|
Bacteria |
Erythro-mycin |
Roxithro- mycin |
Clarithro-mycin |
Dirithro-mycin |
Azithro-mycin |
Mioca-mycin |
Josa-mycin |
Spira-mycin |
Rokita-mycin |
|
Staphylococcus aureus (MSSA) a |
0.1-0.5 |
0.2-0.5 |
0.06-0.5 |
0.02-2 |
0.02-1 |
0.5-4 |
0.5-64 |
0.25-64 |
0.25-4 |
|
Streptococcus pneumoniae |
0.015-1 |
0.05-0.2 |
0.015-0.5 |
0.06-1 |
0.06-2 |
0.12-0.5 |
0.03-0.12 |
0.015-0.03 |
0.12 |
|
Streptococcus pyogenes |
0.03-0.06 |
0.03-0.06 |
0.015-0.015 |
0.03-0.12 |
0.03-0.12 |
0.25-0.5 |
0.06-0.25 |
0.06-0.12 |
0.12-0.25 |
|
Haemophilus influenzae |
1-8 |
1-8 |
1-8 |
0.2-32 |
0.2-4 |
16 ->16 |
4-32 |
4-16 |
4-16 |
|
Chlamydia pneumoniae |
0.06 |
0.25 |
0.007 |
0.5 |
< 2 |
|
4-32 |
4-16 |
|
|
Moraxella catarrhalis |
0.1-0.5 |
0.5-2 |
0.06-2 |
0.1-1 |
0.01-0.1 |
|
0.25 |
4 |
|
|
Legionella pneumophila |
0.1-1 |
0.06-0.5 |
0.1-0.5 |
0.5-4 |
0.125-0.5 |
0.1-0.5 |
0.5-1 |
8-64 |
0.12-0.25 |
|
Helicobacter pylori |
0.1 |
0.07 |
0.03 |
0.06-0.5 |
0.2 |
|
0.5-1 |
8-64 |
|
|
Chlamydia trachomatis |
0.06-1 |
0.015-2 |
0.004-0.2 |
1 |
0.03-0.06 |
0.06 |
|
|
|
|
Borrelia burgdorferi |
0.03-0.12 |
0.015-0.12 |
0.015-0.12 |
< 0.5 |
0.015-0.12 |
|
|
|
|
|
Mycobacterium avium and complex |
32-64 |
8-32 |
0.5-8 |
|
8-32 |
|
|
|
|
a MRSA are usually resistant to macrolides
Data from (45, 174, 175, 177, 327, 345, 354, 358)
|
Bacterial species |
% resistance |
mechanism |
genetic support |
phenotype |
frequency |
references |
|
S. aureus |
> 80 % MRSA ~ 40 % MSSA |
Ribosomal methylation |
erm(A), (C) |
MLSB |
> 80% |
|
|
efflux |
msr(A) |
MSB |
< 10 % |
|||
|
Antibiotic inactivation |
|
|
rare |
|||
|
S. pneumoniae |
~ 30 % |
Ribosomal methylation |
erm(B) |
MLSB |
~ 65 % |
(126) |
|
23S rRNA mutation |
|
ML |
Rare |
|||
|
r-protein mutation |
|
MSB |
Rare |
|||
|
Efflux |
mef(A)- mef(E) |
M |
~ 35 % |
|||
|
S. pyogenes |
~ 10 % |
Ribosomal methylation |
erm(A), (B) |
MLSB |
~ 50 % |
(126) |
|
Efflux |
mef(A) |
M |
~ 50 % |
|||
|
Haemophilus influenzae |
rare |
Ribosomal methylation |
|
MLSB |
|
(331) |
|
23S rRNA mutation |
|
ML |
~ 10 % |
|||
|
r-protein mutation |
|
MSB |
~ 65 % |
|||
|
Efflux |
|
M |
~ 100 % |
|||
|
Helicobacter pylori |
~ 10 % |
23S rRNA mutation |
|
ML |
|
(282) |
|
Mycoplasma |
|
Ribosomal methylation |
|
MLSB |
|
(59) |
|
|
|
23S rRNA mutation |
|
ML |
|
(249) |
|
Mycobacteria |
|
23S rRNA mutation |
|
ML |
|
(249) |
|
Enterobacteriaceae |
|
Antibiotic inactivation |
ere |
M |
|
(249) |
|
Pharmacokinetic parameter |
Erythromycin (45) |
Roxithromycin (349) |
Clarithromycin |
Dirithromycin |
Azithromycin |
Miocamycin (52) |
Josamycin (59) |
Spiramycin |
|
Cmax (mg/l) |
3 |
6.8 |
6.8 |
0.2-0.6 |
0.4 |
2-3 |
1.2 |
1.2 |
|
Tmax (h) |
1.9-4.4 |
2 |
2.7 |
3-5 |
2.5 |
2 |
1 |
2 |
|
T ½ (h) |
2 |
8-13 |
4.4 |
42 |
35-40 |
1 |
2 |
8 |
|
Vd (l/kg) |
0.64 |
|
3-4 |
11 |
23-31 |
|
|
|
|
Bioavailability |
