderson MS, Kakuda TN, Hanley W, Miller J, Kost JT, Stoltz R, Wenning LA, Stone JA, Hoetelmans RM, Wagner JA, Iwamoto M. Minimal pharmacokinetic interaction between the human immunodeficiency virus nonnucleoside reverse transcriptase inhibitor etravirine and the integrase inhibitor raltegravir in healthy subjects. Antimicrob Agents Chemother. 2008;52:4228-32. [PubMed]
2. Barau C, Delaugerre C, Braun J, de Castro N, Furlan V, Charreau I, Gérard L, Lascoux-Combe C, Molina JM, Taburet AM. High Concentration of Raltegravir in Semen of HIV-1 Infected Men: Results from a substudy of the EASIER-ANRS 138 Trial. Antimicrob Agents Chemother. 2010;54:937-39. [PubMed]
3. Best B, Caparelli EV, Stek A, et al. Raltegravir pharmacokinetics during pregnancy [abstract no. H-1668a]. 50th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC). September 12-15, Boston, MA, 2010. [PubMed]
4. Canducci F, Ceresola ER, Boeri E, Spagnuolo V, Cossarini F, Castagna A, Lazzarin A, Clementi M. Cross-resistance profile of the novel integrase inhibitor dolutegravir (S/GSK1349572) using clonal viral variants selected in patients failing raltegravir. J Infect Dis. 2011;204:1811-1815 [PubMed]
5. Clavel C, Peytavin G, Tubiana R, Soulié C, Crenn-Hebert C, Heard I, Bissuel F, Ichou H, Ferreira C, Katlama C, Marcelin AG, Mandelbrot L Raltegravir Concentrations in the Genital Tract of HIV-1 Infected Women Treated with Raltegravir. Antimicrob Agents Chemother. 2011;55(6):3018-21 [PubMed]
6. Colbers A, Molto J, Taylor G, Kabeya K, Rockstroh JK, Wyen C, Weizsäcker K, Sadiq ST, Ivanovic J, Giaquinto C, Taylor GP, Moltó J, Burger DM; PANNA network. A comparison of the pharmacokinetics of raltegravir during pregnancy and post-partum [abstract no. P-18]. 12th International Workshop on Clinical Pharmacology of HIV Therapy. April 13 – 15, Miami, FL, 2011. [PubMed]
7. Croteau D, Letendre S, Best B, Ellis RJ, Breidinger S, Clifford D, Collier A, Gelman B, Marra C, Mbeo G, McCutchan A, Morgello S, Simpson D, Way L, Vaida F, Ueland S, Capparelli E, Grant I; CHARTER Group. Total raltegravir concentrations in cerebrospinal fluid exceed the 50-percent inhibitory concentration for wild-type HIV-1. Antimicrob Agents Chemother. 2010;54:5156-60. [PubMed]
8. Giguere P, la Porte C, Zhang G, Cameron B. Pharmacokinetics of Darunavir, Etravirine and Raltegravir in an HIV-infected patient on haemodialysis. AIDS. 2009; 23: 740-1. [PubMed]
9. Goethals O, Clayton R, Van Ginderen M, Vereycken I, Wagemans E, Geluykens P, Dockx K, Strijbos R, Smits V, Vos A, Meersseman G, Jochmans D, Vermeire K, Schols D, Hallenberger S, Hertogs K. Resistance Mutations in Human Immunodeficiency Virus Type 1 integrase selected with elvitegravir confer reduced susceptibility to a wide range of integrase inhibitors. J Virol. 2008; 82:10366-74. [PubMed]
10. Grinsztejn B, Nguyen BY, Katlama C, Gatell JM, Lazzarin A, Vittecoq D, Gonzalez CJ, Chen J, Harvey CM, Isaacs RD; Protocol 005 Team. Safety and efficacy of the HIV-1 integrase inhibitor raltegravir (MK-0518) in treatment experienced patients with multidrug-resistant virus: a phase II randomised controlled trial. Lancet. 2007;369:1261-9. [PubMed]
