Table 1. Pharmacokinetics of Chloramphenicol [Mean ± SD (Range)] Among Different Patient Populations Receiving Chloramphenicol Succinate1
|
Age |
Cp (mg/ml) |
tmax (hr) |
t1/2 (hr) |
Vd (L/kg) |
CL t (ml/min) |
CL r (ml/min) |
Reference |
|
|
|
|
|
|
|
|
|
|
43-69 Y 2 |
NA |
1.7 (1.0-2.5) |
5.1 (3.1-8.1)
|
0.55 (0.37-1.00) |
88.3 3 (53.4-189.0) |
10.53 (0-30.0) |
(119) |
|
19-64 Y |
15.4±5.8 4 |
0.3±0.2 |
3.2±1.0 |
0.81±0.18 |
228.0±113.4 |
22.4±9.0 |
(19)
|
|
1 M-6 Y |
NA |
NA |
4.0± 1.8 (2.1-8.3) |
1.39±0.34 (0.78-2.09)
|
52.8±35.6 (8.8-135.3) |
NA |
(116)
|
|
2.4- 4.8 M
6 M- 2 Y
7- 15 Y |
32.4 5 (16.5-51.1)
20.45 (11.0-37.0)
21.85 (11.8-32.9) |
NA
NA
NA |
5.6 (4.2-7.6)
3.8 (2.0-9.8)
3.0 (2.1-5.2) |
0.79 (0.61-0.88)
1.44 (1.04-2.36)
0.97 (0.84-1.10) |
10.5 (7.7-12.7)
27.6 (14.2-34.7)
130.2 (100.5-159.8)
|
NA
NA
NA |
(18)
(18)
(18) |
|
2.5 M- 20 Y |
29.6±16.2 6 (9.3-66.7) |
NA |
4.0±1.9 (2.1-7.9) |
0.71±0.52 (0.25-1.99) |
30.4±23.8 (1.5-70.0) |
7.1±7.8 (0.7-23.1) |
(96)
|
|
10.4± 3.3 D |
NA |
NA |
16.1± 2.9 |
NA |
1.1±0.2 7 |
NA |
(105) |
1 Patients in the different studies had varying degrees of organ function, which may have contributed to the heterogeneity of the resulting pharmacokinetic parameters.
2 Hepatic or renal abnormalities were noted in 9 out of 10 patients
3 ml/min/1.73m2
4 Peak concentration observed after end of infusion
5 Peak concentration 0.5-4.0 hours after start of infusion
6 Steady state peak concentration
7 ml/min/kg
Abbreviations: Cp = peak concentration; tmax = time of the maximum concentration; t1/2 = elimination half-life; Vd = volume of distribution; CLt = total clearance; CLr = renal clearance; Y = years; M = months; D = days; NA = not available
Table 2. Dosage Formulations of Chloramphenicol
|
Oral Chloramphenicol capsulesa 250 mg Chloramphenicol palmitate solutiona 150 mg/mL
Intravenous Injection, powder for reconstitution, as sodium succinate 1 g vial
Otic Chloramphenicol otic solution 0.5%
Ophthalmic Chloramphenicol ophthalmic solution 0.5% Chloramphenicol ophthalmic ointment 1% Powder for ophthalmic solution a 25 mg/vial |
a Not available in the United States
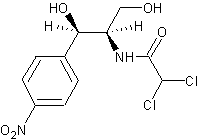
Figure 1. Chemical Structure of Chloramphenicol