25-60 % |
72-85 % |
55 % |
6-14% |
37% |
|
|
|
|
Protein binding |
65-90 |
73-96 |
40-70 |
15-30 |
12-40 |
|
10 |
12 |
|
Tissue/serum concentration |
0.5 |
1-2 |
3-8 |
20-30 |
50-1150 |
|
2-20 b |
1-30 |
|
AUC (mg.h/l) |
4.4-14 |
70 |
4.1 |
3.8 |
2-3.4 |
3 |
7.9 |
8.5 |
|
|
adult |
child |
|
Erythromycin |
500 mg 4 X/day |
12.5 mg/kg 4 X/day |
|
Roxithromycin |
150 mg 2 X/day |
3 mg/kg 2 X/ day |
|
Clarithromycin a |
250 mg-1000 mg 2 X/day |
7.5 mg/kg 2 X/day |
|
Dirithromycin |
500 mg 1 X/day |
- |
|
Azithromycin |
500 mg 1 X/day or |
10 mg/kg on day 1 and 5 mg/kg on days 2-5 |
|
Miocamycin a |
600 mg 2 X/day |
25 mg/kg 2 X/day |
|
Spiramycin |
3 Mio U 2-3 X/day |
0.075-0.1 Mio U/kg 2-3 X/day |
|
Josamycin a |
500 mg – 1000 mg 2 X/day |
10-20 mg/kg 2-3 X/day |
a
a 3 X/day
administration should be preferred to an increase of the dose given 2 X/day in
case of less susceptible organism, based on pharmacodynamic considerations
(time above MIC).
|
Macrolide |
Totally contra-indicated drugs |
Drugs to use with caution (requiring a dose reduction and/or a therapeutic monitoring) |
|
Erythromycin |
astemizole cisapride ergotamine terfenadine
|
oral anticoagulants benzodiazepines bromocriptine carbamazepine cyclosporin clozapine digoxin felodipine lovastatin sildenafil theophylline |
|
Roxithromycin |
astemizole cisapride ergotamine terfenadine |
benzodiazepines bromocriptine theophylline |
|
Clarithromycin |
astemizole cisapride ergotamine terfenadine |
oral anticoagulants bromocriptine carbamazepine cyclosporine clozapine digoxin theophylline |
|
Dirithromycin |
astemizole cisapride ergotamine terfenadine |
|
|
Azithromycin |
astemizole cisapride ergotamine terfenadine |
|
|
Miocamycin |
astemizole cisapride ergotamine terfenadine |
carbamazepine cyclosporine
|
|
Josamycin |
astemizole cisapride ergotamine terfenadine |
benzodiazepines bromocriptine carbamazepine cyclosporine theophylline |
|
Spiramycin |
astemizole cisapride ergotamine terfenadine |
|
|
Rokitamycin |
astemizole cisapride ergotamine terfenadine |
|
(based on demonstration of increase in serum level by coadministration with macrolides [interaction with cytochrome P450]) (11, 333, 473)
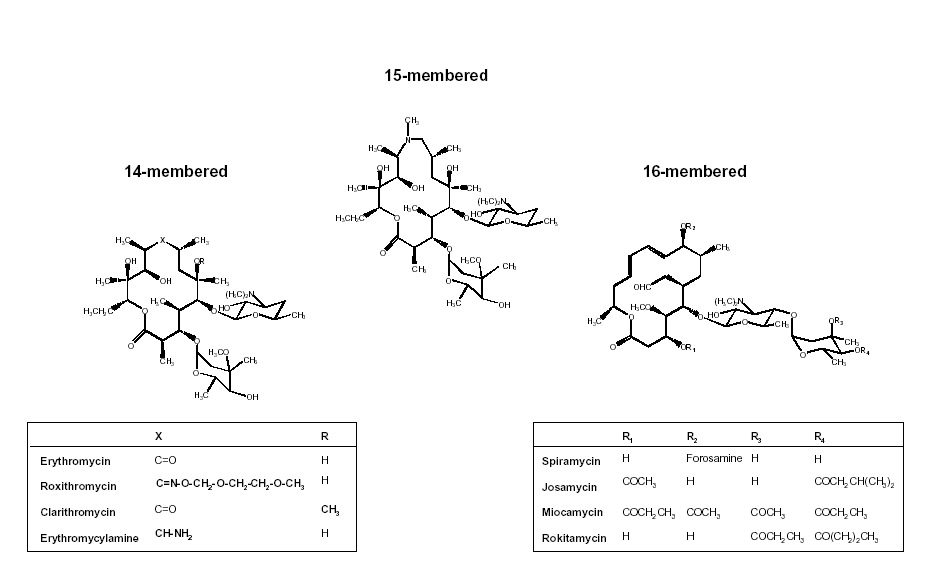
Figure 1. Chemical structure of the macrolide antibiotics currently used in the clinics. Theses are classified according to the number of atoms in the macrocycle. All molecules possess an aminated sugar (desosamine) which confers to them a basic character responsible for their cellular accumulation (azithromycin and erythromycylamine have a second aminated function and are therefore dibasic molecules, which explains their higher level of cellular accumulation). Erythromycylamine is commercialized as a prodrug (dirithromycin) which is intrinsically inactive but regenerates erythromycylamine in vivo or in vitro (230).