11. Hanley WD, Wenning LA, Moreau A, Kost JT, Mangin E, Shamp T, Stone JA, Gottesdiener KM, Wagner JA, Iwamoto M. Effect of tipranavir-ritonavir on pharmacokinetics of raltegravir. Antimicrob Agents Chemother. 2009; 53(7): 2752-5. [PubMed]
12. Hazuda DJ, Miller MD, Nguyen BY. Resistance to the HIV-integrase inhibitor raltegravir: analysis of protocol 005, a phase II study in patients with triple-class resistant HIV-1 infection [abstract no. 8]. Antivi Ther 2007;12: S10. [PubMed]
13. Hazuda DJ, et al. Analysis of resistance to the HIV-1 integrase inhibitor raltegravir: results from the Benchmrk 1 and 2 [abstract J-898]. 48th Interscience Conference on Antimicrobial Agents and Chemotherapy, October 25-28, Washington, D.C., 2008. [PubMed]
14. Isentress (raltegravir): US prescribing information. Whitehouse Station (NJ): Merck and Co, Inc. 2012. [PubMed]
15. Iwamoto M, Wenning LA, Mistry GC, Petry AS, Liou SY, Ghosh K, Breidinger S, Azrolan N, Gutierrez MJ, Bridson WE, Stone JA, Gottesdiener KM, Wagner JA. Atazanavir modestly increases plasma levels of raltegravir in healthy subjects. Clin Infect Dis. 2008;47:137-40. [PubMed]
16. Iwamoto M, Wenning LA, Nguyen BY, Teppler H, Moreau AR, Rhodes RR, Hanley WD, Jin B, Harvey CM, Breidinger SA, Azrolan N, Farmer HF Jr, Isaacs RD, Chodakewitz JA, Stone JA, Wagner JA. Effects of Omeprazole on Plasma Levels of Raltegravir. Clin Infect Dis. 2009;48:489-92. [PubMed]
17. Iwamoto M, Wenning LA, Petry AS, Laethem M, De Smet M, Kost JT, Merschman SA, Strohmaier KM, Ramael S, Lasseter KC, Stone JA, Gottesdiener KM, Wagner JA. Safety, tolerability, and pharmacokinetics of raltegravir after single and multiple doses in healthy subjects. Clin Pharmacol Ther. 2008;83: 293-9. [PubMed]
18. Iwamoto M, Hanley WD, Petry AS, Friedman EJ, Kost JT, Breidinger SA, Lasseter KC, Robson R, Lunde NM, Wenning LA, Stone JA, Wagner JA. Lack of a clinically important effect of moderate hepatic insufficiency and severe renal insufficiency on raltegravir pharmacokinetics. Antimicrob Agents Chemother. 2009; 53(5): 1747-52. [PubMed]
19. Iwamoto M, Wenning LA, Petry AA. Minimal effect of ritonavir (RTV) and efavirenz (EFV) on the pharmacokinetics (PK) of MK-0518. 46th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, September 27-30, 2006. A-0373. [PubMed]
20. Joint United Nations Programme on HIV/AIDS. 2012 report on the global AIDS epidemic (online). http://www.unaids.org/en/resources/publications/2012/name,76121,en.asp (Accessed 2013 Mar 12). [PubMed]
21. Kassahun K, McIntosh I, Cui D, Hreniuk D, Merschman S, Lasseter K, Azrolan N, Iwamoto M, Wagner JA, Wenning LA. Metabolism and Disposition in Humans of raltegravir (MK-0518), an anti-AIDS drug targeting the HIV-1 integrase enzyme. Drug Metab Dispos 2007;35:1657-63. [PubMed]
22. Kim D, Wheeler W, Ziebell R, et al. Prevalence of Transmitted Antiretroviral Drug Resistance among Newly-diagnosed HIV-1-infected Persons, US, 2007. 17th CROI 2010, February 16-19 San Francisco, CA, Abstract #580. [PubMed]
23. Lennox JL, Dejesus E, Lazzarin A, Pollard RB, Madruga JV, Berger DS, Zhao J, Xu X, Williams-Diaz A, Rodgers AJ, Barnard RJ, Miller MD, DiNubile MJ, Nguyen BY, Leavitt R, Sklar P; STARTMRK investigators. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet. 2009;374(9692):796-806. [PubMed]
24. Lennox JL, DeJesus E, Berger DS, Lazzarin A, Pollard RB, Ramalho Madruga JV, Zhao J, Wan H, Gilbert CL, Teppler H, Rodgers AJ, Barnard RJ, Miller MD, Dinubile MJ, Nguyen BY, Leavitt R, Sklar P; STARTMRK Investigators. Raltegravir versus efavirenz regimens in treatment naïve HIV-1 infected patients: 96- week efficacy, durability, subgroup, safety and metabolic analyses. J Acquir Immune Defic Syndr. 2010;55(1):39-48. [PubMed]
25. Llibre JM, Schapiro JM, Clotet B. Clinical implications of genotypic resistance to the newer antiretroviral drugs in HIV-1 infected patients with virologic failure. Clin Infect Dis. 2010;50(6): 872-81. [PubMed]
26. Marinello J, Marchande C, Mott BT, Bain A, Thomas CJ, Pommier Y. Comparison of Raltegravir and Elvitegravir on HIV-1 Integrase Catalytic Reactions and on a series of drug-resistant Integrase Mutants. Biochemistry. 2008;47:9345-54. [PubMed]
27. Markowitz M, Morales-Ramirez JO, Nguyen BY, Kovacs CM, Steigbigel RT, Cooper DA, Liporace R, Schwartz R, Isaacs R, Gilde LR, Wenning L, Zhao J, Teppler H. Antiretroviral activity, pharmacokinetics, and tolerability of MK-0518, a novel inhibitor of HIV-1 integrase, dosed as monotherapy for 10 days in treatment naïve HIV-1 infected individuals. J Acquir Immune Defic Syndr. 2006;43:509-15. [PubMed]
28. Markowitz M, Nguyen BY, Gotuzzo E, Mendo F, Ratanasuwan W, Kovacs C, Prada G, Morales-Ramirez JO, Crumpacker CS, Isaacs RD, Campbell H, Strohmaier KM, Wan H, Danovich RM, Teppler H; Protocol 004 Part II Study Team. Sustained antiretroviral effect of raltegravir after 96 weeks of combination therapy in treatment naïve patients with HIV-1 Infection. J Acquir Immune Defic Syndr. 2009;52:350-6. [PubMed]
29. Markowitz M, Nguyen BY, Gotuzzo E, Mendo F, Ratanasuwan W, Kovacs C, Prada G, Morales-Ramirez JO, Crumpacker CS, Isaacs RD, Gilde LR, Wan H, Miller MD, Wenning LA, Teppler H; Protocol 004 Part II Study Team. Rapid and durable antiretroviral effect of the HIV-1 Integrase inhibitor raltegravir as part of combination therapy in treatment-naive patients with HIV-1 infection: results of a 48-week controlled study. J Acquir Immune Defic Syndr. 2007;46:125-33. [PubMed]
30. Markowitz M, Morales-Ramierz JO, Nguyen BY, Kovac CM, Steigbigel RT, Cooper DA, Liporace R, Schwartz R, Isaacs R, Gilde LR, Wenning L, Zhao J, Teppler H. Antiretroviral activity, pharmacokinetics, and tolerability of MK-0518, a novel inhibitor of HIV-1 Integrase, dosed as monotherapy for 10 days in treatment naïve HIV-1- infected individuals. J Acquired Immune Defic Syndr. 2006;43:509-15. [PubMed]
31. Molto J, Sanz-Moreno J, Valle M, Cedeño S, Bonal J, Bouarich H, Clotet B. Minimal removal of raltegravir by hemodialysis in HIV-infected patients with end-stage renal disease. Antimicrob Agents Chemother. 2010;54:3047-3048. [PubMed]