Figure 2. Mechanism responsible for the inactivation of erythromycin in acidic medium. The ketone in position 9 reacts with the hydroxyl in position 6 to generate a hemiketal, which reacts again with the hydroxyl in 12 to produce a ketal. Both the hemiketal and the ketal are microbiologically inactive. Neomacrolides (see Figure 1) were made are acidostable by either removing the 9-keto function and replacing it with another function (roxithromycin, erythromycylamine, azithromycin), or by substituting the 6-hydroxyl group (clarithromycin; the same approach has been followed for telithromycin). 16-membered derivatives are intrinsically stable because of the absence of a ketone function in the cycle. Adapted from (231)
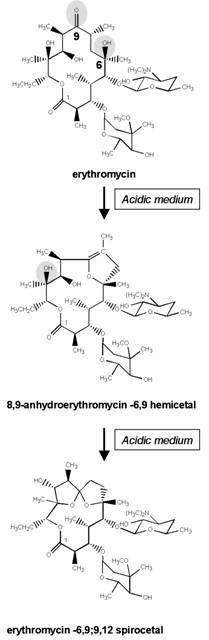
Figure 3. Macrolides in interaction with their ribosomal target. Upper panel: 50S ribosomal subunit of Deinococcus radiodurans in cross section, showing the path of the peptide through the tunnel from the peptidyl -transferase site to emerge of the subunit. The elongating peptide is shown in light blue, the macrolide bound to the ribosome in red, and tRNA in green. Lower panel: erythromycin in interaction with the ribosome, with the desosamine interacts with adenine 2058 shown in grey. Color codes: dark blue: domain V; dark mauve: contacts between erythromycin and domain V, violet: domain II, light blue: domain IV; yellow ribbon; L4 protein; green ribbon, L22 protein. The left panel is a view from the tunnel to erythromycin and the peptidyl transferase site; the right panel is a view from the peptidyl transferase site to the tunnel. The figure also shows the position of the bases involved in the interaction with the antibiotic. Figure prepared by J.M. Harms, Max-Planck-Research Unit for Ribosomal Structure, Hamburg, Germany.
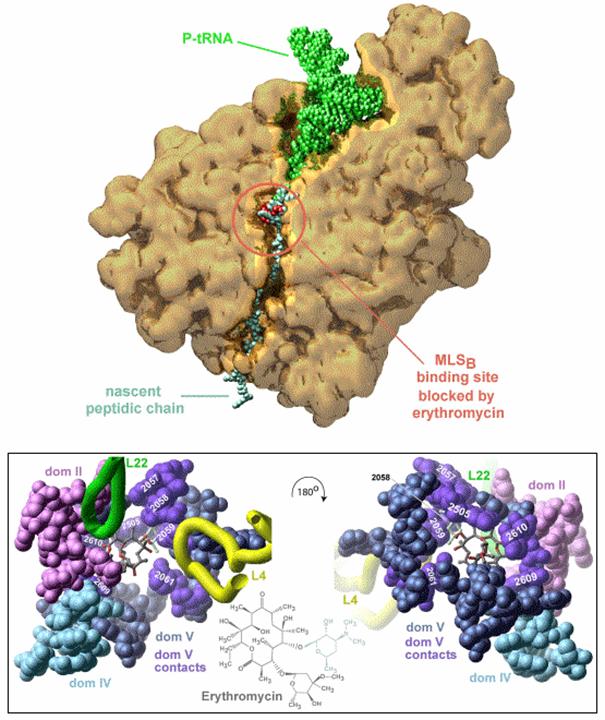
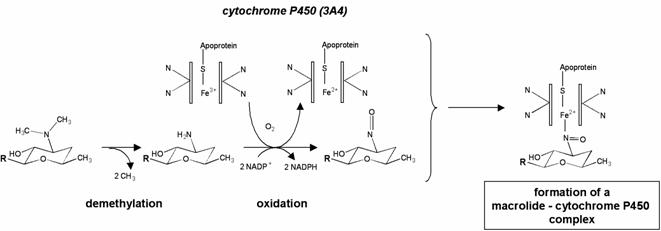
Figure 4. Metabolization of erythromycin by cytochrome P450 and formation of an inactive complex. The tertiary amine of the desosamine is metabolized in nitroso-alkane, which forms a stable, inactive complex with the ion Fe2+ of the cytochrome. This mechanism is responsible for the inhibition by macrolides of the metabolization of other drugs. Adapted from (333).