32. Nachman S, Acosta E, Samson P, et al. Pharmacokinetic (PK), Safety and Efficacy Data on Cohort IIA; youth aged 6-11 from IMPAACT P1066: A Phase I/II Study to Evaluate Raltegravir (RAL) in HIV-1 Infected Youth [poster no 873]. 17th Conference on Retroviruses and Opportunistic Infections. February 16-19, San Francisco, CA, 2010. [PubMed]
33. Nachman S, Acosta E, Samson P, et al. Interim Results from IMPAACT P1066: Raltegravir Oral Chewable Tablet Formulation in Children 6 to 11 Years. [paper 161 lb]. 17th Conference on Retrovirruses and Opportunistic Infections. February 16-19, San Francisco, CA, 2010. 27. [PubMed]
34. Nachman S, Acosta E, Wiznia A, Teppler H, Long M, Homony B, Graham B, Worrell C, Sampson P, Handelsman E. Raltegravir (RAL) pharmacokinetics (PK) in adolescents: Preliminary results from IMPAACT P1066 [Abstract H-4059a]. Program and Abstracts of the 48th Interscience Conference on Antimicrobial Agents and Chemotherapy. October 25-28, Washington, D.C., 2008. [PubMed]
35. Pommier Y, Johnson AA, Marchand C. Integrase Inhibitors to treat HIV/AIDS. Nat Rev Drug Discov. 2005;4:236-48. [PubMed]
36. Richman D, Morton S, Wrin T, Hellmann N, Berry S, Shapiro MF, Bozzette SA. The prevalence of antiretroviral drug resistance in the United States. AIDS. 2004;18:1393-401. [PubMed]
37. Rockstroh JK, Teppler H, Zhao J, Sklar P, Harvey C, Strohmaier K, Leavitt R, Nguyen BY. Safety and efficacy of raltegravir in patients with HIV-1 and hepatitis B and/or hepatitis C co-infection. HIV Medicine. 2012; 13(2): 127-31. [PubMed]
38. Roquebert B, Damond F, Collin G, Matheron S, Peytavin G, Bénard A, Campa P, Chêne G, Brun-Vézinet F, Descamps D; French ANRS HIV-2 Cohort (ANRS CO 05 VIH-2). HIV-2 integrase gene polymorphism and phenotypic susceptibility of HIV-2 clinical isolates to the integrase inhibitors raltegravir and elvitegravir in vitro. J Antimicrob Chemother. 2008;62:914-20. [PubMed]
39. Steigbigel RT, Cooper DA, Teppler H, Eron JJ, Gatell JM, Kumar PN, Rockstroh JK, Schechter M, Katlama C, Markowitz M, Yeni P, Loutfy MR, Lazzarin A, Lennox JL, Clotet B, Zhao J, Wan H, Rhodes RR, Strohmaier KM, Barnard RJ, Isaacs RD, Nguyen BY; BENCHMRK Study Teamsa. Long-term efficacy and safety of raltegravir combined with optimized background therapy in treatment-experienced patients with drug-resistant HIV infection: week 96 results of the BENCHMRK 1 and 2 Phase III trials. Clin Infect Dis. 2010;50:605-12. [PubMed]
40. Stribild (elvitegravir; cobicistat; emtricitabine; tenofovir) package insert. Foster City, CA: Bristol-Myers Squibb, Gilead Sciences, LLC; 2012 Aug. [PubMed]
41. Vispo E, Mena A, Maida I, Blanco F, Cordoba M, Labarga P, Rodriguez-Novoa S, Alvarez E, Jimenez-Nacher I, Soriano V. Hepatic safety profile of raltegravir in HIV-infected patients with chronic hepatitis C. J Antimicrob Chemother. 2010;65:543-7. [PubMed]
42. Wenning L, Anderson M, Petry A, et al. Raltegravir (RAL) dose proportionality and effect of food [abstract no. H-1046]. 47th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2007 Sep 17-20; Chicago, IL, USA. [PubMed]
43. Wenning LA, Petry A, Kost JT, Jin B, Breidinger SA, DeLepeleire I, Carlini EJ, Young S, Rushmore T, Wagner F, Lunde NM, Bieberdorf F, Greenberg H, Stone JA, Wagner JA, Iwamoto M. Pharmacokinetics of raltegravir in individuals with UGT1A1 polymorphisms. Clin Pharmacol Ther. 2009;85:623-7. [PubMed]
44. Wenning LA, Nguyen BY, Sun X, Hwang E, Chen Y, Teppler H, Harvey C, et al. Pharmacokinetic/Pharmaco-dynamic (PK/PD) Analyses for Raltegravir in Phase II and III Studies in Treatment Experienced HIV Infected Patients. 9th International Workshop on Clinical Pharmacology of HIV. April 8, 2008. [PubMed]
45. Wenning LA, Hanley WD, Brainard DM, Petry AS, Ghosh K, Jin B, Mangin E, Marbury TC, Berg JK, Chodakewitz JA, Stone JA, Gottesdiener KM, Wagner JA, Iwamoto M. Effect of rifampin, a potent inducer of drug-metabolizing enzymes, on the pharmacokinetics of raltegravir. Antimicrob Agents Chemother. 2009; 53(7): 2852-6. [PubMed]
46. Wensing AM, van de Vijver DA, Angarano G, Asjö B, Balotta C, Boeri E, Camacho R, Chaix ML, Costagliola D, De Luca A, Derdelinckx I, Grossman Z, Hamouda O, Hatzakis A, Hemmer R, Hoepelman A, Horban A, Korn K, Kücherer C, Leitner T, Loveday C, MacRae E, Maljkovic I, de Mendoza C, Meyer L, Nielsen C, Op de Coul EL, Ormaasen V, Paraskevis D, Perrin L, Puchhammer-Stöckl E, Ruiz L, Salminen M, Schmit JC, Schneider F, Schuurman R, Soriano V, Stanczak G, Stanojevic M, Vandamme AM, Van Laethem K, Violin M, Wilbe K, Yerly S, Zazzi M, Boucher CA; SPREAD Programme. Prevalence of drug-resistant HIV-1 variants in untreated individuals in Europe: implications for clinical management. J Infect Dis. 2005; 192:958-66. [PubMed]
47. Yilmaz A, Gisslen M, Spudich S, Lee E, Jayewardene A, Aweeka F, Price RW. Raltegravir cerebrospinal fluid concentrations in HIV-1 infection. PLoS One. 2009;4:e6877. [PubMed]
48. Zhu K, Dobard C, Chow SA. Requirement for integrase during reverse transcription of human immunodeficiency virus type 1 and the effect of cysteine mutations of integrase on its interactions with reverse transcriptase. J Virol. 2004;78:5045- 5055. [PubMed]
Tables
Table 1. Adverse Events seen at 96 weeks in trials comparing raltegravir plus optimized background therapy with optimized background therapy alone (39).
| Drug Related Adverse Events | Raltegravir (n=462) | Placebo (n=237) |
|---|---|---|
| Abdominal Distention | 2.2 (1.2) | 1.7 (1.5) |
| Diarrhea | 3.2 (1.8) | 5.1 (4.5) |
| Nausea | 4.1 (2.3) | 4.6 (4.1) |
| Vomiting | 1.5 (0.8) | 2.1 (1.9) |
| Fatigue | 3.2 (1.8) | 0.8 (0.7) |
| Pyrexia | 0.9 (0.5) | 2.5 (2.2) |
| Headache | 4.8 (2.7) | 5.1 (4.5) |
Table 2. Adverse events seen at 96 weeks in trials comparing raltegravir versus efavirenz both combined with tenofovir/lamivudine in treatment naive patients (28).
| Drug Related Adverse Events | Raltegravir (n=160) | Efavirenz (n=38) |
|---|---|---|
| Flatulence | 5.6% | 2.6% |
| Diarrhea | 6.9% | 10.5% |
| Nausea | 12.5% | 13.2% |
| Vomiting | 2.5% | 7.9% |
| Fatigue | 5% | 5.3% |
| Insomnia | 8.1% | 10.5% |
| Headache | 8.8% | 23.7% |
| Dizziness | 8.8% | 28.9% |
| Abnormal Dreams | 6.3% | 18.4% |
| Anxiety | 1.3% | 5.3% |
| Disturbance in Attention | 0.6% | 5.3% |
Table 3. Grade 3 or 4 laboratory events seen at 96 weeks in trials comparing raltegravir versus efavirenz both combined with tenofovir/lamivudine in treatment naive patients (28).
| Drug Related Laboratory Event | Raltegravir (n=160) | Efavirenz (n=38) |
|---|---|---|
| Absolute Neutrophil Count | 0.6% | 0.0% |
| Fasting LDL Cholesterol | 0.6% | 5.3% |
| Fasting total Cholesterol | 0.0% | 5.3% |
| Fasting Triglycerides | 0.0% | 7.9% |
| Creatine Phosphokinase | 6.3% | 2.6% |
Table 4. Adverse events seen at 96 weeks in trials comparing raltegravir versus efavirenz both combined with tenofovir/emtricitabine in treatment naive patients (24)
| Drug Related Adverse Events | Raltegravir (n=281) | Efavirenz (n=282) |
|---|---|---|
| Diarrhea | 1.1% | 2.8% |
| Nausea | 2.8% | 3.5% |
| Fatigue | 1.8% | 2.8% |
| Insomnia | 3.6% | 3.2% |
| Headache | 3.9% | 4.6% |
| Dizziness | 1.4% | 6.4% |
| Rash | 0.0% | 6.7% |
Table 5. 96 week BENCHMARK trial results (p<0.001)
| Outcome | Raltegravir (N - 462) | Placebo (N-237) |
|---|---|---|
| HIV RNA < 400 copies /ml | 61% | 28% |
| HIV RNA < 50 copies /ml | 57% | 26% |
| Mean HIV RNA change from baseline (log 10 copies /ml) | - 1.5 | - 0.6 |
| Virologic failure (confirmed) | 150 (33%) | 148 (62%) |
Table 6. 96 week STARTMARK trial results (p<0.001)
| Outcome | Raltegravir (N – 245) | Efavirenz (N – 232) |
|---|---|---|
| HIV RNA < 50 copies/ml (week 48) | 86% | 82% |
| HIV RNA < 50 copies /ml (week 96) | 81% | 79% |
| Virologic failure (confirmed) | 39 patients | 45 patients |
Table 7. Mean decease in viral baseload in treatment-naive patients with raltegravir therapy (29)
| Treatment Group (Twice daily) | HIV RNA < 400 copies /ml, % (95% CI) | HIV RNA < 50 copies /ml, % (95% CI) |
|---|---|---|
| 100 mg | 57 (18 to 90) | 14 (0 to 58) |
| 200 mg | 57 (18 to 90) | 29 (4 to 71) |
| 400 mg | 50 (12 to 88) | 17 (0 to 64) |
| 600 mg | 50 (16 to 84) | 13 (0 to 53) |
| Placebo | 0 (0 to 41) | 0 (0 to 41) |
Table 8. Mean decrease in patient viral baseload in trials comparing raltegravir and efavirenz combination therapies (30)
| Treatment | N | HIV RNA copies / ml, % (95% CI) | |||
|---|---|---|---|---|---|
| < 400 | < 50 | ||||
| Week 24 | Week 48 | Week 24 | Week 48 | ||
| RTG 100 mg | 39 | 95 (83,99) | 97 (87,100) | 87 (73,96) | 85 (70,94) |
| RTG 200 mg | 40 | 85 (70, 94) | 85 (70,94) | 85 (70,94) | 83 (67,93) |
| RTG 400 mg | 41 | 98 (87,100) | 98 (87,100) | 93 (80,99) | 88 (74,96) |
| RTG 600 mg | 40 | 95 (83,99) | 90 (76,97) | 95 (83,99) | 88 (73,96) |
| Placebo | 38 | 95 (82,99) | 87 (72,96) | 92 (78,98) | 87 (72,96) |
Table 9. Table 8. Mean decrease in patient viral baseload in trials comparing raltegravir and efavirenz combination therapies (2) (10)
| Treatment | N | HIV RNA copies / ml, % (95% CI) | |||
|---|---|---|---|---|---|
| < 400 | < 50 | ||||
| Week 24 | Week 48 | Week 24 | Week 48 | ||
| RTG 200 mg | 43 | 70 (54,83) | 69 (53,82) | 65 (49,79) | 64 (48,78) |
| RTG 400 mg | 45 | 71 (56,84) | 64 (48,78) | 56 (40,70) | 46 (30,61) |
| RTG 600 mg | 45 | 71 (56,84) | 71 (56,84) | 67 (51,80) | 53 (38,68) |
| Placebo | 45 | 16 (7,30) | 13 (5,27) | 13 (5,27) | 9 (3,21) |
What's New
Blanco JL, et al. HIV-1 Integrase Inhibitor Resistance and Its Clinical Implications. J Infect Dis 2011;203:1204-1214.
